Factors Influencing the Effectiveness of Botulinum Toxin Therapy in Bruxism Management
Abstract
1. Introduction
2. Results
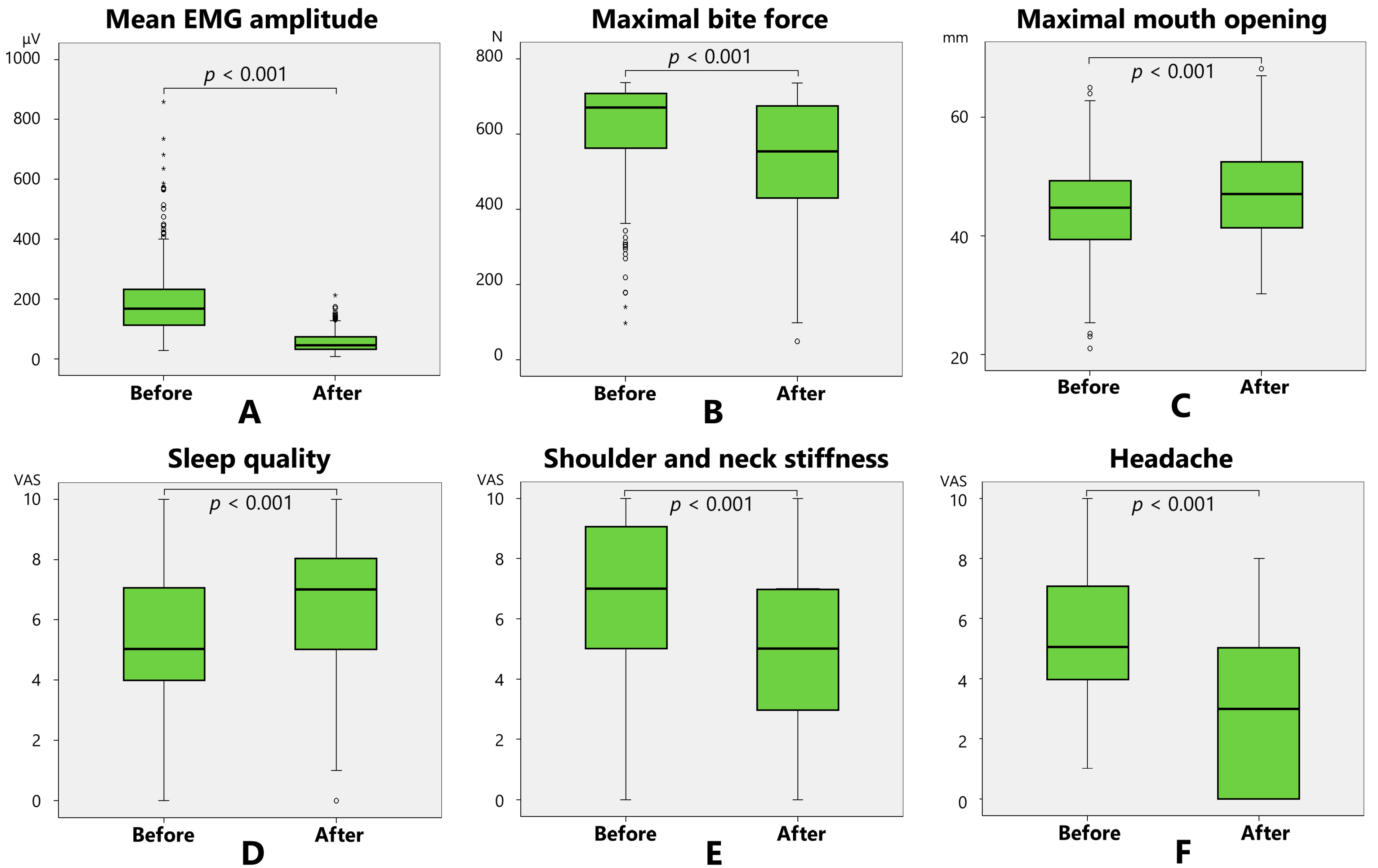
2.1. Changes in the Main Measurement Items After Treatment
2.2. Factors Affecting Symptoms and Effects
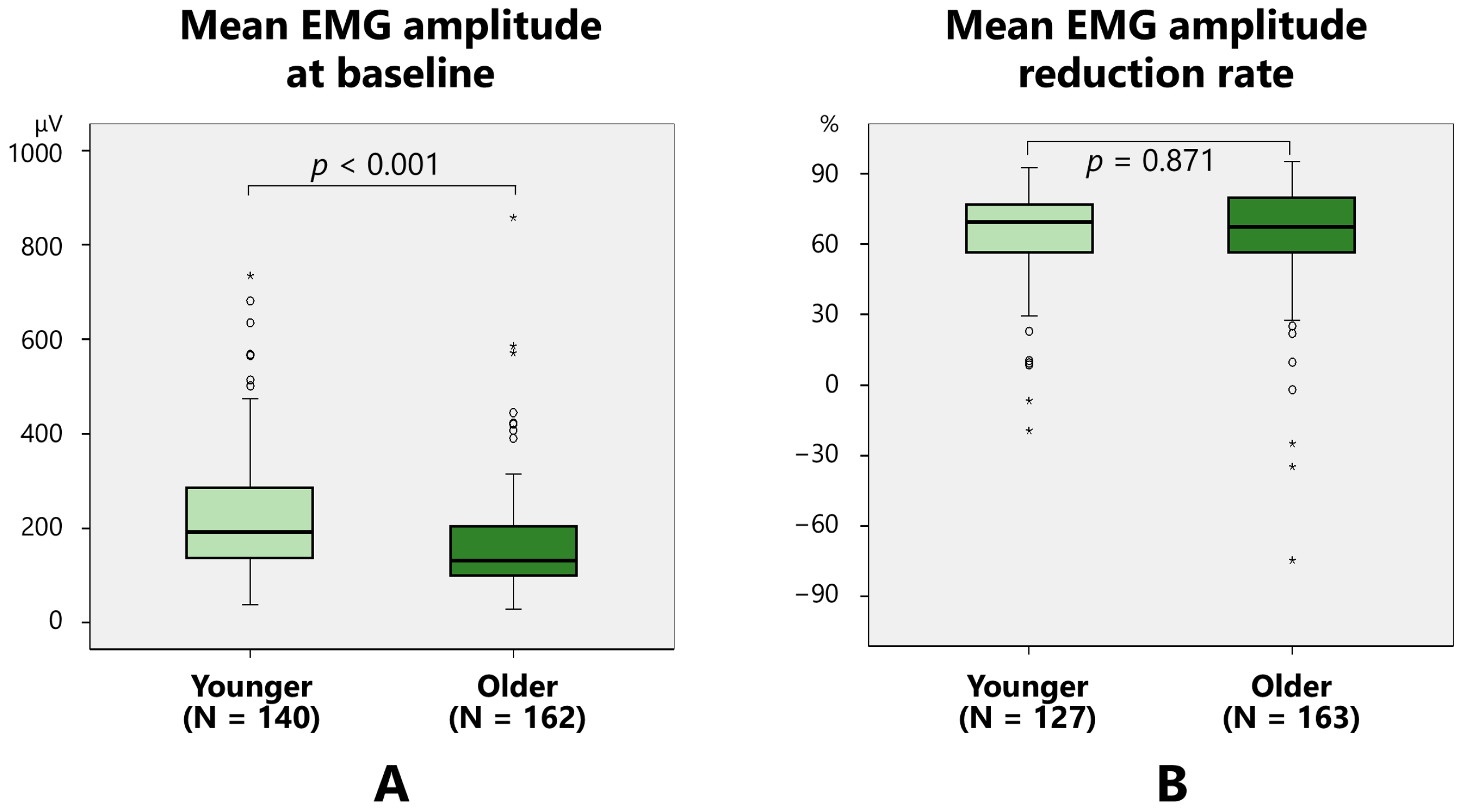
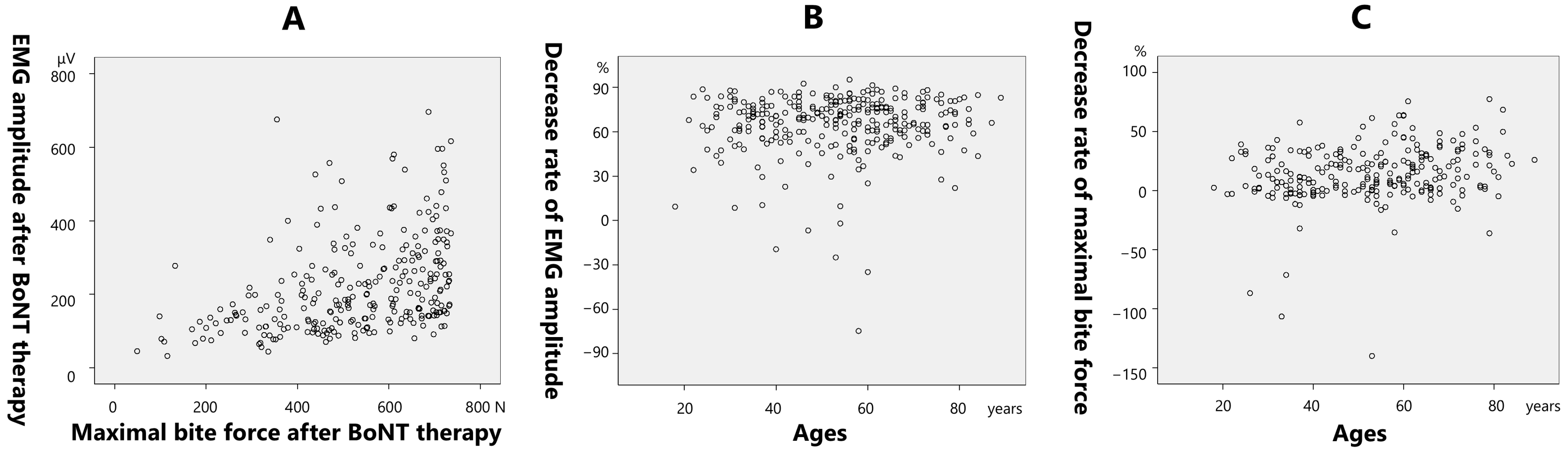
2.2.1. Mean EMG Amplitude
2.2.2. Maximal Bite Force
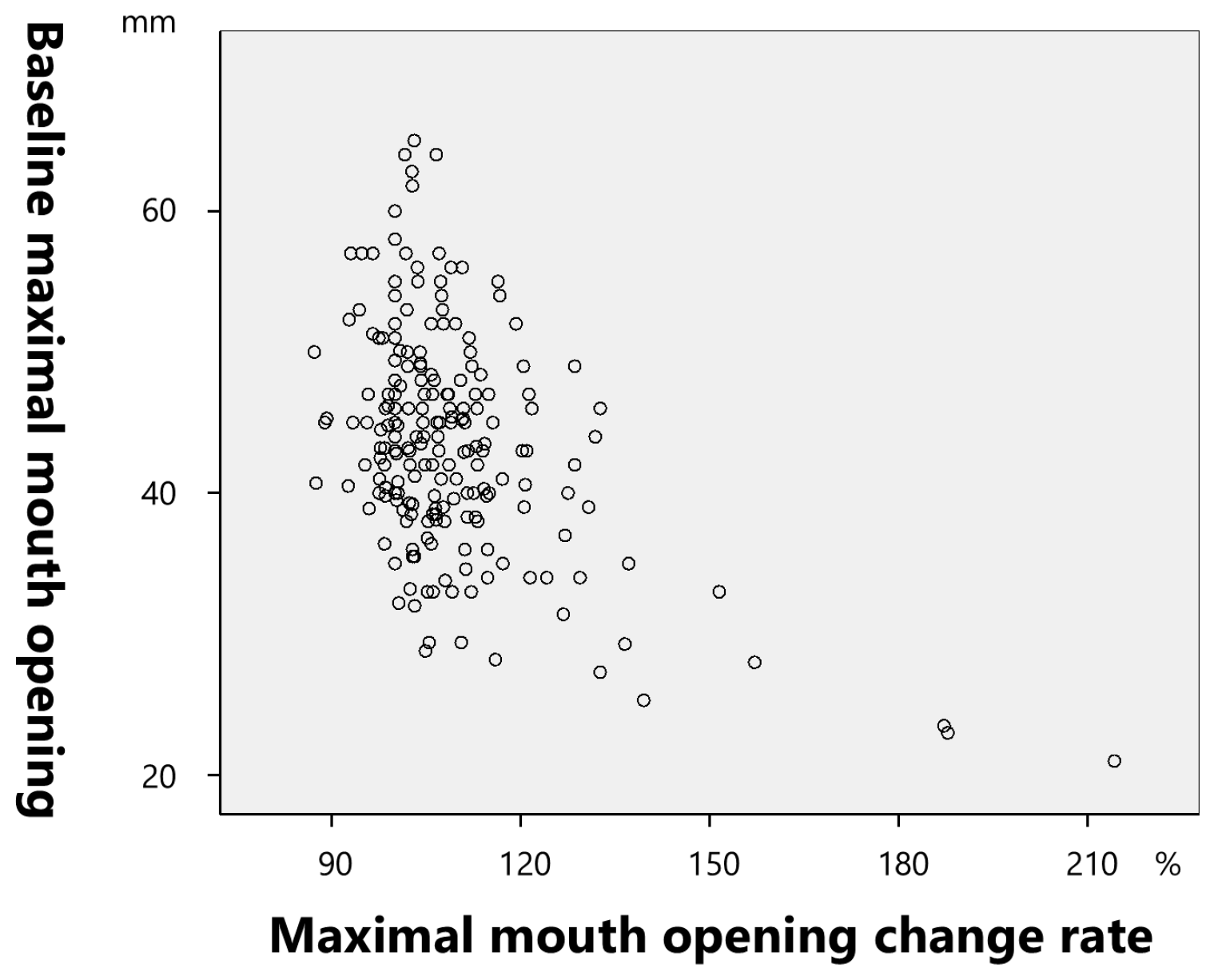
2.2.3. Maximal Mouth Opening
2.2.4. Sleep Quality
2.2.5. Shoulder and Neck Stiffness
2.2.6. Headaches
3. Discussion
3.1. Diagnosis of Bruxism
3.2. Methodology of BoNT Therapy for Bruxism
3.3. Muscle Pain
3.4. Muscle Activity and Bite Force
3.5. Sleep Quality
3.6. Other Secondary Outcomes
3.7. Adverse Effects
3.8. Future Directions
4. Conclusions
5. Materials and Methods
5.1. Participants
5.2. Injection Method
5.3. Clinical Symptoms Measurement Items
5.3.1. Maximal Action Potential of the Masseter Muscles
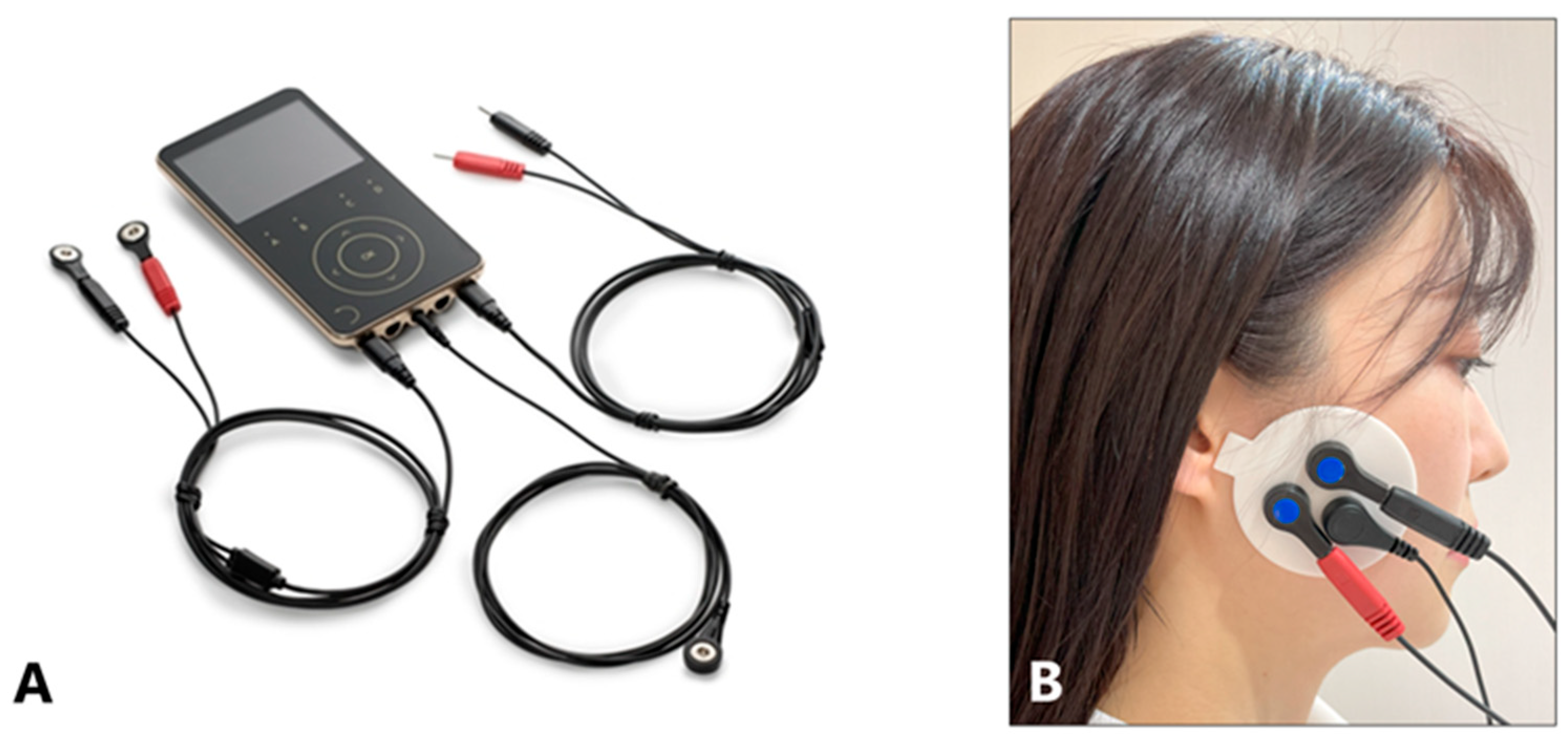
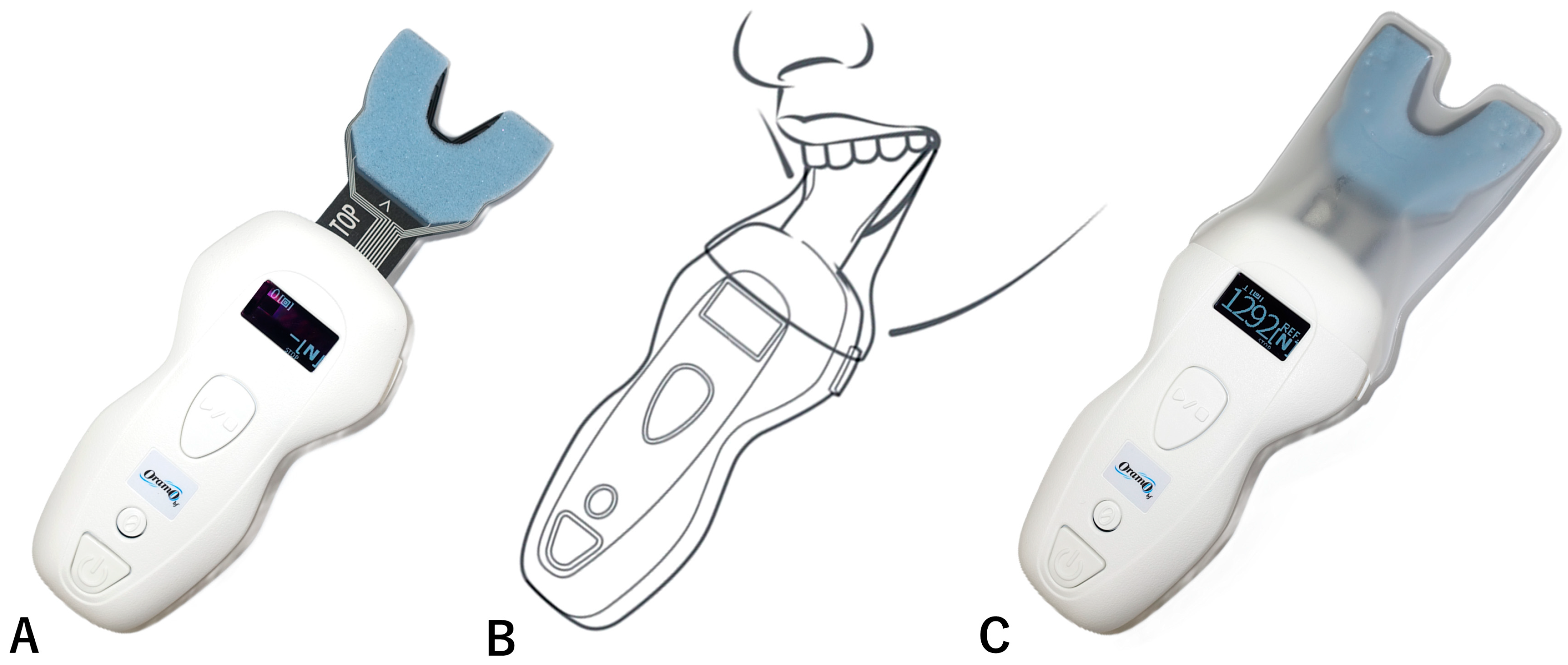
5.3.2. Maximal Bite Force (N)
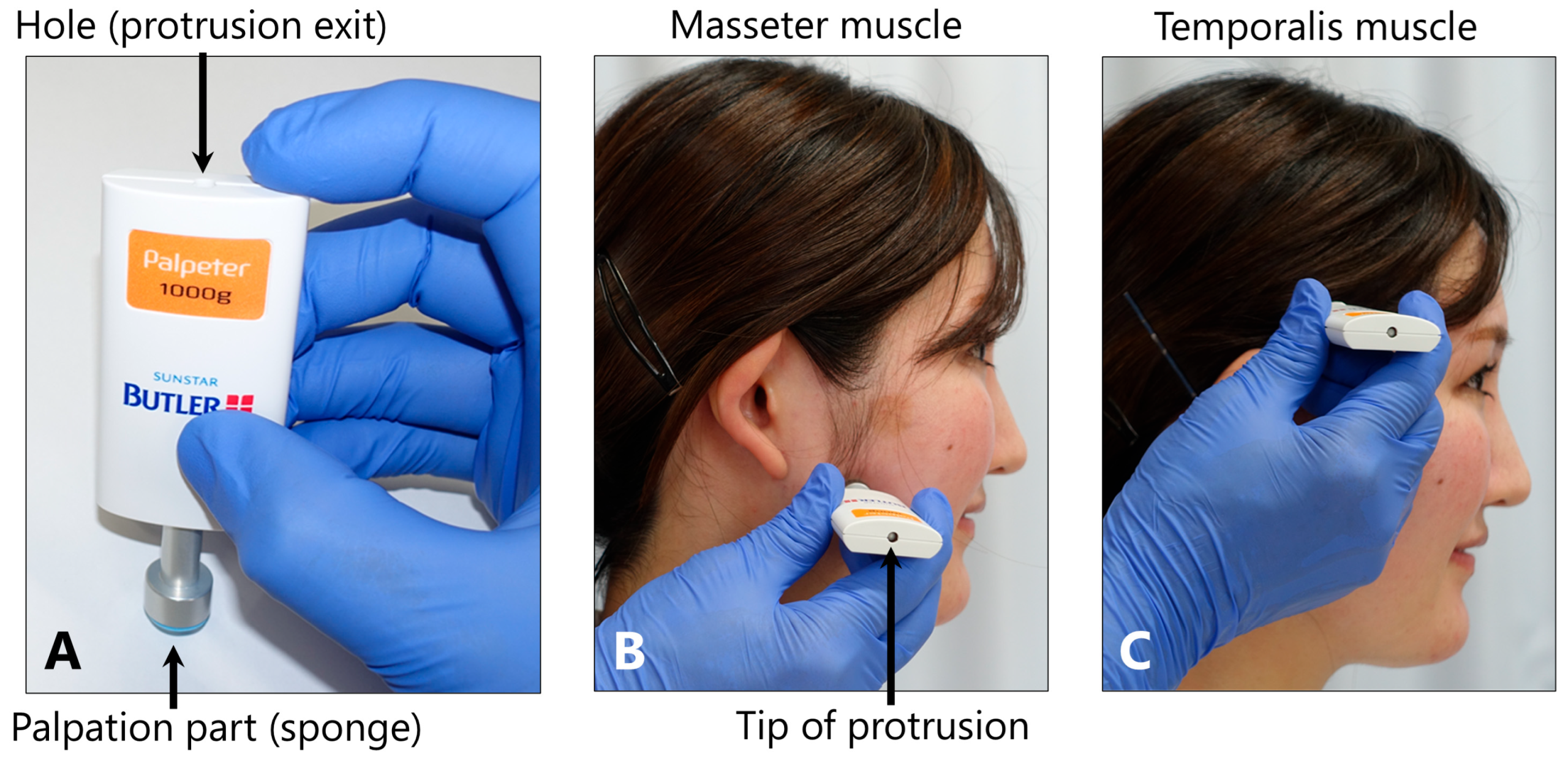
5.3.3. Tenderness of the Masticatory Muscles
5.3.4. Maximal Mouth Opening
5.3.5. Sleep Quality
5.3.6. Headache and Shoulder and Neck Stiffness
5.4. Statistical Analysis
- Changes in the Measurement Items after Treatment
- Factors Affecting the Symptoms and Effects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BoNT | Botulinum neurotoxin |
| TMJ | Temporomandibular joint |
| EMG | Electromyography |
References
- Lobbezoo, F.; Ahlberg, J.; Raphael, K.G.; Wetselaar, P.; Glaros, A.G.; Kato, T.; Santiago, V.; Winocur, E.; de Laat, A.; de Leeuw, R.; et al. International consensus on the assessment of bruxism: Report of a work in progress. J. Oral Rehabil. 2018, 45, 837–844. [Google Scholar] [CrossRef]
- Melo, G.; Duarte, J.; Pauletto, P.; Porporatti, A.L.; Stunginski-Barbosa, J.; Winocur, E.; Flores-Mir, C.; De Luca Canto, G. Bruxism: An umbrella review of systematic reviews. J. Oral Rehabil. 2019, 46, 666–690. [Google Scholar] [CrossRef]
- Polmann, H.; Réus, J.C.; Massignan, C.; Serra-Negra, J.M.; Dick, B.D.; Flores-Mir, C.; Lavigne, G.L.; De Luca Canto, G. Association between sleep bruxism and stress symptoms in adults: A systematic review and meta-analysis. J. Oral Rehabil. 2021, 48, 621–631. [Google Scholar] [CrossRef]
- Thymi, M.; Lobbezoo, F.; Aarab, G.; Ahlberg, J.; Baba, K.; Carra, M.C.; Gallo, L.M.; De Laat, A.; Manfredini, D.; Lavigne, G.; et al. Signal acquisition and analysis of ambulatory electromyographic recordings for the assessment of sleep bruxism: A scoping review. J. Oral Rehabil. 2021, 48, 846–871. [Google Scholar] [CrossRef]
- Wetselaar, P.; Vermaire, E.J.H.; Lobbezoo, F.; Schuller, A.A. The prevalence of awake bruxism and sleep bruxism in the Dutch adult population. J. Oral Rehabil. 2019, 46, 617–623. [Google Scholar] [CrossRef]
- Yacoub, S.; Ons, G.; Khemiss, M. Efficacy of botulinum toxin type A in bruxism management: A systematic review. Dent. Med. Probl. 2025, 62, 145–160. [Google Scholar] [CrossRef]
- Guarda-Nardini, L.; Manfredini, D.; Salamone, M.; Salmaso, L.; Tonello, S.; Ferronato, G. Efficacy of botulinum toxin in treating myofascial pain in bruxers: A controlled placebo pilot study. Cranio 2008, 26, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; McCall, W.D.; Kim, Y.K.; Chung, S.C.; Chung, J.W. Effect of botulinum toxin injection on nocturnal bruxism: A randomized controlled trial. Am. J. Phys. Med. Rehabil. 2010, 89, 16–23. [Google Scholar] [CrossRef]
- Shim, Y.J.; Lee, M.K.; Kato, T.; Park, H.U.; Heo, K.; Kim, S.T. Effects of botulinum toxin on jaw motor events during sleep in sleep bruxism patients: A polysomnographic evaluation. J. Clin. Sleep Med. 2014, 10, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Al-Wayli, H. Treatment of chronic pain associated with nocturnal bruxism with botulinum toxin. A prospective and randomized clinical study. J. Clin. Exp. Dent. 2017, 9, e112–e117. [Google Scholar] [CrossRef] [PubMed]
- Jadhao, V.A.; Lokhande, N.; Habbu, S.G.; Sewane, S.; Dongare, S.; Goyal, N. Efficacy of botulinum toxin in treating myofascial pain and occlusal force characteristics of masticatory muscles in bruxism. Indian J. Dent. Res. 2017, 28, 493–497. [Google Scholar] [CrossRef]
- Ondo, W.G.; Simmons, J.H.; Shahid, M.H.; Hashem, V.; Hunter, C.; Jankovic, J. Onabotulinum toxin—A injections for sleep bruxism. Neurology 2018, 90, e559–e564. [Google Scholar] [CrossRef]
- Shim, Y.J.; Lee, H.J.; Park, K.J.; Kim, H.T.; Hong, I.L.; Kim, S.T. Botulinum toxin therapy for managing sleep bruxism: A randomized and placebo-controlled trial. Toxins 2020, 12, 168. [Google Scholar] [CrossRef] [PubMed]
- Kaya, D.I.; Ataoglu, H. Botulinum toxin treatment of temporomandibular joint pain in patients with bruxism: A prospective and randomized clinical study. Niger. J. Clin. Pract. 2021, 24, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Alqutaibi, A.Y.; Aboalrejal, A.; Elawady, D.M. Botulinum toxin and occlusal splints for the management of sleep bruxism in individuals with implant overdentures: A randomized controlled trial. Saudi. Dent. J. 2021, 33, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Cruse, B.; Dharmadasa, T.; White, E.; Hollis, C.; Evans, A.; Sharmin, S.; Kalincik, T.; Kiers, L. Efficacy of botulinum toxin type a in the targeted treatment of sleep bruxism: A double-blind, randomised, placebo-controlled, cross-over study. BMJ Neurol. Open 2022, 4, e000328. [Google Scholar] [CrossRef]
- Shehri, Z.G.; Alkhouri, I.; Hajeer, M.Y.; Haddad, I.; Abu Hawa, M.H. Evaluation of the efficacy of low-dose botulinum toxin injection into the masseter muscle for the treatment of nocturnal bruxism: A randomized controlled clinical trial. Cureus 2022, 14, e32180. [Google Scholar] [CrossRef]
- Alwayli, M.; Abdulrahman, B.I.; Rastogi, S. Does botulinum toxin have any role in the management of chronic pain associated with bruxism? Cranio 2024, 42, 215–222. [Google Scholar] [CrossRef]
- Yoshida, K. Botulinum toxin therapy for oromandibular dystonia and other movement disorders in the stomatognathic system. Toxins 2022, 14, 282. [Google Scholar] [CrossRef]
- Yoshida, K. How do I inject botulinum toxin into the lateral and medial pterygoid muscles? Mov. Disord. Clin. Pract. 2016, 4, 285. [Google Scholar] [CrossRef]
- Yoshida, K. Movement disorders of the stomatognathic system: A blind spot between dentistry and medicine. Dent. Med. Probl. 2024, 61, 613–625. [Google Scholar] [CrossRef]
- Yoshida, K. Coronoidotomy as treatment for trismus due to jaw-closing oromandibular dystonia. Mov. Disord. 2006, 21, 1028–1031. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Effects of botulinum toxin type A on pain among trigeminal neuralgia, myofascial temporomandibular disorders, and oromandibular dystonia. Toxins 2021, 13, 605. [Google Scholar] [CrossRef]
- Weijs, W.A.; Hillen, B. Cross-sectional areas and estimated intrinsic strength of the human jaw muscles. Acta Morphol. Neerl. Scand. 1985, 23, 267–274. [Google Scholar] [PubMed]
- Porter, M.M.; Vandervoort, A.A.; Lexell, J. Aging of human muscle: Structure, function and adaptability. Scand. J. Med. Sci. Sports 1995, 5, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.; Visser, M.; De Meersman, R.E.; Sepúlveda, D.; Baumgartner, R.N.; Pierson, R.N.; Harris, T.; Heymsfield, S.B. Appendicular skeletal muscle mass: Effects of age, gender, and ethnicity. J. Appl. Physiol. 1997, 83, 229–239. [Google Scholar] [CrossRef]
- Newton, J.P.; Abel, E.W.; Robertson, E.M.; Yemm, R. Changes in human masseter and medial pterygoid muscles with age: A study by computed tomography. Gerodontics 1987, 3, 151–154. [Google Scholar]
- Hatch, J.P.; Shinkai, R.S.; Sakai, S.; Rugh, J.D.; Paunovich, E.D. Determinants of masticatory performance in dentate adults. Arch. Oral Biol. 2001, 46, 641–648. [Google Scholar] [CrossRef]
- Bakke, M.; Holm, B.; Jensen, B.L.; Michler, L.; Möller, E. Unilateral, isometric bite force in 8–68-year-old women and men related to occlusal factors. Scand. J. Dent. Res. 1990, 98, 149–158. [Google Scholar] [CrossRef]
- Jost, W.H. How Do I treat cervical dystonia with botulinum toxin by using ultrasound? Mov. Disord. Clin. Pract. 2017, 4, 647. [Google Scholar] [CrossRef]
- Fietzek, U.M.; Nene, D.; Schramm, A.; Appel-Cresswell, S.; Košutzká, Z.; Walter, U.; Wissel, J.; Berweck, S.; Chouinard, S.; Bäumer, T. The role of ultrasound for the personalized botulinum toxin treatment of cervical dystonia. Toxins 2021, 13, 365. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.N.; Beiu, C.; Iliescu, C.A.; Racoviță, A.; Berteanu, M.; Iliescu, M.G.; Stănescu, A.M.A.; Radaschin, D.S.; Popa, L.G. Ultrasound-guided botulinum toxin-A injections into the masseter muscle for both medical and aesthetic purposes. Toxins 2024, 16, 413. [Google Scholar] [CrossRef]
- Peng, H.P.; Peng, J.H. Complications of botulinum toxin injection for masseter hypertrophy: Incidence rate from 2036 treatments and summary of causes and preventions. J. Cosmet. Dermatol. 2018, 17, 33–38. [Google Scholar] [CrossRef]
- Raphael, K.G.; Tadinada, A.; Bradshaw, J.M.; Janal, M.N.; Sirois, D.A.; Chan, K.C.; Lurie, A.G. Osteopenic consequences of botulinum toxin injections in the masticatory muscles: A pilot study. J. Oral Rehabil. 2014, 41, 555–563. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, S.J.; Lee, K.J.; Yu, H.S.; Baik, H.S. Repeated injections of botulinum toxin into the masseter muscle induce bony changes in human adults: A longitudinal study. Korean J. Orthod. 2017, 47, 222–228. [Google Scholar] [CrossRef]
- Rosales, R.L.; Ng, A.R.; Santos, M.M.D.-D.; Fernandez, H.H. The broadening application of chemodenervation in X-linked dystonia-parkinsonism (Part II): An open-label experience with botulinum toxin-A (Dysport®) injections for oromandibular, lingual, and truncal-axial dystonias. Int. J. Neurosci. 2011, 121 (Suppl. S1), 44–56. [Google Scholar] [CrossRef] [PubMed]
- Buzatu, R.; Luca, M.M.; Castiglione, L.; Sinescu, C. Efficacy and safety of botulinum toxin in the management of temporomandibular symptoms associated with sleep bruxism: A systematic review. Dent. J. 2024, 12, 156. [Google Scholar] [CrossRef] [PubMed]






| Baseline Mean (SD) | After BoNT Therapy Mean (SD) | p-Value | |
|---|---|---|---|
| Mean EMG amplitude (μV) | 189.0 (120.5) | 55.4 (32.9) | p < 0.001 |
| Maximal bite force (N) | 618.4 (128.0) | 527.3 (163.0) | p < 0.001 |
| Maximal mouth opening (mm) | 44.0 (7.8) | 47.3 (7.6) | p < 0.001 |
| Sleep quality (VAS: 0–10) | 5.3 (2.0) | 6.6 (1.8) | p < 0.001 |
| Shoulder and neck stiffness (VAS: 0–10) | 6.7 (2.9) | 4.7 (2.9) | p < 0.001 |
| Headache (VAS: 0–10) | 5.4 (2.3) | 2.9 (2.5) | p < 0.001 |
| Estimate | Standard Error | Standardized Partial Regression Coefficients | p-Value | |
|---|---|---|---|---|
| Mean EMG amplitude reduction rate (%) | ||||
| Constant | 41.322 | 5.541 | ||
| Age (years) | 0.269 | 0.0833 | 0.215 | p = 0.001 |
| Sex | −9.999 | 2.732 | −0.23 | p < 0.001 |
| Mean EMG amplitude at baseline (µV) | 0.0718 | 0.0119 | 0.396 | p < 0.001 |
| Maximal bite force reduction rate (%) | ||||
| Constant | −28.112 | 11.406 | ||
| Age (years) | 0.438 | 0.11 | 0.28 | p < 0.001 |
| Sex | −9.845 | 3.618 | −0.181 | p = 0.007 |
| Maximal bite force at baseline (N) | 0.0356 | 0.0133 | 0.187 | p = 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furuhata, A.; Yoshida, K.; Isono, S. Factors Influencing the Effectiveness of Botulinum Toxin Therapy in Bruxism Management. Toxins 2025, 17, 384. https://doi.org/10.3390/toxins17080384
Furuhata A, Yoshida K, Isono S. Factors Influencing the Effectiveness of Botulinum Toxin Therapy in Bruxism Management. Toxins. 2025; 17(8):384. https://doi.org/10.3390/toxins17080384
Chicago/Turabian StyleFuruhata, Azusa, Kazuya Yoshida, and Shiroh Isono. 2025. "Factors Influencing the Effectiveness of Botulinum Toxin Therapy in Bruxism Management" Toxins 17, no. 8: 384. https://doi.org/10.3390/toxins17080384
APA StyleFuruhata, A., Yoshida, K., & Isono, S. (2025). Factors Influencing the Effectiveness of Botulinum Toxin Therapy in Bruxism Management. Toxins, 17(8), 384. https://doi.org/10.3390/toxins17080384







