Abstract
Aedes aegypti, also known as the yellow fever mosquito, presents a major public health challenge, highlighting the need for effective biorational agents for mosquito control. Here, we investigated the synergistic effects of essential oil mixtures derived from Hypenia irregularis that is a mint-family shrub native to Brazil’s Cerrado biome, known as “alecrim do Cerrado”, in combination with essential oils from noni (Morinda citrifolia), Brazilian mint (“salva-do-Marajó”, Hyptis crenata), and lemongrass (Cymbopogon citratus) against Ae. aegypti. We conducted phytochemical analyses and assessed larvicidal, repellent, and oviposition deterrent activities. Using in silico methods, we predicted molecular interactions between key essential oil components and physiological targets involved in repellent action (odorant-binding protein AeagOBP1 and olfactory receptor Or31) and larvicidal activity (GABA and octopamine receptors, TRP channels, and acetylcholinesterase [AChE]). Major compounds identified included octanoic acid (23%; Hipe. irregularis × M. citrifolia), 2,5-dimethoxy-p-cymene (21.9%; Hipe. irregularis × Hypt. crenata), and citral (23.0%; Hipe. irregularis × C. citratus). Although individual oils showed strong larvicidal activity (Hipe. irregularis LC50 = 2.35 µL/mL; Hypt. crenata = 2.37 µL/mL; M. citrifolia and C. citratus = 2.71 µL/mL), their mixtures did not display synergistic effects. Similarly, repellency and oviposition deterrence were comparable to DEET for individual oils but were not enhanced in mixtures. Notably, the Hipe. irregularis × C. citratus essential oil blend reduced oviposition deterrence. Molecular docking confirmed strong binding of major oil components to AeagOBP1 and Or31, supporting their role in repellency. For larvicidal effects, AChE showed the highest predicted binding affinity. Overall, our findings suggest that H. irregularis, Hypt. crenata, C. citratus, and M. citrifolia (alone or in 1:1 mixture) are promising, sustainable agents for A. aegypti control.
Keywords:
plant-based biorational insecticides; deterrence oviposition; molecular docking; biorational repellents Key Contribution:
This manuscript reinforces the larvicidal and repellent activity of Hypenia irregularis essential oils. However; mixing essential oil of Hype. irregularis with essential oil from other plant species (e.g., Hyptis crenata; Morinda citrifolia; or Cymbopogon citratus) did not enhance efficacy; suggesting that activity depends more on dominant constituents than on synergic actions. Molecular docking analyses indicated acetylcholinesterase; odorant-binding protein AeagOBP1; and olfactory receptor Or31 as likely targets for the Hype. Irregularis bioactivities on Aedes aegypti mosquitoes.
1. Introduction
The yellow fever mosquito, Aedes aegypti (Diptera: Culicidae) (Linnaeus, 1762), is a primary vector responsible for the transmission of several arboviruses, including dengue, yellow fever, chikungunya, and Zika. Consequently, it poses significant public health concerns. These vectors contribute to greater human morbidity and mortality than any other arthropod-borne viral diseases in tropical and subtropical regions worldwide [1]. In 2022, the World Health Organization (WHO) estimated approximately 390 million annual dengue virus infections globally, with 70% occurring in Asia [1]. In particular, Brazil reported nearly 3 million dengue cases in 2023 [2]. As of February 2024, over 512,000 cases had already been recorded—three times the number reported during the same period in 2023 [3]. These data underscore the urgent need for improved control strategies targeting A. aegypti, especially in Brazil, where its impact on public health is especially critical.
Aedes aegypti mosquitoes develop in a wide range of natural and artificial containers during their aquatic immature stages. As adults, females require blood meals to complete their reproductive cycle. If infected with a virus, they can transmit pathogens responsible for the aforementioned diseases through biting, resulting in thousands of human deaths annually [1,4]. As a preventive measure, public health authorities often apply synthetic insecticides in open areas and residential settings. However, extensive use of these chemical agents has raised environmental concerns, including harmful effects on non-target organisms and mammals [5,6]. Notably, N,N-diethyl-meta-toluamide (DEET) and its derivatives—widely used mosquito repellents for over 70 years—are associated with such adverse effects [6]. Furthermore, the growing resistance of A. aegypti populations to conventional insecticides presents a major challenge [7,8]. These issues have intensified interest in plant-derived products with biological activity against mosquito vectors, particularly essential oils, which have demonstrated both toxic effects and behavioral disruption in Ae. aegypti.
In this context, essential oils have emerged as promising agents with toxic effects against both adult and larval stages of mosquitoes, while offering the advantage of selectivity toward natural enemies [9,10,11,12]. Their chemical complexity, consisting of diverse molecular constituents, reduces the likelihood of resistance development in insect populations. Pavela and Benelli [13] noted that the biological efficacy of essential oils may arise from synergistic interactions among all their components or from the activity of major compounds present at higher concentrations. As a result, essential oils can act in multifaceted ways on various targets within the insect organism. Several studies have shown that these biomolecules interfere with key physiological and biochemical processes, including the inhibition or modulation of cytochrome P450 enzymes, acetylcholinesterase (AChE), glutathione S-transferase (GST), γ-aminobutyric acid (GABA) receptors, transient receptor potential (TRP) channels, and the cholinergic and octopaminergic systems [13,14,15,16,17,18,19,20,21,22]. Furthermore, essential oils are considered safe for human use and are already widely employed in pharmaceutical, food, cosmetic, and perfumery industries, as well as in aromatherapy and natural medicine [23].
The plants Hypenia irregularis (Benth.) Harley (Lamiaceae), Morinda citrifolia L. (Rubiaceae), Hyptis crenata Pohl ex Benth (Lamiaceae), and Cymbopogon citratus (DC.) Stapf (Poaceae) have demonstrated notable insecticidal, fungicidal, and acaricidal properties [24,25,26,27,28,29]. Moreover, the major constituents of their essential oils have been linked to behavioral and physiological changes in insects, including oviposition deterrence, antifeedant activity, and other behavioral modifications [30,31,32,33,34]. The distribution of these plants reflects a remarkable adaptation to diverse habitats and soil-climatic conditions [35,36,37,38]. While Hype. irregularis is a mint-family shrub mint native to the Central Brazilian Cerrado, where acidic, nutrient-poor soils and a seasonally dry tropical climate contribute to its unique secondary metabolism [35], Hypt. crenata plants, also known as Brazilian mint (“salva-do-Marajó”) occurs in the Amazon, especially near the Amazon River and Marajó Island, thriving in humid, sandy soils with variable rainfall and high temperatures, which shape its essential oil profile [36]. Morinda citrifolia grows widely across tropical regions from Southeast Asia to the Caribbean, tolerating diverse soils and climates, including drought and salinity [37]. Cymbopogon citratus, i.e., lemongrass, plants are cultivated globally in tropical areas, favoring well-drained, moderately fertile soil and warm, sunny conditions with high rainfall, but is sensitive to frost [38].
Oviposition deterrence, antifeedant activity, and other behavioral modifications effects mediated by plant-based essential oil are particularly relevant in vector species and should be incorporated into A. aegypti management strategies. For instance, repellency minimizes human–vector contact, a function typically targeted by commercial chemical repellents. In addition, oviposition deterrence can reduce egg density in high-risk areas, while oviposition attraction may be leveraged in attractant-baited traps as part of integrated control strategies. Therefore, both effects warrant further exploration. Although numerous studies have documented the efficacy of essential oils against mosquito vectors and attributed these effects to the synergistic actions of their chemical constituents [7,39], relatively few investigations have explored the potentially enhanced effects of combining two different essential oils.
Plant-derived green products are increasingly recognized as safe alternatives for vector control, acting either as preventive agents (e.g., repellents and oviposition deterrents) or as curative agents through their toxic effects, especially in vulnerable environments such as residential areas. Given the documented biological activity of Hype. irregularis, M. citrifolia, Hypt. crenata, and C. citratus, along with their natural availability in Brazil, these species represent a potentially sustainable source of biorational products that can synergistically affect A. aegypti.
Here, considering that the individual insecticidal potential of essential oils from Hype. irregularis, M. citrifolia, Hypt. crenata, and C. citratus has already been reported, we aimed to investigate whether Hype. irregularis essential oil exhibits synergistic effects against A. aegypti when combined with the essential oils of the other three plant species. Larvicidal activity (lethality), repellency, and oviposition deterrence were evaluated to compare the efficacy of Hype. irregularis essential oil alone and in combination with the others. Additionally, we conducted in silico predictions to explore the potential involvement of TRP channels, AChE, GABA and octopamine receptors in the toxicity of the major compounds identified in the essential oil mixtures. We also evaluated molecular targets related to repellency (e.g., odorant-binding protein AeagOBP1 and olfactory receptor Or31). These targets, which are structurally characterized in A. aegypti and associated with major classes of insecticides and repellents, were selected to provide preliminary insights into the potential modes of action of the essential oil combinations.
2. Results
2.1. Identification of Peaks in GC–MS of Essential Oils
Our results showed an essential oil extraction yield of 0.37% for Hipe. irregularis; of 2.98% for M. citrifolia; of 0.92% for Hypt. crenata and 0.73% for C. citratus. The phytochemical profiles of each essential oil were already described in previous investigations (Supplementary Table S1). Here, we our phytochemistry analysis revealed the octanoic acid (23.0%), 2,5-dimethoxy-p-cymene (19.5%), and α-cymene (10.0%) as the major constituents identified in the combination of Hipe. irregularis and M. citrifolia essential oils (Table 1). In the mixture of Hipe. irregularis with Hypt. crenata, the predominant compounds were 2,5-dimethoxy-p-cymene (21.9%), carvacrol (10.4%), and α-cymene (10.0%) (Table 1). For the combination of Hipe. irregularis and C. citratus, the most abundant components were citral A (23.0%), 2,5-dimethoxy-p-cymene (15.5%), and citral B (17.1%) (Table 1).

Table 1.
Relative percentage (area%), obtained by gas chromatography coupled to mass spectrometry (GC–MS) detector of the constituents of the essential oils from the dried leaves of Hypenia irregularis x Morinda citrifolia, Hyptis crenata and Cymbopogon citratus.
2.2. Toxicity of Essential Oils to Aedes aegypti Third-Instar Larvae
When tested individually, Hipe. irregularis essential oil exhibited similar larvicidal potential compared to the essential oils of the other plants (Table 2). When evaluating potential synergistic effects, only numerical variations were recorded among the different combinations and proportions, without presenting any statistical difference (Table 3).

Table 2.
Toxicity of essential oils of Hypenia irregularis, Morinda citrifolia, Hyptis crenata, and Cymbopogon citratus against larvae of Aedes aegypti.

Table 3.
Estimated lethal concentrations (LC) of essential oil mixtures containing Hypenia irregularis x Morinda citrifolia, Hipe. irregularis x Hyptis crenata, and Hipe. irregularis x Cymbopogon citratus against larvae of Aedes aegypti.
2.3. Molecular Modeling Predictions
The selected templates for homology modeling are presented in Supplementary Table S2, along with their sequence identities, Ramachandran favored values, and QMEAN scores used for model validation. The templates selected for AChE, GABA receptor, TRP channel, Octopamine receptor, odorant-binding protein (OBP), and Odorant Receptor AaOr31 were XP_021699617.1, AAA68961.1, AAEL005437, XP_021692997.1, AaegOBP1, and AAEL013217, respectively (Supplementary Table S2).
The major compounds from the different plants were docked with various receptors, forming multiple types of interactions with varying binding affinities, as indicated by the docking assays (Table 4).

Table 4.
Molecular docking results for complexes between major compounds and target of Aedes aegypti.
The best affinity energy results were observed for AChE, OBP, and odorant receptor AaOr31. The molecular interactions of the essential oil major compounds with the active sites of three neurophysiological targets, i.e., AChE, odorant-binding protein (OBP), and odorant receptor 31 (OR31), revealed distinct binding profiles (Figure 1). Within the AChE active site, carvacrol exhibited strong hydrophobic interactions, including pi–alkyl contacts with TYR341 and PHE312 and a pi–pi stacking interaction with TRP86, although an unfavorable steric clash was noted with HIS438. Citral formed multiple hydrophobic contacts, including pi–alkyl, pi–sigma, and alkyl interactions, primarily with THR250, PHE295, ALA310, and TRP425, suggesting a relatively flexible accommodation within the catalytic pocket. 2,5-dimethoxy-p-cymene displayed conventional hydrogen bonding with SER254 and ARG255, along with van der Waals forces involving GLY258, LEU310, and GLU379, contributing to the stability of the ligand–protein complex. Octanoic acid engaged mainly in conventional hydrogen bonds with ASP74 and ASN96, as well as van der Waals interactions with LYS75, ILE95, and PHE151, indicating a more polar interaction profile. Regarding OBP, carvacrol established amide pi-stacked and pi–alkyl interactions with aromatic residues such as TRP109, PHE110, and GLU101, stabilizing the ligand within the binding pocket.
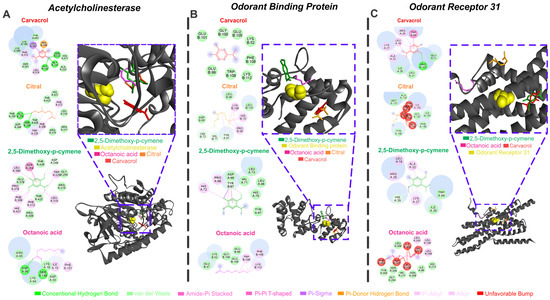
Figure 1.
Carvacrol, citral, 2,5-dimethoxy-p-cymene, and octanoic acid bind with acetylcholinesterase (AChE) (A), Odorant Binding Protein (OBP) (B), and odorant receptor 31 (OR31) (C) target complexes of Aedes aegypti; the 2D maps of molecular interactions with amino acids in each target active site (yellow) are also shown.
Citral engaged in a network of pi–donor hydrogen bonds and pi–alkyl contacts involving ASP66, VAL67, LYS110, and TYR97, supporting moderate affinity and a flexible orientation. 2,5-dimethoxy-p-cymene formed hydrogen bonds with ASP96 and TYR97, along with van der Waals interactions involving HIS70, PRO98, and LEU73, reflecting a balanced hydrophobic and polar interaction pattern.
Octanoic acid exhibited alkyl, pi–alkyl, and pi–sigma interactions with GLU105, VAL100, and LYS112, anchoring the ligand within the hydrophobic core of the protein. In the OR31 binding domain, carvacrol formed pi–alkyl interactions with ARG23 and TRP49, while also presenting an unfavorable steric clash near LYS24, potentially compromising binding stability. Citral primarily engaged in hydrophobic interactions, including alkyl and pi–alkyl contacts with MET131, PHE134, ILE141, and ALA133, suggesting tight packing within the transmembrane region. 2,5-dimethoxy-p-cymene displayed hydrogen bonding with HIS25 and ARG26 and van der Waals interactions with LEU18, TRP94, and LYS27, indicating a strong and stable binding configuration. Finally, octanoic acid interacted through alkyl and van der Waals contacts with ILE43, ILE45, SER109, and PHE108, consistent with a predominantly hydrophobic binding mode.
Additionally, molecular interactions with the GABA receptor, TRP channel, and Octopamine receptor, targets that exhibited lower binding affinities, are also shown Supplementary Materials (Figure S1 and corresponding text).
2.4. Repellence of Essential Oils Against Aedes aegypti Adults
In the analysis of protective activity, essential oils of Hipe. Irregularis, M. citrifolia and Hypt. crenata repelled at least 80% of A. aegypti adults, for up to 140 mines, at concentrations above 17.0 nL/cm (Figure 2A–C). Essential oil of C. citratus retained such repellent actions only up to 100 min (Figure 2D). When pure essential oils were applied at concentrations above 33.0 nL/cm2, the repellent performances were similar to the commercial repellent DEET (15%), providing more than 95% protection (Figure 2). Similar oviposition deterrence performances were reported to the 1:1 essential oil mixtures, except for those containing C. citratus essential oil (Figure 3). Mixtures of Hipe. irregularis with either M. citrifolia or Hypt. crenata at concentrations above 17.0 nL/cm2 repelled more than 80% of A. aegypti adults for up to 140 min (Figure 3A–B). In contrast, the repellent effect of the Hipe. irregularis and C. citratus mixture declined over time across all tested concentrations (Figure 3C), suggesting an antagonistic interaction.
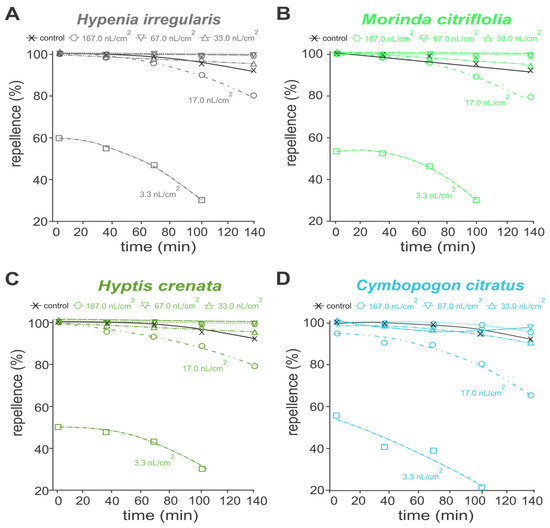
Figure 2.
Protectant activity over time of different concentrations of essential oils of Hypenia irregularis (A); Morinda citrifolia (B); Hyptis crenata (C); and Cymbopogon citratus (D) against adults of Aedes aegypti. (A–D) Control refers to a commercial formulation containing DEET (15%).
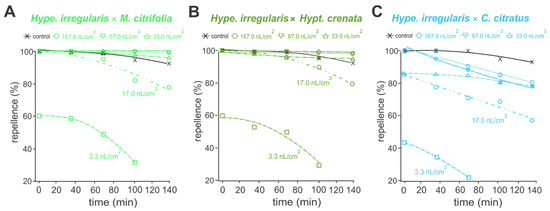
Figure 3.
Protectant activity against adults of Aedes aegypti of a mixture (1:1) of essential oils of Hypenia irregularis × Morinda citrifolia (A), Hypenia irregularis × Hyptis crenata (B) and Hipe. irregularis × Cymbopogon citratus (C) at different concentrations. (A–C) Control refers to a commercial formulation containing DEET (15%).
2.5. Oviposition Deterrence Effects in Aedes aegypti Mediated by Essential Oils
The oviposition deterrent effect of the four essential oils was concentration dependent manner. At the lowest concentration (i.e., 0.083 μL/mL), oviposition was reduced by 50% compared to negative control (untreated solutions), while at the highest concentrations (i.e., 0.200 μL/mL), the reduction reached up to 80% (Figure 4, Supplementary Figure S2). Similar patterns were recorded for the mixtures (1:1 proportions) between the essential oils of Hipe. irregularis and Hypt. crenata or M. citrifolia (Figure 4, Supplementary Figure S2). The mixture between essential oils of Hipe. irregularis and C. citratus provided significant oviposition reductions only at concentrations above 0.130 μL/mL (Supplementary Figure S2), indicating potential antagonistic effects of the mixtures at the essential oil lowest concentrations (i.e., 0.083 μL/mL and 0.130 μL/mL) compared to the oviposition deterrence of the sole essential oils (Figure 4).
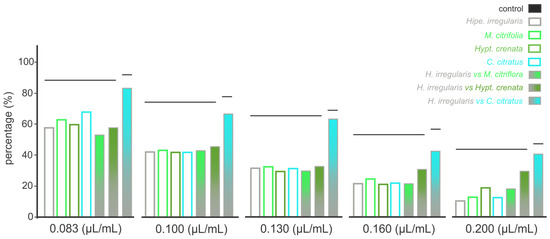
Figure 4.
Oviposition deterrence in Aedes aegypti females mediated by the exposure to different concentrations of “alecrim do Cerrado” (Hypenia irregularis) pure essential oil and its combinations (1:1) with essential oils from noni (Morinda citrifolia), “salva-do-Marajó” (Brazilian mint; Hyptis crenata), and lemongrass (Cymbopogon citratus). The bars represent the percentage of laid eggs in arenas that received essential oil-containing solutions in comparison to the control (i.e., untreated solutions). The oviposition period was 14 days. Bars grouped at the same horizontal line indicate the absence of significant differences according to Tukey’s HSD test (P < 0.05).
3. Discussion
Our results demonstrated that the essential oils of Hype. irregularis, M. citrifolia, Hypt. crenata, and C. citratus exhibit larvicidal activity against A. aegypti larvae, both individually and in 1:1 mixtures. In silico analyses indicated that 2,5-dimethoxy-p-cymene and carvacrol showed the strongest affinities for key A. aegypti targets, including AChE, OBP, and the odorant receptor AaOr31. No significant synergistic or antagonistic differences in LC50 values were recorded for essential oil mixture that combined Hipe. irregularis × M. citrifolia, Hipe. irregularis × H. crenata, and Hipe. irregularis × C. citratus, when compared to the larvicidal activities measured by the application of essential oils alone. When tested at 33.0 nL/cm2, all essential oils provided over 90% repellency against A. aegypti for up to 140 min, which was comparable to the repellence level achieved by the application of a commercial formulation containing DEET (15%). Similarly, no synergistic or antagonistic effects were reported in the repellence performance of essential oil mixtures that combined Hipe. irregularis × M. citrifolia and Hipe. irregularis × Hypt. crenata. However, the mixture that contained Hipe. irregularis × C. citratus essential oils resulted in reduced repellence and oviposition deterrence performances.
Chromatographic analysis revealed that the Hipe. irregularis × M. citrifolia mixture was rich in octanoic acid (23.0%), 2,5-dimethoxy-p-cymene (19.5%), and α-cymene (10%). The Hipe. irregularis × Hypt. crenata mixture was dominated by 2,5-dimethoxy-p-cymene (21.9%), carvacrol (10.4%), and α-cymene (10%). In the Hipe. irregularis × C. citratus mixture, the major compounds were citral A (23.0%), citral B (17.1%), and 2,5-dimethoxy-p-cimene (15.5%). Although thymol has been previously identified as the main compound (approximately 21%) in Hipe. irregularis essential oil [25], its concentration was drastically reduced (approximately 5%) in the mixture of Hipe. irregularis and M. citrifolia essential oils. Similarly, octanoic acid, normally the dominant (at least 64%) constituent [40,41] in M. citrifolia essential oils, remained prevalent in the mixture, but at reduced levels (approximately 23%). Likewise, 1,8-cineole and α-pinene, major components of Hypt. crenata [36,42], were absent in its mixture with Hipe. irregularis. Conversely, the chemical profile of Hipe. irregularis × C. citratus was consistent with known constituents of C. citratus essential oil [24,43]. These findings suggest that blending essential oils may alter their chemical composition due to interactions among constituents, thereby influencing biological activity. Although further investigations such as in-depth comparisons of the chemical composition of the mixed and individual essential oils, the chemotypes of individual plant populations, and electrophysiological and ligand-binding assays, are still needed to draw definitive conclusions, the reduced levels of thymol, α-cymene, and octanoic acid in the mixture of Hype. irregularis and M. citrifolia essential oils may help explain the antagonistic effects observed on repellence and oviposition deterrence of this essential oil mixture at the lowest concentrations. These compounds are typically present at higher concentrations in the pure essential oils.
The toxicity observed in the Hipe. irregularis × M. citrifolia mixture is likely attributable to its major compounds. Octanoic acid has been reported to have insecticidal [44], bactericidal [45], nematicidal [46], and fungicidal [40,41,47] properties and may act synergistically with other compounds [48]. Likewise, essential oils containing 2,5-dimethoxy-p-cymene have demonstrated insecticidal [49,50] and bactericidal [51,52] activity. The larvicidal effect of the Hipe. irregularis × Hypt. crenata mixture may be due not only to 2,5-dimethoxy-p-cymene but also to carvacrol and α-cymene. Carvacrol has broad bioactivity, including bactericidal, acaricidal, and insecticidal effects, and is known to be toxic to disease vectors [53,54,55]. Although our in silico results suggest only moderate affinity of carvacrol for AChE, other studies have shown it to modulate TRP channels, inhibit AChE, act as a positive allosteric modulator of GABA receptors [56,57], and noncompetitively block nicotine binding to nAChRs, leading to neuroinhibition [58,59,60]. It has also been proposed that carvacrol can block the octopamine receptor pathway [61]. Citral, predominant in the Hipe. irregularis × C. citratus mixture, has been shown to inhibit AChE and β-esterase activity in insects [58,62,63].
All essential oils tested exhibited over 90% repellency against A. aegypti when applied at 0.0330 µL/cm3, maintaining effectiveness for 140 min—comparable to DEET. The repellent activity of H. irregularis, M. citrifolia, H. crenata, and C. citratus has been previously documented against various insect species [25,26,27,32,64], but studies on their combined repellent effects are lacking. The repellency observed in the Hipe. irregularis × M. citrifolia mixture may be due to octanoic acid and 2,5-dimethoxy-p-cymene, both associated with arthropod repellency [30,34]. Carvacrol- and α-cymene-containing oils have also shown repellent activity, with carvacrol alone proven effective against mosquitoes and other arthropods [6,31,65,66,67]. Citral is a well-known insect repellent [24,32,65,68], and its efficacy has been demonstrated in pure form [69,70]. Indeed molecular docking analyses revealed that 2,5-dimethoxy-p-cymene and carvacrol interacted with OBP and the odorant receptor AaOr31, which are involved in insect chemoreception and play key roles in olfaction [71,72]. Thireou et al. [73] and Kröber et al. [74] reported strong carvacrol binding to OBPs in Anopheles gambiae, including the same OBP that interacts with DEET and Icaridin. Carvacrol also binds to AgamOBP5 with high affinity [75,76]. AaOr31 has been shown to respond to β-farnesene and mediate pyrethrum repellency in A. aegypti [77,78]. Therefore, these receptors may serve as targets for terpene-based repellents such as 2,5-dimethoxy-p-cymene and carvacrol. Interestingly, Lü and Liu [33] reported that citral can be attractive at low concentrations but repellent at higher doses. While no synergistic or antagonistic repellent effects were seen in Hipe. irregularis × M. citrifolia or Hipe. irregularis × Hypt. crenata combinations, reduced repellency was observed in the Hipe. irregularis × C. citratus blend. Although some studies have demonstrated synergistic effects of C. citratus [79,80], others have reported antagonistic interactions when it is combined with other essential oils [81]. These outcomes likely depend on the specific chemical makeup of the oils, their component interactions, and the target organisms.
A last note is that all tested essential oils also showed concentration-dependent oviposition deterrent activity. But no synergistic or antagonistic effects were noted, except for the Hipe. irregularis × C. citratus combination, which led to increased oviposition—indicating a possible antagonistic effect. Fatty acids, especially octanoic acid, have previously been linked to oviposition deterrence [82,83,84], although some studies have reported the opposite effect [85,86], highlighting species-specific and context-dependent responses. Similarly, essential oils containing carvacrol, as well as carvacrol alone, have demonstrated oviposition deterrence similar to octanoic acid [87,88,89]. Comparable results have also been observed for citral [63,90]. Overall, the predicted differences in binding affinities between the major compounds of the essential oil mixtures and various neurophysiological targets support the hypothesis of multiple modes of action, a beneficial feature for developing environmentally safe biorational insecticides. These findings should be viewed as exploratory and hypothesis-generating.
4. Conclusions
Our findings highlight the potential of essential oils from H. irregularis, M. citrifolia, H. crenata, and C. citratus, all of which are widely available in Brazil, as sustainable sources of biorational products for controlling A. aegypti. These oils exhibit promising dual functionality: acting as larvicidal agents and serving as preventive tools through their oviposition deterrent and repellent properties. The predicted binding affinities suggest multiple modes of action, a beneficial trait for developing environmentally safe biorational insecticides. Although exploratory, our findings provide a basis for future validation through electrophysiological or biochemical assays. Additional targets, such as voltage-gated sodium channels, ryanodine receptors, and nicotinic acetylcholine receptors, may also be involved. Collectively, the findings described here offer valuable insights into the potential mechanisms of essential oil-based insecticides and support their continued investigation.
5. Materials and Methods
5.1. Essential Oil Extraction
Branches of Hipe. irregularis, native to the Central Brazilian Cerrado, containing leaves and flowers were collected in Jalapão, Tocantins (09°57′46″ S, 47°40′38″ W), a region characterized by nutrient-poor, acidic soils and a strongly seasonal climate, which influence the species’ secondary metabolism. In contrast, the other plant species (i.e., C. citratus, Hypt. crenata, and M. citrifolia) were collected at the Federal University of Tocantins, campus of Gurupi (11°43′45″ S, 49°04′07″ W), where edaphoclimatic conditions differ considerably, with generally more fertile soils and a more humid tropical climate. Essential oils were extracted separately from each plant species using healthy leaves that were shade-dried for a period of 10 days. The dried leaves of each species were then cut into small pieces. For each extraction, 200 g of dried leaves were combined with 800 mL of distilled water in a 1000 mL round-bottomed flask and subjected to hydrodistillation using a Clevenger apparatus for three hours. The essential oils were collected individually in amber bottles and stored at 4 °C [91].
5.2. Gas Chromatography (GC) Analysis
The chemical composition of the essential oils was determined at the Analytical Center of the Chemistry Institute, University of São Paulo, using GC–MS. Analyses were performed on a Shimadzu GC-2010 instrument equipped with a QP2010Plus mass selective detector. The GC was fitted with a fused silica capillary column (30 m × 0.25 mm × 0.25 μm film thickness), with the following temperature program: 60 to 240 °C at 3 °C/min. Injector temperature was set to 220 °C. Helium was used as the carrier gas, and the injection was performed in split mode (1:100), using 1 µL of a 1:1000 solution in hexane. For the MS, the following parameters were used: electron impact ionization at 70 eV, with the ion source and interface temperatures set at 200 °C [92]. The components of the essential oils were identified by comparing their mass spectra with those in the spectrophotometer database (Wiley 7, NIST 05, and NIST 05s) and by analyzing their retention indices (RI). To calculate the RI, a mixture of saturated C7–C40 alkanes (Supelco Inc., Bellefonte, PA, USA) was analyzed under the same chromatographic conditions as the essential oil, and the adjusted retention times of the compounds were determined. The resulting RI values were then compared with those reported in the literature [93,94].
5.3. Origin and Maintenance of Aedes aegypti Mosquitoes
Aedes aegypti mosquitoes as used in this study were obtained from local populations collected in Gurupi City (11°43′07″ S, Tocantins State, Brazil) and maintained for several generations at the Laboratory of Integrated Pest Management, Federal University of Tocantins, Campus Gurupi. Adult males were fed a 10% sucrose solution, while adult females were fed on heparinized horse blood. Larvae were reared in plastic containers (35 cm × 5 cm) and fed a sterilized diet consisting of an 80:20 mixture of chick chow and yeast [95].
5.4. Bioassays of Toxicity
Toxicity tests were conducted following the World Health Organization protocol [96], with minor modifications. A stock solution was prepared at a concentration of 10 µL/mL using 1.7% dimethyl sulfoxide (DMSO) in distilled water. From this stock, a series of working concentrations was prepared (0.007, 0.013, 0.020, 0.027, 0.030, 0.050, 0.067, 0.083, 0.100, and 0.130 µL/mL). For each concentration, 30 mL of the test solution and 25 third-instar A. aegypti larvae were placed into disposable 100 mL plastic cups. Three replicates were performed per concentration.
The bioassays were maintained at 27 ± 1 °C, 65 ± 6% relative humidity, with a 12 h photoperiod. After 24 h, larval mortality was recorded, and the median lethal concentration (LC50) was estimated. Using the same procedure, we evaluated potential synergistic effects between Hipe. irregularis and M. citrifolia, H. crenata, or C. citratus in terms of toxicity, as outlined in Table 5.

Table 5.
Proportions tested to determine the synergistic/antagonistic effects of Hypenia irregularis with Morinda citrifolia, Hyptis crenata, and Cymbopogon citratus.
5.5. Molecular Modeling Analysis
The ligands selected for the molecular docking study were the major compounds found in combinations of Hipe. irregularis with M. citrifolia, H. crenata, and C. citratus. The 3D structures of these compounds, in their neutral forms, were constructed using Marvin Sketch 18.10 (ChemAxon, http://www.chemaxon.com).
Amino acid sequences of the target proteins were obtained from the National Center for Biotechnology Information (NCBI) database (https://www.ncbi.nlm.nih.gov/, accessed on 30 October 2024). Their 3D structures were constructed via homology modeling using the Swiss-Model Workspace (https://swissmodel.expasy.org/, https://www.ncbi.nlm.nih.gov/, accessed on 30 October 2024), following the selection of appropriate templates with the BLASTp tool. Templates were obtained from the Protein Data Bank (https://www.rcsb.org/, https://www.ncbi.nlm.nih.gov/, accessed on 30 October 2024), considering quality parameters such as experimental method, resolution, R-value, and complexation with a ligand. The Swiss-Model platform was also used to verify structural integrity and active site conformation [97]. Model validation was performed using Ramachandran plots [98,99] to assess the distribution of backbone torsion angles (ϕ and ψ), thereby evaluating stereochemical quality. The QMEAN score was also used to assess model reliability [100].
Protein targets and ligands were prepared for docking using AutoDock Tools 1.5.7 [101], following the methodology proposed by Souza Moura et al. [102]. Docking calculations were performed using AutoDock Vina [103], generating nine binding poses for each ligand–target interaction and reporting the binding affinity in kcal/mol. The best docking poses were selected based on binding affinity and were visualized and analyzed using PyMOL 2.0 [104] and Discovery Studio 4.5 [105].
5.6. Repellence Test
The repellency test was conducted using the essential oils of Hype irregularis, M. citrifolia, Hypt. crenata, and C. citratus, following the method described by Haris, Azeem, and Binyameen [4]. Three acrylic boxes (24 × 24 × 24 cm) were used: one for testing the essential oils, one as a negative control (ethanol), and one as a positive control (15% DEET). Each box contained 50 adult female A. aegypti mosquitoes aged 5–7 days. In parallel, forearms of five volunteers were cleaned with neutral soap, sanitized with 70% ethanol, and dried. A 300 cm2 area on each forearm was marked for treatment and exposure; the remaining areas were covered with rubber gloves. Essential oil solutions were prepared at concentrations of 0.0033, 0.017, 0.033, 0.067, 0.167, 0.333, and 0.500 μL/cm2 using 99.80% ethanol at a 1:1 ratio. These were applied to the exposed skin areas. Finally, forearms were inserted into the acrylic boxes for 3 min every 30 min, and the number of mosquito bites was recorded. After 140 min, the total number of bites was used to estimate protective efficacy. The same procedure was used to evaluate the synergistic effects of oil combinations (1:1 ratio). Five replicates were performed per concentration, and all tests were conducted during daytime hours. The repellence index was calculated using the formula:
where RI is the percentage of repellence, T is the number of bites in the control, and I is the number in the treatment (essential oil protection).
%RI = ((T − I)/T) × 100
We tested each essential oil alone and the mixture formulations on human volunteer subjects, following approval of the research protocol number CAAE 81727617.3.0000.0003 (https://plataformabrasil.saude.gov.br/, accessed on 4 August 2025, approved on 23 March 2018).
5.7. Oviposition Deterrence Test
Oviposition deterrence was evaluated using essential oil concentrations of 0.0833, 0.1, 0.13, 0.166, and 0.2 μL/mL, prepared as previously described. Synergistic effects of essential oil combinations (1:1 ratio) were also assessed at the same concentrations. For the experiment, entomological cages (35 cm wide × 23 cm deep × 47 cm high) were used. Each cage contained two disposable cups: one with 30 mL of the essential oil solution (pure or in combination), and the other with distilled water containing DMSO as the control. Cups were wrapped in aluminum foil to prevent visual bias. Twenty-five newly emerged female and fifty male A. aegypti mosquitoes were released into each cage. Mosquitoes were fed daily with rodent blood and a 10% sucrose solution and maintained at 28 °C. Egg counts were recorded daily over seven days. The same procedure was followed to assess the effects of oil combinations. Oviposition deterrence was calculated using the formula:
where V is the percentage of oviposition deterrence, T is the number of viable eggs in the control, and I is the number in the treatment.
%V = ((T − I)/T) × 100
5.8. Statistical Analysis
Lethal concentrations (LC50 and LC95) were estimated using the PROBIT analysis method with POLO PLUS statistical software (version 1.0, LeOra Software, Berkeley, CA, USA). By means of SigmaPlot 14.0 software (Systat Software, San Jose, CA, USA), we applied analysis of variance (ANOVA) and Tukey’s HSD test (p < 0.05) to compare results between treatment groups in the repellence and oviposition deterrence analysis using SigmaPlot 14.0 (Systat Software, San Jose, CA, USA).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxins17080402/s1, Figure S1: Carvacrol, citral, 2,5-dimethoxy-p-cymene, and octanoic acid bind with the GABA receptor (A), TRP channel (B) and Octopamine receptor (C) target complexes of Aedes aegypti; the 2D maps of molecular interactions with amino acids in each target active site (yellow) are also shown; Figure S2: Oviposition deterrence in Aedes aegypti females mediated by the exposure to different concentrations of “alecrim do Cerrado”, Hypenia irregularis, essential oil and its combinations (1:1) with essential oils from noni (Morinda citrifolia), (“salva-do-Marajó (Brazilian mint; Hyptis crenata), and lemongrass (Cymbopogon citratus). The bars represent the number of laid eggs in arenas that received essential oil-containing solutions in comparison to the control (i.e., untreated solutions). The oviposition period was 14 days; Table S1: Phytochemical profiles for essential oils of Hypenia irregularis, Morinda citrifolia, Hyptis crenata and Cymbopogon citratus revealed by gas chromatography coupled to mass spectrometry (GC-MS) detectors. These results were obtained in publications available in the literature; Table S2: Target models of Aedes aegypti used to analyze the molecular docking with the major compounds. Refs. [25,40] are also cited in the Supplementary Materials. Refs. [106,107] are cited only in the Supplementary Materials.
Author Contributions
Conceptualization, R.W.S.A., R.D.P., L.O.V.J. and E.E.O.; methodology, R.W.S.A., R.D.P., L.O.V.J. and E.E.O.; software, B.S.A., W.S.M., R.R.F. and O.M.H.; validation, R.W.S.A., W.S.M., B.S.A. and E.E.O.; formal analysis, R.D.P., L.O.V.J., W.S.M., B.S.A., R.R.F., G.R.S. and E.E.O.; investigation, R.D.P. and O.M.H.; resources, R.R.F., R.W.S.A., G.R.S., G.S. and E.E.O.; data curation, W.S.M., R.D.P., O.M.H., R.R.F. and B.S.A.; writing—original draft preparation, L.O.V.J., R.D.P., W.S.M., G.S. and E.E.O.; writing—review and editing, L.O.V.J., W.S.M., R.W.S.A., G.S., G.R.S. and E.E.O.; visualization, L.O.V.J., E.E.O. and W.S.M.; supervision, R.W.S.A., G.R.S., R.R.F., E.E.O.; project administration, R.W.S.A., R.R.F. and E.E.O.; funding acquisition, R.W.S.A., G.R.S. and E.E.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional De Desenvolvimento Científico E Tecnológico (CNPq, Grant Numbers: 313455/2019-8; 427304/2018-0; 308576/2018-7; 408598/2023-9 309890/2022-5), CAPES Foundation (Finance Code 001), Tocantins State Foundation for Research Aid (FAPT-SESAU/TO-DECIT/SCTIE/MS_CNPQ/N° 01/2017/EDITAL FAPT/SEPLAN—Projeto REDE DESER), Federal University of Tocantins (PROPESQ—EDITAL N° 29/2020 PROPESQ, and PPGBIOTEC/UFT/GURUPI—Chamada pública para auxílio de tradução e/ou publicação de artigos científicos—EDITAL N° 011/2020), Fundação de apoio à pesquisa do Distrito Federal (FAPDF—Grant Number: 00193-00002148/2023-27), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG—Grant Numbers APQ-03771-18; APQ-05316-23).
Institutional Review Board Statement
Human volunteer subjects were used to test each essential oil alone or in mixture formulations, and the research protocol was approved by the Institutional Human Ethics Committee (CAAE 81727617.3.0000.0003; https://plataformabrasil.saude.gov.br/), approved on 23 March 2018.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We thank everyone who helped during the biological experiments and manuscript writing.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| Dimethyl sulfoxide | DMSO |
| N,N-diethyl-meta-toluamide | DEET |
| Hypenia iregularis | Hypt. irregularis |
| Morinda citrifolia | M. citrifolia |
| Hyptis crenata | Hypt. crenata |
| Cymbopogom citratus | C. citratus |
| Aedes aegypti | A. aegypti |
| Acetylcholinesterase | AChE |
| Glutathione S-transferase | GST |
| γ-aminobutyric acid | GABA |
| Transient receptor potential | TRP |
| Gas Chromatography coupled with Mass Spectrometry | GC–MS |
References
- Suresh, Y.; Azil, A.H.; Abdullah, S.R. A scoping review on the use of different blood sources and components in the artificial membrane feeding system and its effects on blood-feeding and fecundity rate of Aedes aegypti. PLoS ONE 2024, 19, e0295961. [Google Scholar] [CrossRef]
- Alves, L. Brazil to start widespread dengue vaccinations. Lancet 2024, 403, 133. [Google Scholar] [CrossRef]
- Taylor, L. Dengue fever: Brazil rushes out vaccine as climate change fuels unprecedented surge. BMJ 2024, 384, q483. [Google Scholar] [CrossRef]
- Haris, A.; Azeem, M.; Binyameen, M. Mosquito Repellent Potential of Carpesium abrotanoides Essential Oil and Its Main Components Against a Dengue Vector, Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2022, 59, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Soonwera, M.; Moungthipmalai, T.; Aungtikun, J.; Sittichok, S. Combinations of plant essential oils and their major compositions inducing mortality and morphological abnormality of Aedes aegypti and Aedes albopictus. Heliyon 2022, 8, e09346. [Google Scholar] [CrossRef] [PubMed]
- Sanei-Dehkordi, A.; Hatami, S.; Zarenezhad, E.; Montaseri, Z.; Osanloo, M. Efficacy of nanogels containing carvacrol, cinnamaldehyde, thymol, and a mix compared to a standard repellent against Anopheles stephensi. Ind. Crops Prod. 2022, 189, 115883. [Google Scholar] [CrossRef]
- Norris, E.J.; Bloomquist, J.R. Co-Toxicity factor analysis reveals numerous plant essential oils are synergists of natural pyrethrins against Aedes aegypti mosquitoes. Insects 2021, 12, 154. [Google Scholar] [CrossRef]
- Haddi, K.; Tomé, H.V.V.; Du, Y.; Valbon, W.R.; Nomura, Y.; Martins, G.F.; Dong, K.; Oliveira, E.E. Detection of a new pyrethroid resistance mutation (V410L) in the sodium channel of Aedes aegypti: A potential challenge for mosquito control. Sci. Rep. 2017, 7, 46549. [Google Scholar] [CrossRef]
- Moungthipmalai, T.; Puwanard, C.; Aungtikun, J.; Sittichok, S.; Soonwera, M. Ovicidal toxicity of plant essential oils and their major constituents against two mosquito vectors and their non-target aquatic predators. Sci. Rep. 2023, 13, 2119. [Google Scholar] [CrossRef]
- Radhakrishnan, N.; Karthi, S.; Raghuraman, P.; Ganesan, R.; Srinivasan, K.; Edwin, E.-S.; Ganesh-Kumar, S.; Mohd Esa, N.; Senthil-Nathan, S.; Vasantha-Srinivasan, P.; et al. Chemical screening and mosquitocidal activity of essential oil derived from Mikania scandens (L.) Willd. against Anopheles gambiae Giles and their non-toxicity on mosquito predators. All Life 2023, 16, 2169959. [Google Scholar] [CrossRef]
- Pavela, R.; Govindarajan, M. The essential oil from Zanthoxylum monophyllum a potential mosquito larvicide with low toxicity to the non-target fish Gambusia affinis. J. Pest Sci. 2017, 90, 369–378. [Google Scholar] [CrossRef]
- Costa, L.T.M.; Smagghe, G.; Viteri Jumbo, L.O.; Santos, G.R.; Aguiar, R.W.S.; Oliveira, E.E. Selective actions of plant-based biorational insecticides: Molecular mechanisms and reduced risks to non-target organisms. Curr. Opin. Environ. Sci. Health. 2025, 44, 100601. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential Oils as Ecofriendly Biopesticides? Challenges and Constraints. Trends Plant Sci 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Toledo, P.F.S.; Viteri Jumbo, L.O.; Rezende, S.M.; Haddi, K.; Silva, B.A.; Mello, T.S.; Della Lucia, T.M.C.; Aguiar, R.W.S.; Smagghe, G.; Oliveira, E.E. Disentangling the ecotoxicological selectivity of clove essential oil against aphids and non-target ladybeetles. Sci. Total Environ. 2020, 718, 137328. [Google Scholar] [CrossRef]
- Jumbo, L.O.V.; Corrêa, M.J.M.; Gomes, J.M.; Armijos, M.J.G.; Valarezo, E.; Mantilla-Afanador, J.G.; Machado, F.P.; Rocha, L.; Aguiar, R.W.S.; Oliveira, E.E. Potential of Bursera graveolens essential oil for controlling bean weevil infestations: Toxicity, repellence, and action targets. Ind. Crops Prod. 2022, 178, 114611. [Google Scholar] [CrossRef]
- Shaaya, E.; Rafaeli, A. Essential Oils as Biorational Insecticides–Potency and Mode of Action. In Insecticides Design Using Advanced Technologies; Ishaaya, I., Horowitz, A.R., Nauen, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 249–261. [Google Scholar]
- Duque, J.E.; Urbina, D.L.; Vesga, L.C.; Ortiz-Rodríguez, L.A.; Vanegas, T.S.; Stashenko, E.E.; Mendez-Sanchez, S.C. Insecticidal activity of essential oils from American native plants against Aedes aegypti (Diptera: Culicidae): An introduction to their possible mechanism of action. Sci. Rep. 2023, 13, 2989. [Google Scholar] [CrossRef] [PubMed]
- Chebbac, K.; Benziane Ouaritini, Z.; Allali, A.; Tüzün, B.; Zouirech, O.; Chalkha, M.; El Moussaoui, A.; Lafraxo, S.; Nafidi, H.-A.; Bin Jardan, Y.A.; et al. Promising insecticidal properties of essential oils from Artemisia aragonensis Lam. and Artemisia negrei L. (Asteraceae) by targeting gamma-aminobutyric aacid and Ryanodine Receptor Proteins: In vitro and in silico approaches. Separations 2023, 10, 329. [Google Scholar] [CrossRef]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular targets for components of essential oils in the insect nervous system—A review. Molecules 2018, 23, 34. [Google Scholar] [CrossRef]
- Kostyukovsky, M.; Rafaeli, A.; Gileadi, C.; Demchenko, N.; Shaaya, E. Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: Possible mode of action against insect pests. Pest Manag. Sci 2002, 58, 1101–1106. [Google Scholar] [CrossRef]
- Awad, M.; Alfuhaid, N.A.; Amer, A.; Hassan, N.N.; Moustafa, M.A.M. Towards sustainable Pest Management: Toxicity, biochemical effects, and molecular Docking analysis of Ocimum basilicum (Lamiaceae) essential Oil on Agrotis ipsilon and Spodoptera littoralis (Lepidoptera: Noctuidae). Neotrop. Entomol. 2024, 53, 669–681. [Google Scholar] [CrossRef]
- da Cruz Araujo, S.H.; Mantilla-Afanador, J.G.; Svacina, T.; Nascimento, T.F.; da Silva Lima, A.; Camara, M.B.P.; Viteri Jumbo, L.O.; dos Santos, G.R.; da Rocha, C.Q.; de Oliveira, E.E. Contributions of γ-Aminobutyric Acid (GABA) Receptors for the Activities of Pectis brevipedunculata Essential Oil against Drosophila suzukii and Pollinator Bees. Plants 2024, 13, 1392. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Plata-Rueda, A.; Martínez, L.C.; Rolim, G.d.S.; Coelho, R.P.; Santos, M.H.; Tavares, W.d.S.; Zanuncio, J.C.; Serrão, J.E. Insecticidal and repellent activities of Cymbopogon citratus (Poaceae) essential oil and its terpenoids (citral and geranyl acetate) against Ulomoides dermestoides. Crop. Prot. 2020, 137, 105299. [Google Scholar] [CrossRef]
- Possel, R.D.; Souza, T.P.; Oliveira, D.M.; Ferreira, M.O.; Dias, D.P.; Moraes, G.K.A.; Ferraz, L.F.; Ferreira, T.P.; Fernandes, A.C.; Didonet, J. Larvicide and repellent activity of Hypenia irregularis (Benth.) Harley in the alternative control of mosquito Aedes aegypti. J. Med. Plants Res. 2020, 14, 535–543. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Folorunso, A.S.; Oyebamiji, A.K.; Olugbeko, S.C. Mosquito repellent and antibacterial efficiency of facile and low-cost silver nanoparticles synthesized using the leaf extract of Morinda citrifolia. Plasmonics 2021, 16, 1645–1656. [Google Scholar] [CrossRef]
- Nelson, S.C. Morinda citrifolia L. Rubiaceae (Rubioideae) Coffee Family; Permanent Agriculture Resources: Holualoa, HI, USA, 2003. [Google Scholar]
- Kovendan, K.; Murugan, K.; Shanthakumar, S.P.; Vincent, S.; Hwang, J.-S. Larvicidal activity of Morinda citrifolia L. (Noni) (Family: Rubiaceae) leaf extract against Anopheles stephensi, Culex quinquefasciatus, and Aedes aegypti. Parasitol. Res. 2012, 111, 1481–1490. [Google Scholar] [CrossRef]
- Violante, I.M.P.; Garcez, W.S.; da Silva Barbosa, C.; Garcez, F.R. Chemical composition and biological activities of essential oil from Hyptis crenata growing in the Brazilian cerrado. Nat. Prod. Commun. 2012, 7, 1934578X1200701037. [Google Scholar] [CrossRef]
- Nazzi, F.; Bortolomeazzi, R.; Della Vedova, G.; Del Piccolo, F.; Annoscia, D.; Milani, N. Octanoic acid confers to royal jelly varroa-repellent properties. Naturwissenschaften 2009, 96, 309–314. [Google Scholar] [CrossRef]
- Tabari, M.A.; Youssefi, M.R.; Maggi, F.; Benelli, G. Toxic and repellent activity of selected monoterpenoids (thymol, carvacrol and linalool) against the castor bean tick, Ixodes ricinus (Acari: Ixodidae). Vet. Parasitol. 2017, 245, 86–91. [Google Scholar] [CrossRef]
- Diabate, S.; Martin, T.; Murungi, L.K.; Fiaboe, K.K.M.; Subramanian, S.; Wesonga, J.; Deletre, E. Repellent activity of Cymbopogon citratus and Tagetes minuta and their specific volatiles against Megalurothrips sjostedti. J. Appl. Entomol. 2019, 143, 855–866. [Google Scholar] [CrossRef]
- Lü, J.; Liu, S. The behavioral response of Lasioderma serricorne (Coleoptera: Anobiidae) to citronellal, citral, and rutin. SpringerPlus 2016, 5, 798. [Google Scholar] [CrossRef]
- Hou, X.-Q.; Zhang, D.-D.; Powell, D.; Wang, H.-L.; Andersson, M.N.; Löfstedt, C. Ionotropic receptors in the turnip moth Agrotis segetum respond to repellent medium-chain fatty acids. BMC Biology 2022, 20, 34. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.T.; Costa, D.P.; Vilela, E.C.; Ribeiro, D.G.; Ferreira, H.D.; Santos, S.C.; Seraphin, J.C.; Ferri, P.H. Chemotaxonomic markers in essential oils of Hypenia (Mart. ex Benth.) R. Harley. J. Braz. Chem. Soc. 2012, 23, 1844–1852. [Google Scholar] [CrossRef]
- Lima, M.N.N.d.; Costa, J.S.d.; Guimarães, B.A.; Freitas, J.J.S.; Setzer, W.N.; Silva, J.K.R.d.; Maia, J.G.S.; Figueiredo, P.L.B. Chemometrics of the composition and antioxidant capacity of Hyptis crenata essential oils from Brazil. Molecules 2023, 28, 3371. [Google Scholar] [CrossRef]
- Almeida, É.S.; de Oliveira, D.; Hotza, D. Properties and Applications of Morinda citrifolia (Noni): A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 883–909. [Google Scholar] [CrossRef]
- Mwithiga, G.; Maina, S.; Muturi, P.; Gitari, J. Lemongrass Cymbopogon flexuosus growth rate, essential oil yield and composition as influenced by different soil conditioners under two watering regimes. Heliyon 2024, 10, e25540. [Google Scholar] [CrossRef]
- Santana, A.d.S.; Baldin, E.L.L.; Santos, T.L.B.d.; Baptista, Y.A.; dos Santos, M.C.; Lima, A.P.S.; Tanajura, L.S.; Vieira, T.M.; Crotti, A.E.M. Synergism between essential oils: A promising alternative to control Sitophilus zeamais (Coleoptera: Curculionidae). Crop. Prot. 2022, 153, 105882. [Google Scholar] [CrossRef]
- Dalcin, M.S.; Dias, B.L.; Viteri Jumbo, L.O.; Oliveira, A.C.S.S.; Araújo, S.H.C.; Moura, W.S.; Mourão, D.S.C.; Ferreira, T.P.S.; Campos, F.S.; Cangussu, A.S.R.; et al. Potential action mechanism and inhibition efficacy of Morinda citrifolia essential oil and octanoic acid against Stagonosporopsis cucurbitacearum infestations. Molecules 2022, 27, 5173. [Google Scholar] [CrossRef]
- Osorio, P.R.A.; Dias, F.R.; Mourão, D.S.C.; Araujo, S.H.C.; Toledo, P.F.S.; Silva, A.C.F.; Viera, W.A.S.; Câmara, M.P.S.; Moura, W.S.; Aguiar, R.W.A.; et al. Essential oil of Noni, Morinda citrifolia L., fruits controls the rice stem-rot disease without detrimentally affect beneficial fungi and ladybeetles. Ind. Crops Prod. 2021, 170, 113728. [Google Scholar] [CrossRef]
- de Lima, M.N.N.; Guimarães, B.A.; de Castro, A.L.S.; Ribeiro, K.B.; Miller, D.C.; da Silva, P.I.C.; Freitas, J.J.S.; de Lima, A.B.; Setzer, W.N.; da Silva, J.K.R.; et al. Chemical composition and antinociceptive and anti-inflammatory activity of the essential oil of Hyptis crenata Pohl ex Benth. from the Brazilian Amazon. J. Ethnopharmacol. 2023, 300, 115720. [Google Scholar] [CrossRef]
- Loko, Y.L.E.; Medegan Fagla, S.; Kassa, P.; Ahouansou, C.A.; Toffa, J.; Glinma, B.; Dougnon, V.; Koukoui, O.; Djogbenou, S.L.; Tamò, M.; et al. Bioactivity of essential oils of Cymbopogon citratus (DC) Stapf and Cymbopogon nardus (L.) W. Watson from Benin against Dinoderus porcellus Lesne (Coleoptera: Bostrichidae) infesting yam chips. Int. J. Trop. Insect Sci. 2021, 41, 511–524. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Wrońska, A.K.; Kazek, M.; Boguś, M.I. Octanoic ccid—An insecticidal metabolite of Conidiobolus coronatus (Entomopthorales) that affects two majors antifungal protection systems in Galleria mellonella (Lepidoptera): Cuticular lipids and hemocytes. Int. J. Mol. Sci. 2022, 23, 5204. [Google Scholar] [CrossRef]
- Zhang, H.; Dolan, H.L.; Ding, Q.; Wang, S.; Tikekar, R.V. Antimicrobial action of octanoic acid against Escherichia coli O157:H7 during washing of baby spinach and grape tomatoes. Food Res. Int. 2019, 125, 108523. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Li, Q.-Y.; Ren, L.; Guo, C.; Qu, J.-P.; Gao, Z.; Wang, H.-F.; Zhang, Q.; Zhou, B. Transcriptomic and physiological analysis of the effect of octanoic acid on Meloidogyne incognita. Pestic. Biochem. Physiol. 2023, 193, 105432. [Google Scholar] [CrossRef]
- Holanda, L.; Bezerra, G.B.; Ramos, C.S. Potent antifungal activity of essential oil from Morinda citrifolia fruits rich in short-chain fatty acids. Int. J. Fruit Sci. 2020, 20, S448–S454. [Google Scholar] [CrossRef]
- Rani, S.; Singh, H.; Ram, C. Efficacy and mechanism of carvacrol with octanoic acid against mastitis causing multi-drug-resistant pathogens. Braz. J. Microbiol. 2022, 53, 385–399. [Google Scholar] [CrossRef]
- Owolabi, M.S.; Lajide, L.; Villanueva, H.E.; Setzer, W.N. Essential oil composition and insecticidal activity of Blumea perrottetiana growing in Southwestern Nigeria. Nat. Prod. Commun. 2010, 5, 1135–1138. [Google Scholar] [CrossRef]
- Benelli, G.; Govindarajan, M.; Rajeswary, M.; Senthilmurugan, S.; Vijayan, P.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M. Larvicidal activity of Blumea eriantha essential oil and its components against six mosquito species, including Zika virus vectors: The promising potential of (4E,6Z)-allo-ocimene, carvotanacetone and dodecyl acetate. Parasitol. Res. 2017, 116, 1175–1188. [Google Scholar] [CrossRef]
- Getahun, T.; Sharma, V.; Kumar, D.; Gupta, N. Chemical composition, and antibacterial and antioxidant activities of essential oils from Laggera tomentosa Sch. Bip. ex Oliv. et Hiern (Asteraceae). Turk. J. Chem. 2020, 44, 1539–1548. [Google Scholar] [CrossRef]
- Gonfa, Y.H.; Tessema, F.B.; Gelagle, A.A.; Getnet, S.D.; Tadesse, M.G.; Bachheti, A.; Bachheti, R.K. Chemical compositions of essential oil from aerial parts of Cyclospermum leptophyllum and its application as antibacterial activity against some food spoilage bacteria. J. Chem. 2022, 2022, 5426050. [Google Scholar] [CrossRef]
- Park, J.-H.; Jeon, Y.-J.; Lee, C.-H.; Chung, N.; Lee, H.-S. Insecticidal toxicities of carvacrol and thymol derived from Thymus vulgaris Lin. against Pochazia shantungensis Chou & Lu., newly recorded pest. Sci. Rep. 2017, 7, 40902. [Google Scholar] [CrossRef]
- Youssefi, M.R.; Tabari, M.A.; Esfandiari, A.; Kazemi, S.; Moghadamnia, A.A.; Sut, S.; Dall’Acqua, S.; Benelli, G.; Maggi, F. Efficacy of two monoterpenoids, carvacrol and thymol, and their combinations against eggs and larvae of the west nile vector Culex pipiens. Molecules 2019, 24, 1867. [Google Scholar] [CrossRef]
- Suntres, Z.E.; Coccimiglio, J.; Alipour, M. The bioactivity and toxicological actions of Carvacrol. Crit. Rev. Food Sci. Nutr. 2015, 55, 304–318. [Google Scholar] [CrossRef]
- Kessler, A.; Sahin-Nadeem, H.; Lummis, S.C.R.; Weigel, I.; Pischetsrieder, M.; Buettner, A.; Villmann, C. GABAA receptor modulation by terpenoids from Sideritis extracts. Mol. Nutr. Food Res. 2014, 58, 851–862. [Google Scholar] [CrossRef]
- Trailović, S.M.; Marjanović, D.S.; Nedeljković Trailović, J.; Robertson, A.P.; Martin, R.J. Interaction of carvacrol with the Ascaris suum nicotinic acetylcholine receptors and gamma-aminobutyric acid receptors, potential mechanism of antinematodal action. Parasitol. Res. 2015, 114, 3059–3068. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Q.X.; Song, B. Pesticidal activity and mode of action of monoterpenes. J. Agric. Food Chem. 2022, 70, 4556–4571. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Gross, A.D.; Dolan, M.C.; Coats, J.R. The phenolic monoterpenoid carvacrol inhibits the binding of nicotine to the housefly nicotinic acetylcholine receptor. Pest Manag. Sci 2013, 69, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Gad, H.A.; Ramadan, G.R.M.; El-Bakry, A.M.; El-Sabrout, A.M.; Abdelgaleil, S.A.M. Monoterpenes: Promising natural products for public health insect control- A review. Int. J. Trop. Insect Sci. 2022, 42, 1059–1075. [Google Scholar] [CrossRef]
- Khanikor, B.; Parida, P.; Yadav, R.; Bora, D. Comparative mode of action of some terpene compounds against octopamine receptor and acetyl cholinesterase of mosquito and human system by the help of homology modeling and docking studies. J. Appl. Pharm. Sci. 2013, 3, 006–012. [Google Scholar] [CrossRef]
- Kumar, P.; Mishra, S.; Malik, A.; Satya, S. Housefly (Musca domestica L.) control potential of Cymbopogon citratus Stapf. (Poales: Poaceae) essential oil and monoterpenes (citral and 1,8-cineole). Parasitol. Res. 2013, 112, 69–76. [Google Scholar] [CrossRef]
- Dancewicz, K.; Szumny, A.; Wawrzeńczyk, C.; Gabryś, B. Repellent and antifeedant activities of Citral-Derived lactones against the peach potato aphid. Int. J. Mol. Sci. 2020, 21, 8029. [Google Scholar] [CrossRef]
- Leng, P.H.; Reddy, G.V.P. Bioactivity of selected eco-friendly pesticides against Cylas formicarius (Coleoptera: Brentidae). Fla. Entomol. 2012, 95, 1040–1047. [Google Scholar] [CrossRef]
- Chauhan, N.; Malik, A.; Sharma, S. Repellency potential of essential oils against housefly, Musca domestica L. Environ. Sci. Pollut. Res. Int. 2018, 25, 4707–4714. [Google Scholar] [CrossRef]
- Evergetis, E.; Bellini, R.; Balatsos, G.; Michaelakis, A.; Carrieri, M.; Veronesi, R.; Papachristos, D.P.; Puggioli, A.; Kapsaski-Kanelli, V.-N.; Haroutounian, S.A. From bio-prospecting to field assessment: The case of Carvacrol rich essential oil as a potent mosquito larvicidal and repellent agent. Front. Ecol. Evol. 2018, 6, 204. [Google Scholar] [CrossRef]
- Paudel, P.; Shah, F.M.; Guddeti, D.K.; Ali, A.; Chen, J.; Khan, I.A.; Li, X.-C. Repellency of Carvacrol, Thymol, and their acetates against imported fire ants. Insects 2023, 14, 790. [Google Scholar] [CrossRef]
- Leal, W.S.; Uchida, K. Application of GC-EAD to the determination of mosquito repellents derived from a plant, Cymbopogon citratus. J. Asia-Pac. Entomol. 1998, 1, 217–221. [Google Scholar] [CrossRef]
- Pascual-Villalobos, M.J.; Cantó-Tejero, M.; Vallejo, R.; Guirao, P.; Rodríguez-Rojo, S.; Cocero, M.J. Use of nanoemulsions of plant essential oils as aphid repellents. Ind. Crops Prod. 2017, 110, 45–57. [Google Scholar] [CrossRef]
- Kumar, P.; Mishra, S.; Malik, A.; Satya, S. Biocontrol potential of essential oil monoterpenes against housefly, Musca domestica (Diptera: Muscidae). Ecotoxicol. Environ. Saf. 2014, 100, 1–6. [Google Scholar] [CrossRef]
- Yang, Y.; Tan, S.; Wang, Q.; Wang, F.; Zhang, Y. Key amino acids in odorant-binding protein OBP7 enable Bradysia odoriphaga to recognize host plant volatiles. Int. J. Biol. Macromol. 2025, 284, 138179. [Google Scholar] [CrossRef]
- Leite, N.R.; Krogh, R.; Xu, W.; Ishida, Y.; Iulek, J.; Leal, W.S.; Oliva, G. Structure of an Odorant-Binding Protein from the Mosquito Aedes aegypti Suggests a Binding Pocket Covered by a pH-Sensitive “Lid”. PLoS ONE 2009, 4, e8006. [Google Scholar] [CrossRef] [PubMed]
- Thireou, T.; Kythreoti, G.; Tsitsanou, K.E.; Koussis, K.; Drakou, C.E.; Kinnersley, J.; Kröber, T.; Guerin, P.M.; Zhou, J.-J.; Iatrou, K.; et al. Identification of novel bioinspired synthetic mosquito repellents by combined ligand-based screening and OBP-structure-based molecular docking. Insect Biochem. Mol. Biol. 2018, 98, 48–61. [Google Scholar] [CrossRef]
- Kröber, T.; Koussis, K.; Bourquin, M.; Tsitoura, P.; Konstantopoulou, M.; Awolola, T.S.; Dani, F.R.; Qiao, H.; Pelosi, P.; Iatrou, K.; et al. Odorant-binding protein-based identification of natural spatial repellents for the African malaria mosquito Anopheles gambiae. Insect Biochem. Mol. Biol. 2018, 96, 36–50. [Google Scholar] [CrossRef]
- Liggri, P.G.V.; Tsitsanou, K.E.; Stamati, E.C.V.; Saitta, F.; Drakou, C.E.; Leonidas, D.D.; Fessas, D.; Zographos, S.E. The structure of AgamOBP5 in complex with the natural insect repellents Carvacrol and Thymol: Crystallographic, fluorescence and thermodynamic binding studies. Int. J. Biol. Macromol. 2023, 237, 124009. [Google Scholar] [CrossRef]
- Guo, J.; Liu, P.; Zhang, X.; An, J.; Li, Y.; Zhang, T.; Gao, Z. Characterization of the ligand-binding properties of odorant-binding protein 38 from Riptortus pedestris when interacting with soybean volatiles. Front. Physiol. 2025, 15, 1475489. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Q.; Xu, P.; Andreazza, F.; Valbon, W.R.; Bandason, E.; Chen, M.; Yan, R.; Feng, B.; Smith, L.B.; et al. A dual-target molecular mechanism of pyrethrum repellency against mosquitoes. Nat. Commun. 2021, 12, 2553. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, P.; Andreazza, F.; Liu, Y.; Nomura, Y.; Duran, P.; Jiang, L.; Chen, M.; Takamatsu, G.; Ihara, M.; et al. Identification of multiple odorant receptors essential for pyrethrum repellency in Drosophila melanogaster. PLOS Genet. 2021, 17, e1009677. [Google Scholar] [CrossRef]
- Aungtikun, J.; Soonwera, M.; Sittichok, S. Insecticidal synergy of essential oils from Cymbopogon citratus (Stapf.), Myristica fragrans (Houtt.), and Illicium verum Hook. f. and their major active constituents. Ind. Crops Prod. 2021, 164, 113386. [Google Scholar] [CrossRef]
- Soonwera, M.; Sittichok, S. Adulticidal activities of Cymbopogon citratus (Stapf.) and Eucalyptus globulus (Labill.) essential oils and of their synergistic combinations against Aedes aegypti (L.), Aedes albopictus (Skuse), and Musca domestica (L.). Environ. Sci. Pollut. Res. Int. 2020, 27, 20201–20214. [Google Scholar] [CrossRef]
- Shezryna, S.; Anisah, N.; Saleh, I.; Syamsa, R. Acaricidal activity of the essential oils from Citrus hystrix (Rutaceae) and Cymbopogon citratus (Poaceae) on the cattle tick Rhipicephalus (Boophilus) microplus larvae (Acari: Ixodidae). Trop. Biomed. 2020, 37, 433–442. [Google Scholar]
- Santos, L.M.M.; Nascimento, J.S.; Santos, M.A.G.; Marriel, N.B.; Bezerra-Silva, P.C.; Rocha, S.K.L.; Silva, A.G.; Correia, M.T.S.; Paiva, P.M.G.; Martins, G.F.; et al. Fatty acid-rich volatile oil from Syagrus coronata seeds has larvicidal and oviposition-deterrent activities against Aedes aegypti. Physiol. Mol. Plant Pathol. 2017, 100, 35–40. [Google Scholar] [CrossRef]
- Grant, G.G.; Zhao, B.; Langevin, D. Oviposition response of spruce budworm (Lepidoptera: Tortricidae) to aliphatic carboxylic acids. Environ. Entomol. 2000, 29, 164–170. [Google Scholar] [CrossRef]
- Roh, G.H.; Kendra, P.E.; Zhu, J.J.; Roda, A.; Loeb, G.M.; Tay, J.-W.; Cha, D.H. Coconut oil derived five-component synthetic oviposition deterrent for oriental fruit fly, Bactrocera dorsalis. Pest Manag. Sci. 2023, 79, 3852–3859. [Google Scholar] [CrossRef]
- Álvarez-Ocaña, R.; Shahandeh, M.P.; Ray, V.; Auer, T.O.; Gompel, N.; Benton, R. Odor-regulated oviposition behavior in an ecological specialist. Nat. Commun. 2023, 14, 3041. [Google Scholar] [CrossRef]
- R’Kha, S.; Capy, P.; David, J.R. Host-plant specialization in the Drosophila melanogaster species complex: A physiological, behavioral, and genetical analysis. Proc. Natl. Acad. Sci. USA 1991, 88, 1835–1839. [Google Scholar] [CrossRef]
- Andrade-Ochoa, S.; Sánchez-Aldana, D.; Chacón-Vargas, K.F.; Rivera-Chavira, B.E.; Sánchez-Torres, L.E.; Camacho, A.D.; Nogueda-Torres, B.; Nevárez-Moorillón, G.V. Oviposition deterrent and larvicidal and pupaecidal activity of seven essential oils and their major components against Culex quinquefasciatus Say (Diptera: Culicidae): Synergism–antagonism Effects. Insects 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, E.; Prinsloo, G.J.; Smart, L.E.; Dewhirst, S.Y. Methyl salicylate, thymol and carvacrol as oviposition deterrents for Frankliniella occidentalis (Pergande) on plum blossoms. Arthropod-Plant Interact. 2014, 8, 421–427. [Google Scholar] [CrossRef]
- Damtie, D.; Mekonnen, Y. Toxicity and oviposition deterrent activities of thyme essential oils against Anopheles arabiensis. Psyche J. Entomol. 2021, 2021, 6684156. [Google Scholar] [CrossRef]
- Saxena, K.N.; Basit, A. Inhibition of oviposition by volatiles of certain plants and chemicals in the leafhopper Amrasca devastons (distant). J. Chem. Ecol. 1982, 8, 329–338. [Google Scholar] [CrossRef]
- Fagbemi, K.O.; Aina, D.A.; Olajuyigbe, O.O. Soxhlet Extraction versus Hydrodistillation Using the Clevenger Apparatus: A Comparative Study on the Extraction of a Volatile Compound from Tamarindus indica Seeds. Sci. World J. 2021, 2021, 5961586. [Google Scholar] [CrossRef]
- Yang, N.-Y.; Li, Q.-R.; Zhang, X.; Zeng, F.-L.; Shen, X.-C.; Long, Q.-D. Spectrum-Efficacy Relationships between GC-MS Fingerprints of Essential Oil from Valerianae Jatamansi Rhizoma et Radix and the Efficacy of Inhibiting Microglial Activation. eCAM 2022, 2022, 9972902. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry (ed. 4.1); Allured Publ Crop: Carol Steam, IL, USA, 2017; Available online: http://essentialoilcomponentsbygcms.com/ (accessed on 1 November 2020).
- Montaño-Campaz, M.L.; Oliveira, E.E.; Toro-Restrepo, B.; Bacca, T.; Feuillet-Hurtado, C.; Afanador, J.G.M.; Moreira, R.P.L.; Mendes, L.A.; Aguiar, R.W.S.; Dias, L.G. Siparuna gesnerioides and Siparuna guianensis Essential Oils in Aedes aegypti Control: Phytoanalysis, Molecular Insights for Larvicidal Activity and Selectivity to Non-Target Organisms. Plants 2025, 14, 1322. [Google Scholar] [CrossRef]
- Aguiar, R.W.S.; dos Santos, S.F.; da Silva Morgado, F.; Ascencio, S.D.; de Mendonça Lopes, M.; Viana, K.F.; Didonet, J.; Ribeiro, B.M. Insecticidal and Repellent Activity of Siparuna guianensis Aubl. (Negramina) against Aedes aegypti and Culex quinquefasciatus. PLoS ONE 2015, 10, e0116765. [Google Scholar] [CrossRef]
- WHO. Guidelines for Laboratory and Field Testing of Mosquito Larvicides; WHO: Geneva, Switzerland, 2005. [Google Scholar]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T. A P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, G.N.; Sasisekharan, V. Conformation of Polypeptides and Proteins. In Advances in Protein Chemistry; Anfinsen, C.B., Anson, M.L., Edsall, J.T., Richards, F.M., Eds.; Academic Press: Cambridge, MA, USA, 1968; Volume 23, pp. 283–437. [Google Scholar]
- Haas, J.; Barbato, A.; Behringer, D.; Studer, G.; Roth, S.; Bertoni, M.; Mostaguir, K.; Gumienny, R.; Schwede, T. Continuous Automated Model EvaluatiOn (CAMEO) complementing the critical assessment of structure prediction in CASP12. Proteins:Struct., Funct., Bioinf. 2018, 86, 387–398. [Google Scholar] [CrossRef]
- Benkert, P.; Biasini, M.; Schwede, T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 2010, 27, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph Model 1999, 17, 57–61. [Google Scholar]
- Souza Moura, W.; de Souza, S.R.; Campos, F.S.; Sander Rodrigues Cangussu, A.; Macedo Sobrinho Santos, E.; Silva Andrade, B.; Borges Gomes, C.H.; Fernandes Viana, K.; Haddi, K.; Oliveira, E.E.; et al. Antibacterial activity of Siparuna guianensis essential oil mediated by impairment of membrane permeability and replication of pathogenic bacteria. Ind. Crops Prod. 2020, 146, 112142. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Schrodinger, LLC. The PyMOL Molecular Graphics System Version 1.8; Schrödinger LLC: New York, NY, USA, 2015. [Google Scholar]
- Biovia, D.S. Discovery Studio Modeling Environment; Dassault Systèmes: San Diego, CA, USA, 2017. [Google Scholar]
- Coelho-de-Souza, A.; Alves-Soares, R.; Oliveira, H.; Gomes-Vasconcelos, Y.; Souza, P.; Santos-Nascimento, T.; Oliveira, K.; Diniz, L.; Guimarães-Pereira, J.; Leal-Cardoso, J. The essential oil of Hyptis crenata Pohl ex Benth. presents an antiedematogenic effect in mice. Braz. J. Med. Biol. Res. 2021, 54, e9422. [Google Scholar] [CrossRef]
- Mourão, D.D.S.C.; Ferreira de Souza Pereira, T.; Souza, D.J.d.; Chagas Júnior, A.F.; Dalcin, M.S.; Veloso, R.A.; Leão, E.U.; Santos, G.R.d. Essential Oil of Cymbopogon citratus on the Control of the Curvularia Leaf Spot Disease on Maize. Medicines 2017, 4, 62. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).