Comparative Efficacy of pHA130 Haemoadsorption Combined with Haemodialysis Versus Online Haemodiafiltration in Removing Protein-Bound and Middle-Molecular-Weight Uraemic Toxins: A Randomized Controlled Trial
Abstract
1. Introduction
2. Results
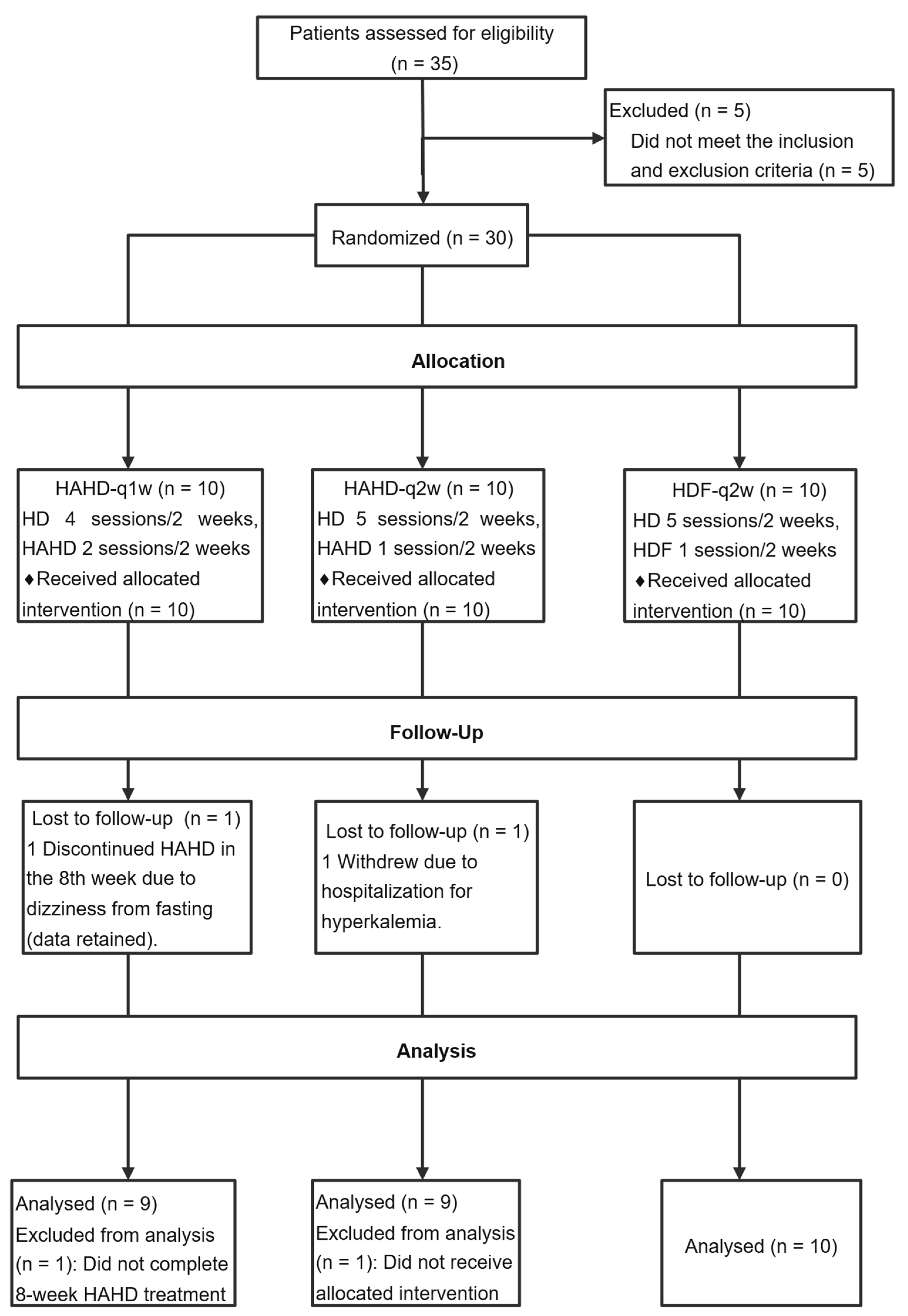
2.1. Patient Characteristics
2.2. Primary Outcome: Single-Session Toxin Reduction
2.3. Longitudinal Toxin Levels and Frequency Effects
2.4. Postintervention Rebound
2.5. Patient-Reported Outcomes
2.6. Generalized Estimating Equation (GEE) Analysis
2.7. Safety Outcomes
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Design
5.2. Interventions
5.3. Treatment Protocols
5.4. Outcomes
5.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosner, M.H.; Reis, T.; Husain-Syed, F.; Vanholder, R.; Hutchison, C.; Stenvinkel, P.; Blankestijn, P.J.; Cozzolino, M.; Juillard, L.; Kashani, K.; et al. Classification of Uremic Toxins and Their Role in Kidney Failure. Clin. J. Am. Soc. Nephrol. CJASN 2021, 16, 1918–1928. [Google Scholar] [CrossRef]
- Borges, N.A.; Barros, A.F.; Nakao, L.S.; Dolenga, C.J.; Fouque, D.; Mafra, D. Protein-Bound Uremic Toxins from Gut Microbiota and Inflammatory Markers in Chronic Kidney Disease. J. Ren. Nutr. 2016, 26, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Wolley, M.J.; Hutchison, C.A. Large uremic toxins: An unsolved problem in end-stage kidney disease. Nephrol. Dial. Transpl. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2018, 33, iii6–iii11. [Google Scholar] [CrossRef]
- Graboski, A.L.; Redinbo, M.R. Gut-Derived Protein-Bound Uremic Toxins. Toxins 2020, 12, 590. [Google Scholar] [CrossRef]
- Yamamoto, S.; Fuller, D.S.; Komaba, H.; Nomura, T.; Massy, Z.A.; Bieber, B.; Robinson, B.; Pisoni, R.; Fukagawa, M. Serum total indoxyl sulfate and clinical outcomes in hemodialysis patients: Results from the Japan Dialysis Outcomes and Practice Patterns Study. Clin. Kidney J. 2021, 14, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Chuang, C.K.; Jayakumar, T.; Liu, H.L.; Pan, C.F.; Wang, T.J.; Chen, H.H.; Wu, C.J. Serum p-cresyl sulfate predicts cardiovascular disease and mortality in elderly hemodialysis patients. Arch. Med. Sci. 2013, 9, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Wojtaszek, E.; Oldakowska-Jedynak, U.; Kwiatkowska, M.; Glogowski, T.; Malyszko, J. Uremic Toxins, Oxidative Stress, Atherosclerosis in Chronic Kidney Disease, and Kidney Transplantation. Oxidative Med. Cell. Longev. 2021, 2021, 6651367. [Google Scholar] [CrossRef]
- Cozzolino, M.; Magagnoli, L.; Ciceri, P. From Physicochemical Classification to Multidimensional Insights: A Comprehensive Review of Uremic Toxin Research. Toxins 2025, 17, 295. [Google Scholar] [CrossRef]
- Nielsen, T.L.; Plesner, L.L.; Warming, P.E.; Pallisgaard, J.L.; Dalsgaard, M.; Schou, M.; Høst, U.; Rydahl, C.; Brandi, L.; Køber, L.; et al. YKL-40 in patients with end-stage renal disease receiving haemodialysis. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem. 2018, 23, 357–363. [Google Scholar] [CrossRef]
- Lorenz, G.; Schmalenberg, M.; Kemmner, S.; Haller, B.; Steubl, D.; Pham, D.; Schreiegg, A.; Bachmann, Q.; Schmidt, A.; Haderer, S.; et al. Mortality prediction in stable hemodialysis patients is refined by YKL-40, a 40-kDa glycoprotein associated with inflammation. Kidney Int. 2018, 93, 221–230. [Google Scholar] [CrossRef]
- Krieter, D.H.; Kerwagen, S.; Rüth, M.; Lemke, H.D.; Wanner, C. Differences in Dialysis Efficacy Have Limited Effects on Protein-Bound Uremic Toxins Plasma Levels over Time. Toxins 2019, 11, 47. [Google Scholar] [CrossRef]
- Ronco, C.; Clark, W.R. Haemodialysis membranes. Nat. Rev. Nephrol. 2018, 14, 394–410. [Google Scholar] [CrossRef]
- Himmelfarb, J.; Vanholder, R.; Mehrotra, R.; Tonelli, M. The current and future landscape of dialysis. Nat. Rev. Nephrol. 2020, 16, 573–585. [Google Scholar] [CrossRef]
- Sarhaan, I.I.; Hassan, M.S.; Samea, M.S.A.E.; Mahmoud, A.M.; Gharib, M.S. Is online hemodiafiltration superior to hemodialysis in removal of indoxyl sulfate in chronic hemodialysis patients? A comparative study. J. Egypt. Soc. Nephrol. Transpl. 2023, 23, 149–155. [Google Scholar]
- Niwa, T. Removal of protein-bound uraemic toxins by haemodialysis. Blood Purif. 2013, 35 (Suppl. 2), 20–25. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Kazama, J.J.; Omori, K.; Matsuo, K.; Takahashi, Y.; Kawamura, K.; Matsuto, T.; Watanabe, H.; Maruyama, T.; Narita, I. Continuous Reduction of Protein-Bound Uraemic Toxins with Improved Oxidative Stress by Using the Oral Charcoal Adsorbent AST-120 in Haemodialysis Patients. Sci. Rep. 2015, 5, 14381. [Google Scholar] [CrossRef]
- Sternkopf, M.; Thoröe-Boveleth, S.; Beck, T.; Oleschko, K.; Erlenkötter, A.; Tschulena, U.; Steppan, S.; Speer, T.; Goettsch, C.; Jankowski, V.; et al. A Bifunctional Adsorber Particle for the Removal of Hydrophobic Uremic Toxins from Whole Blood of Renal Failure Patients. Toxins 2019, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Ankawi, G.; Bagshaw, S.M.; Baldwin, I.; Basu, R.; Bottari, G.; Cantaluppi, V.; Clark, W.; De Rosa, S.; Forni, L.G.; et al. Hemoadsorption: Consensus report of the 30th Acute Disease Quality Initiative workgroup. Nephrol. Dial. Transpl. 2024, 39, 1945–1964. [Google Scholar] [CrossRef] [PubMed]
- Meijers, B.; Vega, A.; Juillard, L.; Kawanishi, H.; Kirsch, A.H.; Maduell, F.; Massy, Z.A.; Mitra, S.; Vanholder, R.; Ronco, C.; et al. Extracorporeal Techniques in Kidney Failure. Blood Purif. 2024, 53, 343–357. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Wang, Y.; Jiang, A.; Cao, N.; He, Y.; Wang, J.; Guo, Z.; Liu, W.; Shi, W.; et al. Randomized Control Study on Hemoperfusion Combined with Hemodialysis versus Standard Hemodialysis: Effects on Middle-Molecular-Weight Toxins and Uremic Pruritus. Blood Purif. 2022, 51, 812–822. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Deng, W.; Meng, L.; Gong, W.; Yao, H. The Effect of Combination Use of Hemodialysis and Hemoperfusion on Microinflammation in Elderly Patients with Maintenance Hemodialysis. Blood Purif. 2022, 51, 739–746. [Google Scholar] [CrossRef]
- Sun, L.; Hua, R.X.; Wu, Y.; Zou, L.X. Effect of different hemodialysis modalities on hepcidin clearance in patients undergoing maintenance hemodialysis. Semin. Dial. 2023, 36, 240–246. [Google Scholar] [CrossRef]
- Cheng, W.; Luo, Y.; Wang, H.; Qin, X.; Liu, X.; Fu, Y.; Ronco, C. Survival Outcomes of Hemoperfusion and Hemodialysis versus Hemodialysis in Patients with End-Stage Renal Disease: A Systematic Review and Meta-Analysis. Blood Purif. 2022, 51, 213–225. [Google Scholar] [CrossRef]
- Damianaki, A.; Stambolliu, E.; Alexakou, Z.; Petras, D. Expanding the potential therapeutic options of hemoperfusion in the era of improved sorbent biocompatibility. Kidney Res. Clin. Pract. 2023, 42, 298–311. [Google Scholar] [CrossRef]
- Ronco, C. Combined Hemoperfusion-Hemodialysis in End-Stage Renal Disease Patients. Contrib. Nephrol. 2023, 200, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Peng, X.; Hu, Z.; Yu, M.; Fu, J.; Huang, Y. Fabrication of a novel nitrogen-containing porous carbon adsorbent for protein-bound uremic toxins removal. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111879. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y.; Peng, X.; Wei, Y.; Shao, K. Simultaneous hypercrosslinking and functionalization of porous polystyrene adsorbent for protein-bound uraemic toxins removal. React. Funct. Polym. 2025, 214, 106265. [Google Scholar] [CrossRef]
- Ramirez-Guerrero, G.; Reis, T.; Segovia-Hernandez, B.; Aranda, F.; Verdugo, C.; Pedreros-Rosales, C.; Marcello, M.; Leon, J.; Rojas, A.; Galli, F.; et al. Efficacy of HA130 Hemoadsorption in Removing Advanced Glycation End Products in Maintenance Hemodialysis Patients. Artif. Organs 2025, 49, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Marcello, M.; Ramírez-Guerrero, G.; Reis, T.; Ronco, C. Blood purification for the treatment of chronic kidney disease-associated pruritus. Integr. Med. Nephrol. Androl. 2024, 11, e24-00005. [Google Scholar] [CrossRef]
- Brendolan, A.; Lorenzin, A.; De Cal, M.; Virzi, G.M.; Cantaluppi, V.; Marengo, M.; Lentini, P.; Ronco, C. Hemoadsorption Combined with Hemodialysis and the “Inflammation Mitigation Hypothesis”. Integr. Med. Nephrol. Androl. 2024, 11, e00006. [Google Scholar] [CrossRef]
- Poesen, R.; Viaene, L.; Verbeke, K.; Claes, K.; Bammens, B.; Sprangers, B.; Naesens, M.; Vanrenterghem, Y.; Kuypers, D.; Evenepoel, P.; et al. Renal clearance and intestinal generation of p-cresyl sulfate and indoxyl sulfate in CKD. Clin. J. Am. Soc. Nephrol. CJASN 2013, 8, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Meijers, B.K.; De Loor, H.; Bammens, B.; Verbeke, K.; Vanrenterghem, Y.; Evenepoel, P. p-Cresyl sulfate and indoxyl sulfate in hemodialysis patients. Clin. J. Am. Soc. Nephrol. CJASN 2009, 4, 1932–1938. [Google Scholar] [CrossRef] [PubMed]
- Canaud, B.; Stuard, S.; Laukhuf, F.; Yan, G.; Canabal, M.I.G.; Lim, P.S.; Kraus, M.A. Choices in hemodialysis therapies: Variants, personalized therapy and application of evidence-based medicine. Clin. Kidney J. 2021, 14, i45–i58. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, C.; Yang, T.; Zhao, J.; Wang, X.; Zhang, L.; Li, Y.; Shen, Y.; Gao, Y.; Zhang, H. Long-Term Safety of “4-Hour” Hemoadsorption Combined with Hemodialysis in Maintenance Hemodialysis Patients: A Multicenter Prospective Cohort Study. Blood Purif. 2025, 413–423. [Google Scholar] [CrossRef]
- Bergström, J.; Wehle, B. No change in corrected beta 2-microglobulin concentration after cuprophane haemodialysis. Lancet 1987, 1, 628–629. [Google Scholar] [CrossRef] [PubMed]
| Parameters | HAHD-q1w (n = 10) | HAHD-q2w (n = 10) | HDF-q2w (n = 10) | All (n = 30) | F | p |
|---|---|---|---|---|---|---|
| Age (years) | 52.50 ± 8.82 | 52.30 ± 11.25 | 51.60 ± 14.03 | 52.13 ± 11.16 | 0.02 | 0.983 |
| Dialysis duration (months) | 78.10 ± 42.81 | 82.40 ± 38.20 | 66.40 ± 42.17 | 75.63 ± 40.26 | 0.41 | 0.671 |
| Systolic blood pressure (SBP) (mmHg) | 142.40 ± 10.86 | 135.70 ± 12.37 | 137 ± 15.51 | 138.37 ± 12.94 | 0.74 | 0.486 |
| Diastolic blood pressure (DBP) (mmHg) | 78.90 ± 10.57 | 73.80 ± 11.42 | 78.60 ± 12.88 | 77.10 ± 11.50 | 0.60 | 0.555 |
| Pre-dialysis heart rate (beats/min) | 77.80 ± 13.81 | 77.40 ± 10.94 | 73.30 ± 7.30 | 76.17 ± 10.82 | 0.51 | 0.605 |
| Red blood cell (RBC) count (×1012/L) | 3.53 ± 0.70 | 3.76 ± 0.80 | 3.76 ± 0.56 | 3.68 ± 0.68 | 0.38 | 0.689 |
| Packed cell volume (PCV) (L/L) | 0.34 ± 0.06 | 0.35 ± 0.03 | 0.36 ± 0.04 | 0.35 ± 0.05 | 0.39 | 0.679 |
| White blood cell (WBC) count (×109/L) | 6.05 ± 1.27 | 5.77 ± 1.14 | 5.89 ± 0.74 | 5.90 ± 1.04 | 0.18 | 0.837 |
| Hemoglobin (Hb) (g/L) | 106.50 ± 20.10 | 109.50 ± 7.11 | 114.50 ± 13.45 | 110.17 ± 14.44 | 0.77 | 0.472 |
| Platelet (PLT) count (×109/L) | 146.30 ± 47.75 | 161.40 ± 29.36 | 171.20 ± 32.39 | 159.63 ± 37.54 | 1.13 | 0.339 |
| Previous heparin dosage (mL) | 0.33 ± 0.05 | 0.34 ± 0.04 | 0.31 ± 0.07 | 0.32 ± 0.05 | 0.40 | 0.677 |
| Baseline heparin dosage (mL) | 0.37 ± 0.05 | 0.34 ± 0.04 | 0.32 ± 0.07 | 0.34 ± 0.06 | 2.61 | 0.092 |
| Pre-dialysis body weight (kg) | 65.55 ± 8.24 | 64.93 ± 10.85 | 61.59 ± 8.19 | 64.02 ± 9.03 | 0.54 | 0.590 |
| Post-dialysis body weight (kg) | 63.46 ± 7.65 | 62.66 ± 10.93 | 59.47 ± 7.92 | 61.86 ± 8.82 | 0.56 | 0.580 |
| Interdialytic weight gain (kg) | 2.09 ± 1.05 | 2.27 ± 0.73 | 2.12 ± 0.78 | 2.16 ± 0.84 | 0.12 | 0.884 |
| Duo Pruritus Score (points) | 13 ± 5.73 | 8.80 ± 2.62 | 9.50 ± 5.04 | 10.43 ± 4.87 | 2.33 | 0.116 |
| PSQI score (points) | 10.30 ± 3.50 | 9.60 ± 2.67 | 7.50 ± 3.60 | 9.13 ± 3.39 | 1.97 | 0.159 |
| Pre-dialysis Indoxyl Sulfate (mg/L) | 33.26 ± 15.32 | 31.26 ± 13.97 | 44.63 ± 22.78 | 36.38 ± 18.18 | 1.64 | 0.212 |
| Pre-dialysis p-Cresyl Sulfate (mg/L) | 17.79 ± 23.70 | 16.70 ± 13.29 | 30.88 ± 22.53 | 21.79 ± 20.73 | 1.50 | 0.241 |
| Pre-dialysis β2-Microglobulin (mg/L) | 32.69 ± 4.54 | 31.55 ± 3.25 | 33.12 ± 3.51 | 32.45 ± 3.74 | 0.45 | 0.640 |
| Pre-dialysis YKL-40 (pg/mL) | 260.53 ± 162.05 | 253.55 ± 173.99 | 242.20 ± 86.07 | 252.09 ± 141.08 | 0.04 | 0.961 |
| Parameters | HAHD Group (n = 38) | HDF Group (n = 20) | All (n = 58) | t | p |
|---|---|---|---|---|---|
| IS RR (%) | 46.89 ± 15.54 | 31.78 ± 40.07 | 41.68 ± 27.28 | 2.06 | 0.044 |
| PCS RR (%) | 44.59 ± 10.25 | 31.36 ± 21.80 | 40.03 ± 16.34 | 3.15 | 0.003 |
| YKL-40 RR (%) | 29.83 ± 10.19 | 23.86 ± 26.13 | 27.77 ± 17.41 | 0.98 | 0.336 |
| Parameters | HAHD-q1w (n = 10) | HAHD-q2w (n = 10) | HDF-q2w (n = 10) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | After 8 Weeks | p | Baseline | After 8 Weeks | p | Baseline | After 8 Weeks | p | |
| Red blood cell count (×1012/L) | 3.53 ± 0.70 | 3.42 ± 0.68 | 0.709 | 3.84 ± 0.81 | 3.95 ± 0.88 | 0.805 | 3.76 ± 0.56 | 3.79 ± 0.38 | 0.873 |
| Packed cell volume (L/L) | 0.34 ± 0.06 | 0.33 ± 0.06 | 0.589 | 0.35 ± 0.03 | 0.36 ± 0.04 | 0.592 | 0.36 ± 0.04 | 0.37 ± 0.02 | 0.753 |
| White blood cell count (×109/L) | 6.05 ± 1.27 | 6.99 ± 1.46 | 0.094 | 5.68 ± 1.17 | 6.87 ± 1.58 | 0.154 | 5.89 ± 0.74 | 6.37 ± 0.84 | 0.202 |
| Hemoglobin (g/L) | 106.50 ± 20.10 | 102.70 ± 18.43 | 0.559 | 110.33 ± 7.00 | 112.44 ± 9.61 | 0.623 | 114.50 ± 13.45 | 116.10 ± 8.03 | 0.759 |
| Platelet count (×109/L) | 146.30 ± 47.75 | 156.60 ± 43.74 | 0.444 | 162.44 ± 30.95 | 164.89 ± 34.41 | 0.854 | 171.20 ± 32.39 | 182.10 ± 34.80 | 0.485 |
| Duo pruritus score (points) | 13.00 ± 5.73 | 8.30 ± 3.43 | <0.001 | 8.44 ± 2.51 | 6.11 ± 0.93 | 0.009 | 9.50 ± 5.04 | 8.80 ± 5.71 | 0.132 |
| PSQI (points) | 10.30 ± 3.50 | 5.80 ± 1.87 | <0.001 | 9.67 ± 2.83 | 5.67 ± 1.66 | <0.001 | 7.50 ± 3.60 | 7.90 ± 4.68 | 0.724 |
| Indoxyl sulfate (mg/L) | 33.26 ± 15.32 | 22.54 ± 9.66 | 0.001 | 31.15 ± 14.82 | 21.27 ± 9.85 | 0.004 | 44.63 ± 22.78 | 32.55 ± 19.04 | 0.001 |
| p-Cresyl sulfate (mg/L) | 17.79 ± 23.70 | 17.41 ± 17.47 | 0.905 | 14.86 ± 12.68 | 20.22 ± 14.53 | 0.042 | 30.88 ± 22.53 | 29.32 ± 19.66 | 0.736 |
| YKL-40 (pg/mL) | 260.53 ± 162.05 | 284.91 ± 206.00 | 0.271 | 253.55 ± 173.99 | 225.51 ± 131.17 | 0.318 | 242.20 ± 86.07 | 207.54 ± 111.39 | 0.381 |
| Parameters | HAHD-q1w (n = 10) | HAHD-q2w (n = 10) | HDF-q2w (n = 10) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Week 8 | Week 12 | p | Week 8 | Week 12 | p | Week 8 | Week 12 | p | |
| Red blood cell count (×1012/L) | 3.42 ± 0.68 | 3.48 ± 0.73 | 0.410 | 3.95 ± 0.88 | 4.09 ± 0.67 | 0.316 | 3.79 ± 0.38 | 3.76 ± 0.36 | 0.707 |
| Packed cell volume (L/L) | 0.33 ± 0.06 | 0.34 ± 0.07 | 0.485 | 0.36 ± 0.04 | 0.37 ± 0.03 | 0.211 | 0.37 ± 0.02 | 0.36 ± 0.02 | 0.780 |
| White blood cell count (×109/L) | 6.99 ± 1.46 | 6.08 ± 1.67 | 0.011 | 6.87 ± 1.58 | 6.86 ± 1.84 | 0.979 | 6.37 ± 0.84 | 5.97 ± 1.08 | 0.239 |
| Hemoglobin (g/L) | 102.70 ± 18.43 | 104.50 ± 21.54 | 0.473 | 112.44 ± 9.61 | 117.44 ± 12.75 | 0.228 | 116.10 ± 8.03 | 114.80 ± 6.44 | 0.614 |
| Platelet count (×109/L) | 156.60 ± 43.74 | 160.60 ± 45.27 | 0.549 | 164.89 ± 34.41 | 171.67 ± 34.76 | 0.208 | 182.10 ± 34.80 | 179.30 ± 40.65 | 0.737 |
| Duo pruritus score (points) | 8.30 ± 3.43 | 5.40 ± 3.34 | <0.001 | 6.11 ± 0.93 | 4.22 ± 1.86 | 0.003 | 8.80 ± 5.71 | 10.00 ± 4.57 | 0.154 |
| PSQI (points) | 5.80 ± 1.87 | 4.50 ± 2.46 | 0.057 | 5.67 ± 1.66 | 4.44 ± 2.13 | 0.038 | 7.90 ± 4.68 | 8.60 ± 6.38 | 0.322 |
| Indoxyl sulfate (mg/L) | 22.54 ± 9.66 | 26.34 ± 11.32 | 0.062 | 21.27 ± 9.85 | 21.14 ± 12.45 | 0.961 | 32.55 ± 19.04 | 31.70 ± 14.44 | 0.803 |
| p-Cresyl sulfate (mg/L) | 17.41 ± 17.47 | 14.47 ± 18.23 | 0.097 | 20.22 ± 14.53 | 14.46 ± 12.32 | 0.055 | 29.32 ± 19.66 | 21.10 ± 14.05 | 0.045 |
| YKL-40 (pg/mL) | 284.91 ± 206.00 | 260.36 ± 177.70 | 0.185 | 225.51 ± 131.17 | 256.96 ± 143.16 | 0.190 | 207.54 ± 111.39 | 243.90 ± 97.00 | 0.023 |
| Group | Estimation (B) | S.E. | 95% CI | p | |
|---|---|---|---|---|---|
| Duo pruritus score (points) | HDF | 0 | - | - | - |
| HAHD-q1w | 1.53 | 2.10 | −2.58 to 5.64 | 0.465 | |
| HAHD-q2w | −1.44 | 1.71 | −4.80 to 1.92 | 0.401 | |
| PSQI (points) | HDF | 0 | - | - | - |
| HAHD-q1w | 0.44 | 1.39 | −2.30 to 3.17 | 0.754 | |
| HAHD-q2w | 0.05 | 1.30 | −2.49 to 2.60 | 0.967 | |
| Indoxyl sulfate (mg/L) | HDF | 0 | - | - | - |
| HAHD-q1w | −10.63 | 7.12 | −24.59 to 3.32 | 0.135 | |
| HAHD-q2w | −11.26 | 7.03 | −25.04 to 2.52 | 0.109 | |
| p-Cresyl sulfate (mg/L) | HDF | 0 | - | - | - |
| HAHD-q1w | −12.03 | 8.17 | −28.05 to 3.99 | 0.141 | |
| HAHD-q2w | −10.77 | 6.82 | −24.13 to 2.60 | 0.114 | |
| YKL-40 (pg/mL) | HDF | 0 | - | - | - |
| HAHD-q1w | 49.09 | 59.90 | −68.32 to 166.50 | 0.413 | |
| HAHD-q2w | 15.93 | 49.93 | −81.93 to 113.78 | 0.750 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Yuan, H.; Xiong, X.; Zhu, Y.; Fu, P. Comparative Efficacy of pHA130 Haemoadsorption Combined with Haemodialysis Versus Online Haemodiafiltration in Removing Protein-Bound and Middle-Molecular-Weight Uraemic Toxins: A Randomized Controlled Trial. Toxins 2025, 17, 392. https://doi.org/10.3390/toxins17080392
Yu S, Yuan H, Xiong X, Zhu Y, Fu P. Comparative Efficacy of pHA130 Haemoadsorption Combined with Haemodialysis Versus Online Haemodiafiltration in Removing Protein-Bound and Middle-Molecular-Weight Uraemic Toxins: A Randomized Controlled Trial. Toxins. 2025; 17(8):392. https://doi.org/10.3390/toxins17080392
Chicago/Turabian StyleYu, Shaobin, Huaihong Yuan, Xiaohong Xiong, Yalin Zhu, and Ping Fu. 2025. "Comparative Efficacy of pHA130 Haemoadsorption Combined with Haemodialysis Versus Online Haemodiafiltration in Removing Protein-Bound and Middle-Molecular-Weight Uraemic Toxins: A Randomized Controlled Trial" Toxins 17, no. 8: 392. https://doi.org/10.3390/toxins17080392
APA StyleYu, S., Yuan, H., Xiong, X., Zhu, Y., & Fu, P. (2025). Comparative Efficacy of pHA130 Haemoadsorption Combined with Haemodialysis Versus Online Haemodiafiltration in Removing Protein-Bound and Middle-Molecular-Weight Uraemic Toxins: A Randomized Controlled Trial. Toxins, 17(8), 392. https://doi.org/10.3390/toxins17080392





