Determination of Fumonisins B1 and B2 in Food Matrices: Optimisation of a Liquid Chromatographic Method with Fluorescence Detection
Abstract
1. Introduction
2. Results and Discussion
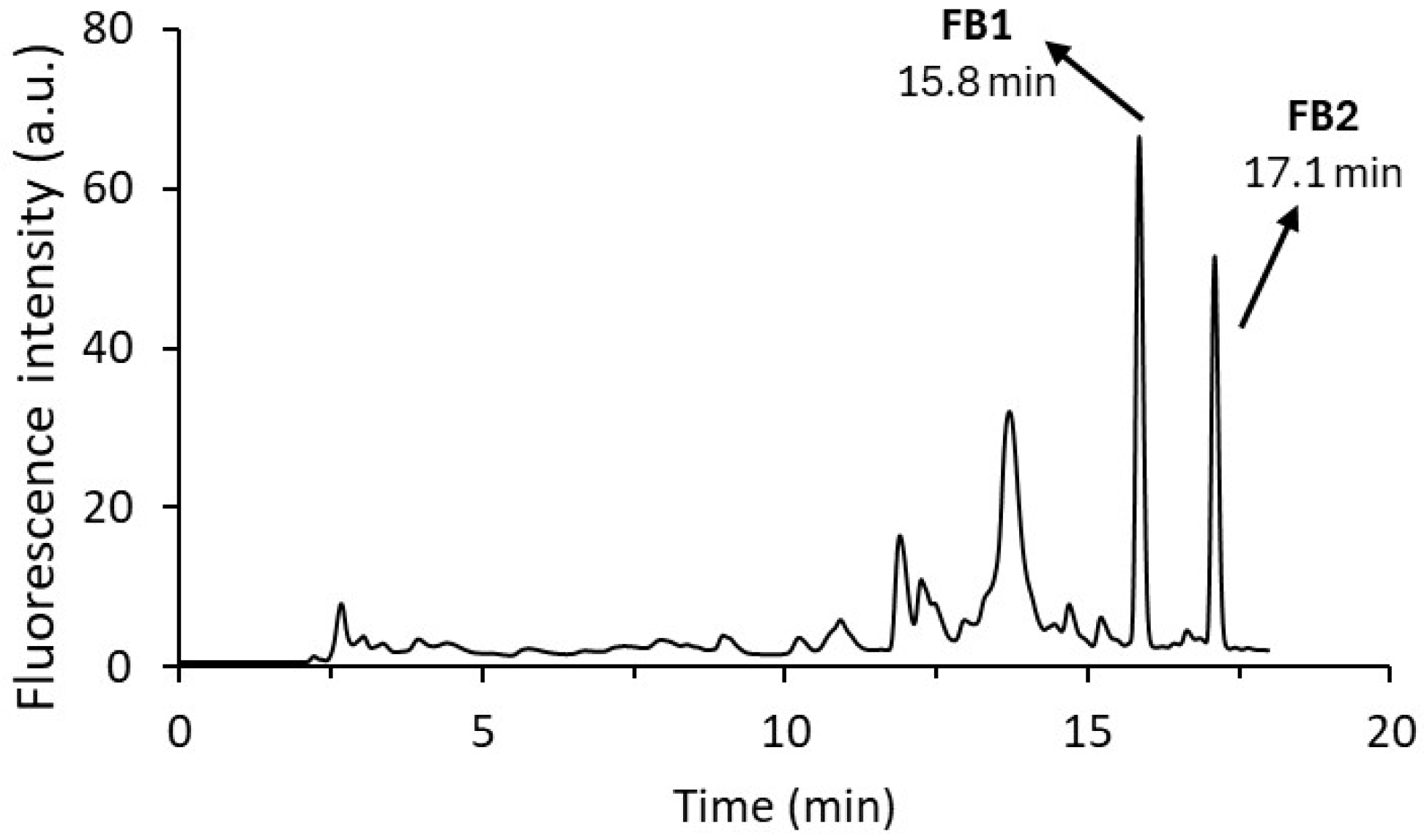
2.1. Chromatographic Conditions Optimisation
2.2. Automatic Derivatisation Optimisation
2.3. Method Validation and Analytical Figures of Merit
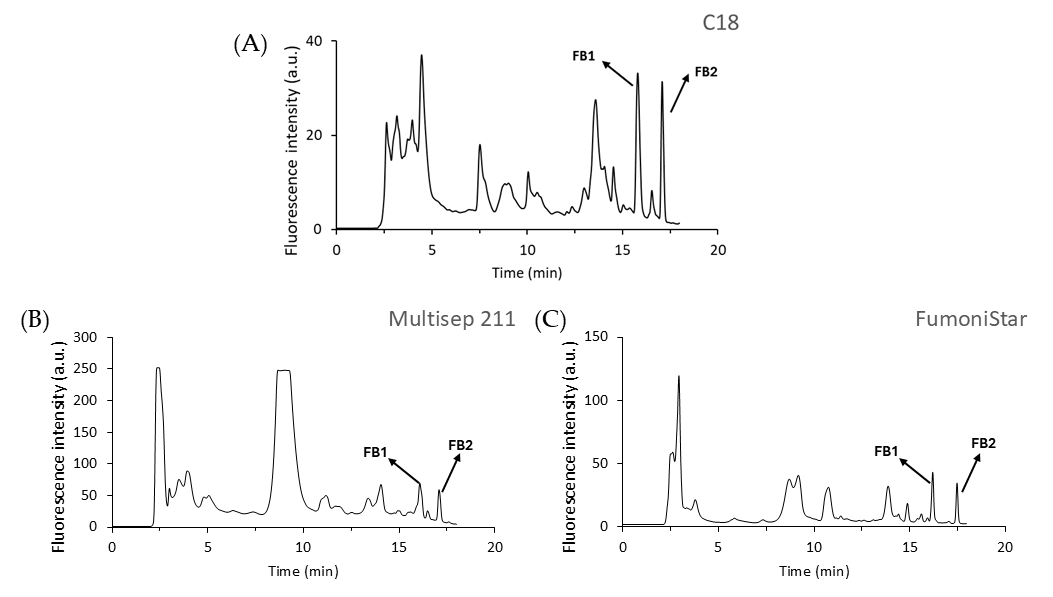
2.4. Application of the Method to Different Food Matrices
3. Conclusions
4. Materials and Methods
4.1. Reagents and Standards
4.2. Precolumn Automatic Derivatisation Optimisation
4.3. HPLC-FLD Analysis

4.4. Calibration Curve and Figures of Merit
4.5. Food Matrices Preparation and Clean-Up Procedures
- (A)
- C18 cartridge (Thermo Fisher Scientific, Waltham, MA, USA): The supernatant (2 mL) was collected and diluted with 5 mL of water. Samples were loaded onto a C18 cartridge, which was preconditioned with 5 mL MeOH and 5 mL water. After washing with 5 mL water and 2 mL ACN:H2O (1:9 v/v), fumonisins were eluted with 2 mL ACN:H2O (1:1 v/v). Samples were filtered through a 0.22 μm PTFE filter and stored at 4 °C until FB1 and FB2 analysis (<12 h).
- (B)
- MultiSep 211 Fumonisins (Romer Labs, Getzersdorf, Austria): The supernatant was filtered, and the pH was adjusted to a range of 6–9. Next, 3 mL extract was mixed with 8 mL of MeOH: H2O (3:1 v/v) and applied to the MultiSep 211 column, previously prewashed with 5 mL of MeOH followed by 5 mL of MeOH: H2O (3:1 v/v). After the sample passed through the cartridge, the column was washed with 8 mL of MeOH:H2O (3:1, v/v), followed by 3 mL of MeOH. Fumonisins were eluted with 10 mL of ACN/water (1:1 v/v) and evaporated to dryness with a Rotavapor. The resulting residue was dissolved in 0.5 mL of ACN:H2O (1:1 v/v) and stored at 4 °C until its HPLC analysis (<12 h).
- (C)
- FumoniStar IAC (Romer Labs): First, 1 mL of the extracted sample was added to 4 mL of PBS, mixed, and filtered. Then, 1 mL of the diluted extract was passed through an IAC and washed with 1 mL of PBS at an approximate flow rate of 1 mL/min. Fumonisins were eluted using 3 mL of ACN:H2O (1:1, v/v). The eluate was evaporated and redissolved in 0.5 mL of ACN:H2O (1:1, v/v). Samples were filtered through a 0.22 μm PTFE filter and stored at 4 °C until FB1 and FB2 analyses (<12 h).
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FB | Fumonisin |
| OPA | o-phthaldialdehyde |
| SPE | Solid-phase extraction |
| IAC | Immunoaffinity columns |
| BBD | Box–Behnken design |
References
- Sultan, Y.; Magan, N.; Medina, A. Efficacy of Different C18 HPLC Analytical Columns in the Analysis of Fumonisins B1 and B2 in Different Matrices. Biointerface Res. Appl. Chem. 2022, 12, 1721–1734. [Google Scholar] [CrossRef]
- Li, T.; Li, J.; Wang, J.; Xue, K.S.; Su, X.; Qu, H.; Duan, X.; Jiang, Y. The occurrence and management of fumonisin contamination across the food production and supply chains. J. Adv. Res. 2024, 60, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Zentai, A.; Szeitzné-Szabó, M.; Mihucz, G.; Szeli, N.; Szabó, A.; Kovács, M. Occurrence and Risk Assessment of Fumonisin B1 and B2 Mycotoxins in Maize-Based Food Products in Hungary. Toxins 2019, 11, 709. [Google Scholar] [CrossRef]
- Voss, K.A.; Riley, R.T. Fumonisin Toxicity and Mechanism of Action: Overview and Current Perspectives. Food Saf. 2013, 1, 49–69. [Google Scholar] [CrossRef]
- Rao, Z.-X.; Tokach, M.D.; Woodworth, J.C.; DeRouchey, J.M.; Goodband, R.D.; Calderón, H.I.; Dritz, S.S. Effects of Fumonisin-Contaminated Corn on Growth Performance of 9 to 28 kg Nursery Pigs. Toxins 2020, 12, 604. [Google Scholar] [CrossRef]
- Arumugam, T.; Ghazi, T.; Chuturgoon, A.A. Molecular and Epigenetic Modes of Fumonisin B1 Mediated Toxicity and Carcinogenesis and Detoxification Strategies. Crit. Rev. Toxicol. 2021, 51, 76–94. [Google Scholar] [CrossRef]
- Yayeh, T.; Jeong, H.R.; Park, Y.S.; Moon, S.; Sur, B.; Yoo, H.-S.; Oh, S. Fumonisin B1-Induced Toxicity Was Not Exacerbated in Glutathione Peroxidase-1/Catalase Double Knock Out Mice. Biomol. Ther. 2021, 29, 52–57. [Google Scholar] [CrossRef]
- Martins, F.A.; Ferreira, F.M.D.; Ferreira, F.D.; Bando, É.; Nerilo, S.B.; Hirooka, E.Y.; Machinski, M. Daily Intake Estimates of Fumonisins in Corn-Based Food Products in the Population of Paraná, Brazil. Food Control. 2012, 26, 614–618. [Google Scholar] [CrossRef]
- Anumudu, C.K.; Ekwueme, C.T.; Uhegwu, C.C.; Ejileugha, C.; Augustine, J.; Okolo, C.A.; Onyeaka, H. A Review of the Mycotoxin Family of Fumonisins, Their Biosynthesis, Metabolism, Methods of Detection and Effects on Humans and Animals. Int. J. Mol. Sci. 2025, 26, 184. [Google Scholar] [CrossRef]
- Kaltner, F.; Rampl, C.; Rychlik, M. Development and Validation of a Cost-Effective HPLC-FLD Method for Routine Analysis of Fumonisins B1 and B2 in Corn and Corn Products. Food Anal. Methods 2017, 10, 1349–1358. [Google Scholar] [CrossRef]
- De Girolamo, A.; Solfrizzo, M.; von Holst, C.; Visconti, A. Comparison of Different Extraction and Clean-Up Procedures for the Determination of Fumonisins in Maize and Maize-Based Food Products. Food Addit. Contam. 2001, 18, 59–67. [Google Scholar] [CrossRef]
- Esposito, F.; Fasano, E.; Scognamiglio, G.; Nardone, A.; Triassi, M.; Cirillo, T. Exposure Assessment to Fumonisins B1, B2 and B3 through Consumption of Gluten-Free Foodstuffs Intended for People Affected by Celiac Disease. Food Chem. Toxicol. 2016, 97, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, I.; Falavigna, C.; Dall’Asta, C.; Proctor, R.H.; Galaverna, G.; Battilani, P. Fumonisins B, A and C Profile and Masking in Fusarium verticillioides Strains on Fumonisin-Inducing and Maize-Based Media. Int. J. Food Microbiol. 2012, 159, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Cano-Sancho, G.; Ramos, A.J.; Marín, S.; Sanchís, V. Occurrence of Fumonisins in Catalonia (Spain) and an Exposure Assessment of Specific Population Groups. Food Addit. Contam. Part A 2012, 29, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Ndube, N.; van der Westhuizen, L.; Green, I.R.; Shephard, G.S. HPLC Determination of Fumonisin Mycotoxins in Maize: A Comparative Study of Naphthalene-2,3-Dicarboxaldehyde and o-Phthaldialdehyde Derivatization Reagents for Fluorescence and Diode Array Detection. J. Chromatogr. B 2011, 879, 2239–2243. [Google Scholar] [CrossRef]
- Ye, H.; Lai, X.; Liu, C. Determination of Fumonisin B1 and B2 in Corn Using Matrix-Phase Dispersion Coupled to High Performance Liquid Chromatography. Asian J. Chem. 2013, 25, 6807–6810. [Google Scholar] [CrossRef]
- Anfossi, L.; Calderara, M.; Baggiani, C.; Giovannoli, C.; Arletti, E.; Giraudi, G. Development and Application of a Quantitative Lateral Flow Immunoassay for Fumonisins in Maize. Anal. Chim. Acta 2010, 682, 104–109. [Google Scholar] [CrossRef]
- Jakšić, S.; Živkov-Baloš, M.; Mihaljev, Ž.; Mašić, Z.; Jajić, I.; Banić, N.; Abramović, B. Extraction without Organic Solvents in the Determination of Fumonisins B1, B2, and B3 in Maize by HPLC–FLD and ELISA Tests. Food Anal. Methods 2015, 8, 1446–1455. [Google Scholar] [CrossRef]
- Coronel, M.B.; Vicente, S.; Resnik, S.L.; Alzamora, S.M.; Pacin, A. Fumonisins in Maize and Gluten Meal Analysed in Argentinean Wet Milling Industrial Plants by ELISA Compared with HPLC-FLD Method. Food Control 2016, 67, 285–291. [Google Scholar] [CrossRef]
- Hinojo, M.J.; Medina, A.; Valle-Algarra, F.M.; Gimeno-Adelantado, J.V.; Jiménez, M.; Mateo, R. Fumonisin Production in Rice Cultures of Fusarium verticillioides under Different Incubation Conditions Using an Optimized Analytical Method. Food Microbiol. 2006, 23, 119–127. [Google Scholar] [CrossRef]
- Kosoglu, I.; Aksoy, U.; Pehlivan, R. Fumonisin B1 and B2 occurrence in dried fig fruits (Ficus carica L.) under Meander Valley’s climatic conditions and relationship with fruit quality. Food Addit. Contam. Part A 2011, 28, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, P.B.; Mogensen, J.M.; Larsen, T.O.; Nielsen, K.F. Occurrence of fumonisins B2 and B4 in retail raisins. J. Agric. Food Chem. 2011, 59, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Petrarca, M.H.; Rodrigues, M.I.; Rossi, E.A.; de Sylos, C.M. Optimisation of a Sample Preparation Method for the Determination of Fumonisin B1 in Rice. Food Chem. 2014, 158, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on Agriculture, Food, and Human Health and Their Management Strategies. Toxins 2019, 11, 328. [Google Scholar] [CrossRef]
- AOAC International. Fumonisins B1 and B2 in Corn and Corn Flakes. Liquid Chromatography with Immunoaffinity Column Cleanup. J. AOAC Int. 2001, 84, 1828–1837. [Google Scholar] [CrossRef]
- Williams, L.D.; Meredith, F.I.; Riley, R.T. Fumonisin-ortho-phthalaldehyde derivative is stabilized at low temperature. J. Chromatogr. B 2004, 806, 311–314. [Google Scholar] [CrossRef]
- Commission Regulation (EU). 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union L 2023, 119, 103–157. Available online: https://eur-lex.europa.eu/eli/reg/2023/915/oj (accessed on 27 July 2025).
- Ocampo-Acuña, Y.D.; Salazar-Rios, E.; Ramírez-Cisneros, M.Á.; Rios, M.Y. Comprehensive Review of Liquid Chromatography Methods for Fumonisin Determination, a 2006–2022 Update. Arab. J. Chem. 2023, 16, 104716. [Google Scholar] [CrossRef]
- Abdallah, M.F.; Krska, R.; Sulyok, M. Occurrence of ochratoxins, fumonisin B2, aflatoxins (B1 and B2), and other secondary fungal metabolites in dried date palm fruits from Egypt: A mini-survey. J. Food Sci. 2018, 83, 559–564. [Google Scholar] [CrossRef]
- Commission Implementing Regulation (EU). 2023/2782 of 14 December 2023 Laying Down the Methods of Sampling and Analysis for the Control of the Levels of Mycotoxins in Food and Repealing Regulation (EC) No 401/2006. Off. J. Eur. Union L 2023, 2782. Available online: http://data.europa.eu/eli/reg_impl/2023/2782/oj (accessed on 27 July 2025).
- Palumbo, J.D.; O’Keeffe, T.L.; McGarvey, J.A. Incidence of fumonisin B2 production within Aspergillus section Nigri populations isolated from California raisins. J. Food Prot. 2011, 74, 672–675. [Google Scholar] [CrossRef]
- Galán, A.J.; Martín, A.; Torres-Vila, L.M.; Ruiz-Moyano, S.; Galván, A.I.; Serradilla, M.J.; López-Corrales, M. Quantification and identification of damage caused by pests and fungi in dried figs from orchards with different levels of agronomic management in the main production areas of Extremadura (SW Spain). Crop Prot. 2023, 172, 106334. [Google Scholar] [CrossRef]
- Pereira, V.L.; Fernandes, J.O.; Cunha, S.C. Mycotoxins in Cereals and Related Foodstuffs: A Review on Occurrence and Recent Methods of Analysis. Trends Food Sci. Technol. 2014, 36, 96–136. [Google Scholar] [CrossRef]
- Kim, E.K.; Scott, P.M.; Lau, B.P.Y.; Lewis, D.A. Extraction of fumonisins B1 and B2 from white rice flour and their stability in white rice flour, cornstarch, cornmeal, and glucose. J. Agric. Food Chem. 2002, 50, 3614–3620. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Appropriateness to set a group health-based guidance value for fumonisins and their modified forms. EFSA J. 2018, 16, e05172. [Google Scholar] [CrossRef]
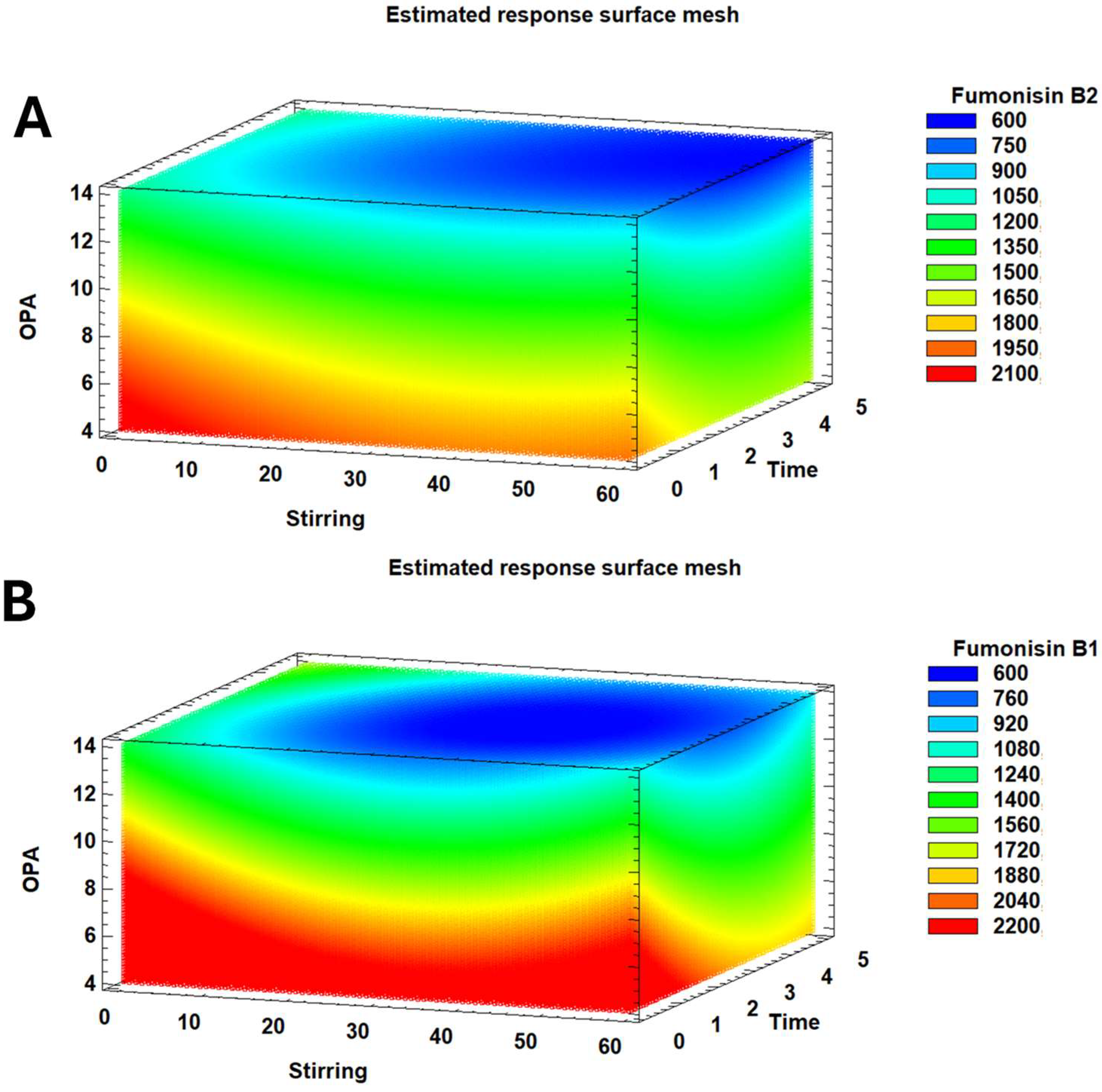
| ANOVA | FB1 | FB2 | ||
|---|---|---|---|---|
| p-Values | Reg. Coeff. 1 | p-Values | Reg. Coeff. | |
| Constant | 3514.3 | 2386.9 | ||
| A: Stirring | 0.023 2 | −29.8 | 0.016 | −12.4 |
| B: Incubation Time | 0.002 | −349.3 | 0.044 | −127.7 |
| C: OPA | 0.000 | −144.7 | 0.000 | −34.4 |
| AA | 0.000 | 0.4 | 0.075 | 0.1 |
| AB | 0.027 | −1.6 | 0.276 | −0.9 |
| AC | 0.803 | 0.1 | 0.978 | 0.0 |
| BB | 0.001 | 46.7 | 0.070 | 22.0 |
| BC | 0.004 | 13.2 | 0.796 | 1.0 |
| CC | 0.439 | −0.9 | 0.056 | −3.8 |
| R-squared statistics | ||||
| R2 | 0.996 | 0.988 | ||
| R2 (adjusted by g.l.) | 0.989 | 0.966 | ||
| Optimal SFE conditions | ||||
| Stirring (times) | 10 | 10 | ||
| Time (min) | 1 | 5 | ||
| Derivatasing reagent (OPA; µL) | 4 | 4 | ||
| Function | Quantity (Volume or Times) | Compound |
|---|---|---|
| Draw | 2 µL 1 | Derivatisation reagent |
| Needle wash | 2 times | MeOH |
| Draw | 16 µL | Sample |
| Needle wash | 2 times | MeOH |
| Draw | 2 µL 1 | Derivatisation reagent |
| Needle wash | 2 times | MeOH |
| Mix | 10 times 1 | Air |
| Incubation | 1 min 1 | |
| Inject | 20 µL | Reaction mixture |
| FB1 | FB2 | |
|---|---|---|
| Linear range (µg mL−1) | 0.05–5.0 | 0.05–5.0 |
| Regression eq. (SD) | y = 528 (4) x – 12 (1) | y = 410 (5) x + 10 (2) |
| Determination Coefficient (r2) | 0.9993 | 0.9987 |
| LOD (µg mL−1) | 0.006 | 0.012 |
| LOQ (µg mL−1) | 0.017 | 0.038 |
| Intraday repeatability (RSD %) | 0.85 | 0.83 |
| Interday repeatability (RSD %) | 2.4 | 5.9 |
| Recovery (%) | C18 Cartridge | MultiSep 211 Fumonisins | FumoniStar Immunoaffinity Columns | |||
|---|---|---|---|---|---|---|
| FB1 | FB2 | FB1 | FB2 | FB1 | FB2 | |
| Dried figs | 89.9 b* ± 7.4 | 72.2 2 ± 2.3 | 115.9 c ± 3.1 | 105.4 3 ± 2.8 | 60.0 a ± 2.2 | 65.2 1 ± 6.2 |
| Raisins | 38.9 a ± 2.3 | <20 1 | 124.5 c ± 6.2 | 77.9 2 ± 1.5 | 72.6 b ± 4.9 | 86.1 3 ± 5.5 |
| Dates | <20 a | 49.7 2 ± 5.5 | <20 a | <20 1 | 81.3 b ± 0.6 | 92.5 3 ± 6.2 |
| Corn | <20 a | 77.5 2 ± 8.5 | 125.6 c ± 4.9 | <20 1 | 97.3 b ± 3.1 | 99.6 3 ± 1.4 |
| Cornmeal | 35.2 a ± 7.6 | 83.9 2 ± 0.3 | 118.2 c ± 8.6 | <20 1 | 87.4 b ± 8.8 | 101.4 3 ± 9.8 |
| Wheat flour | 66.5 b ± 4.3 | 78.6 ± 3.8 2 | <20 a | <20 1 | 75.2 c ± 6.4 | 83.7 2 ± 4.7 |
| Rice | <20 a | <20 1 | <20 a | <20 1 | 74.4 b ± 3.8 | 85.1 b ± 8.7 |
| Block | Stirring Cycles | Incubation Time(s) | OPA Reagent (µL) |
|---|---|---|---|
| 1 | 60 | 1 | 9 |
| 2 | 10 | 3 | 14 |
| 3 | 10 | 1 | 9 |
| 4 | 35 | 1 | 4 |
| 5 | 60 | 3 | 4 |
| 6 | 10 | 5 | 9 |
| 7 | 60 | 5 | 9 |
| 8 | 35 | 5 | 14 |
| 9 | 35 | 5 | 4 |
| 10 | 10 | 3 | 4 |
| 11 | 60 | 3 | 14 |
| 12 | 35 | 1 | 14 |
| 13 | 35 | 3 | 9 |
| 14 | 35 | 3 | 9 |
| 15 | 35 | 3 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cebadero-Domínguez, Ó.; Ruiz-Moyano, S.; Martín, A.; Martín-Tornero, E. Determination of Fumonisins B1 and B2 in Food Matrices: Optimisation of a Liquid Chromatographic Method with Fluorescence Detection. Toxins 2025, 17, 391. https://doi.org/10.3390/toxins17080391
Cebadero-Domínguez Ó, Ruiz-Moyano S, Martín A, Martín-Tornero E. Determination of Fumonisins B1 and B2 in Food Matrices: Optimisation of a Liquid Chromatographic Method with Fluorescence Detection. Toxins. 2025; 17(8):391. https://doi.org/10.3390/toxins17080391
Chicago/Turabian StyleCebadero-Domínguez, Óscar, Santiago Ruiz-Moyano, Alberto Martín, and Elisabet Martín-Tornero. 2025. "Determination of Fumonisins B1 and B2 in Food Matrices: Optimisation of a Liquid Chromatographic Method with Fluorescence Detection" Toxins 17, no. 8: 391. https://doi.org/10.3390/toxins17080391
APA StyleCebadero-Domínguez, Ó., Ruiz-Moyano, S., Martín, A., & Martín-Tornero, E. (2025). Determination of Fumonisins B1 and B2 in Food Matrices: Optimisation of a Liquid Chromatographic Method with Fluorescence Detection. Toxins, 17(8), 391. https://doi.org/10.3390/toxins17080391





