Botulinum Toxin Effects on Biochemical Biomarkers Related to Inflammation-Associated Head and Neck Chronic Conditions: A Systematic Review of Preclinical Research
Abstract
1. Introduction
2. Results
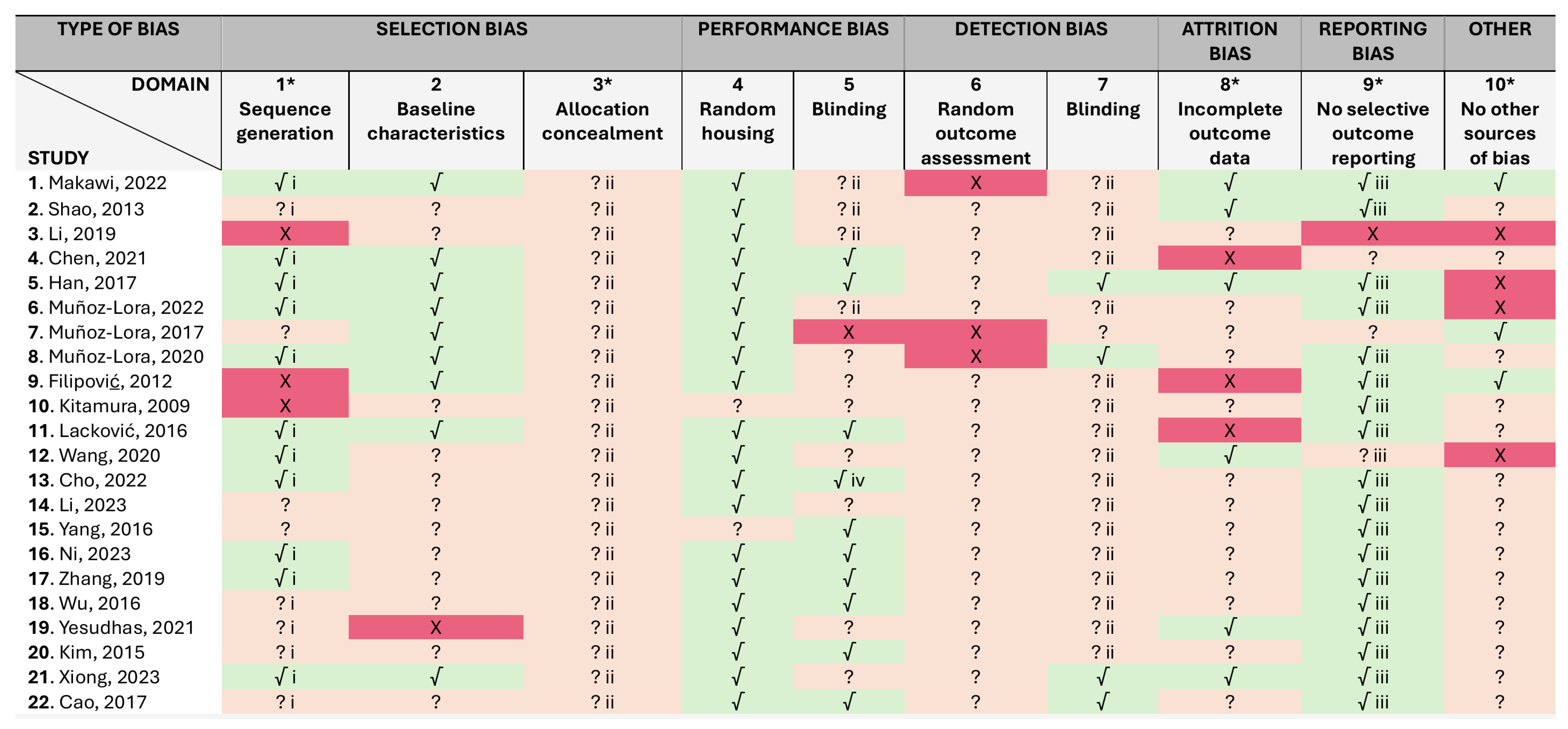
2.1. Risk of Bias
2.2. Results of Individual Studies
2.2.1. Biomarkers
2.2.2. Botulinum Toxin Key Effect
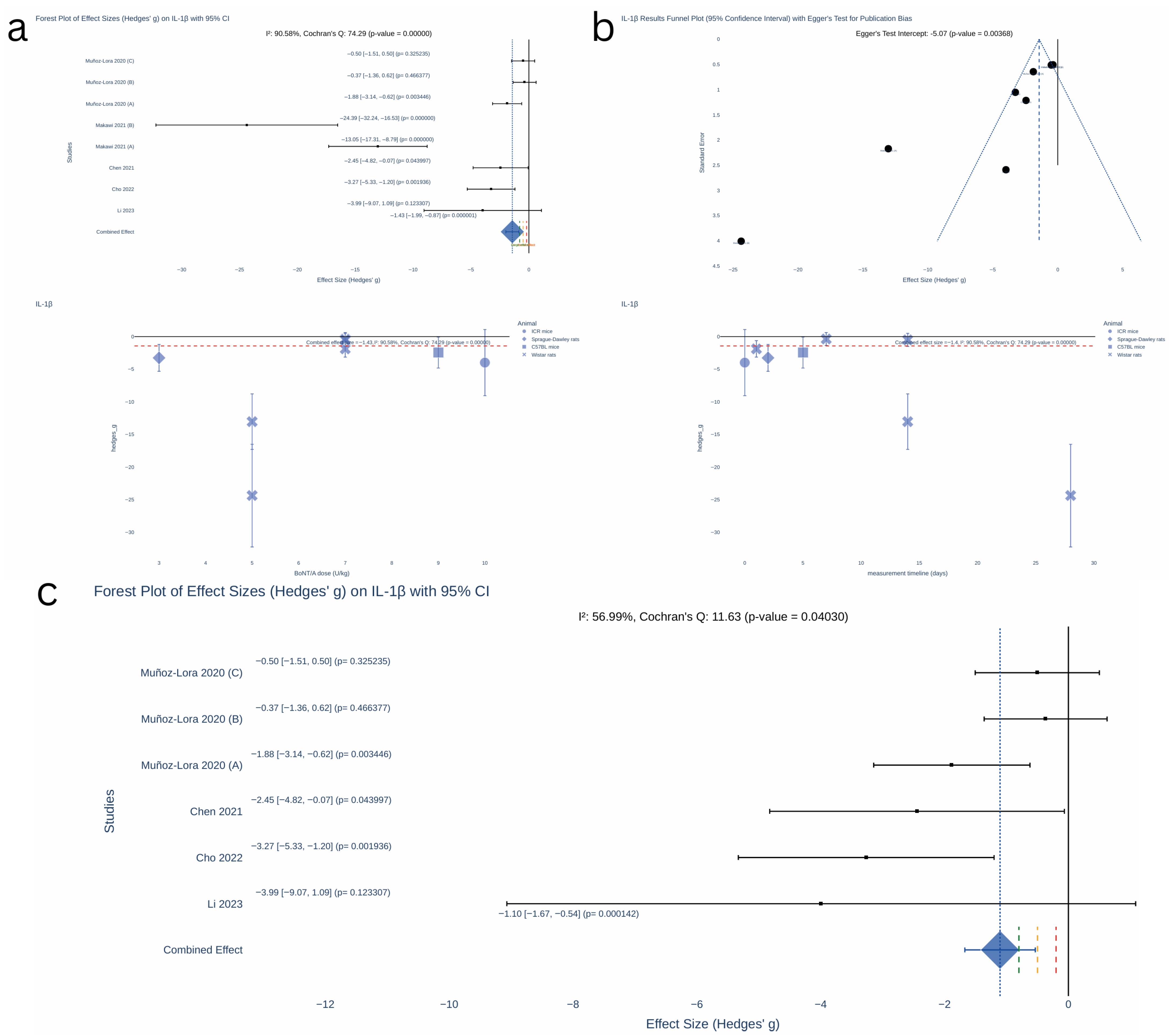
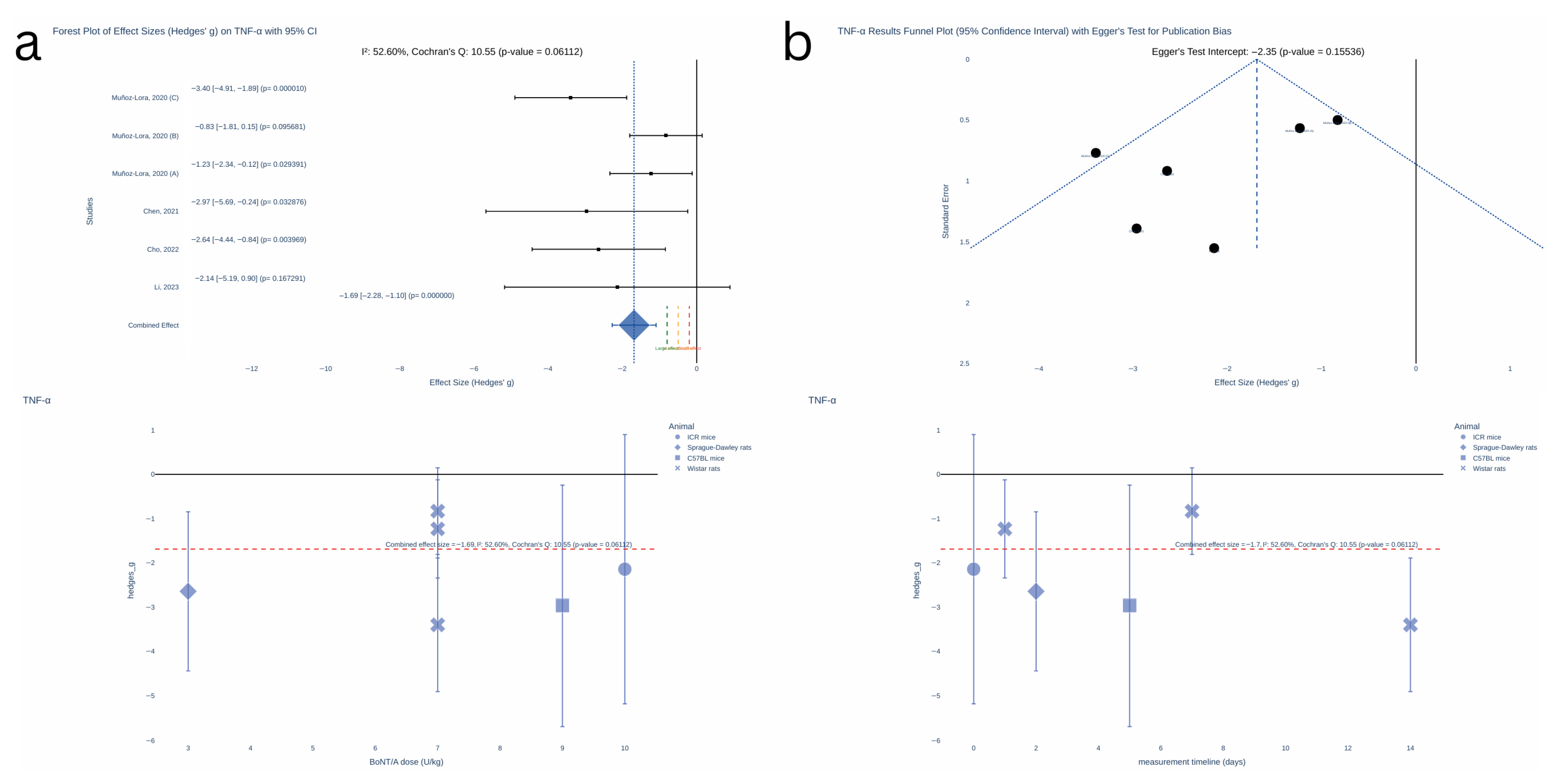
2.3. Results of Synthesis
Sample Size for Future Studies
2.4. Certainty of Evidence
3. Discussion
3.1. Limitations
3.2. Future Directions
4. Conclusions
5. Materials and Methods
5.1. Inclusion and Exclusion Criteria
5.2. Search—Sources and Strategy
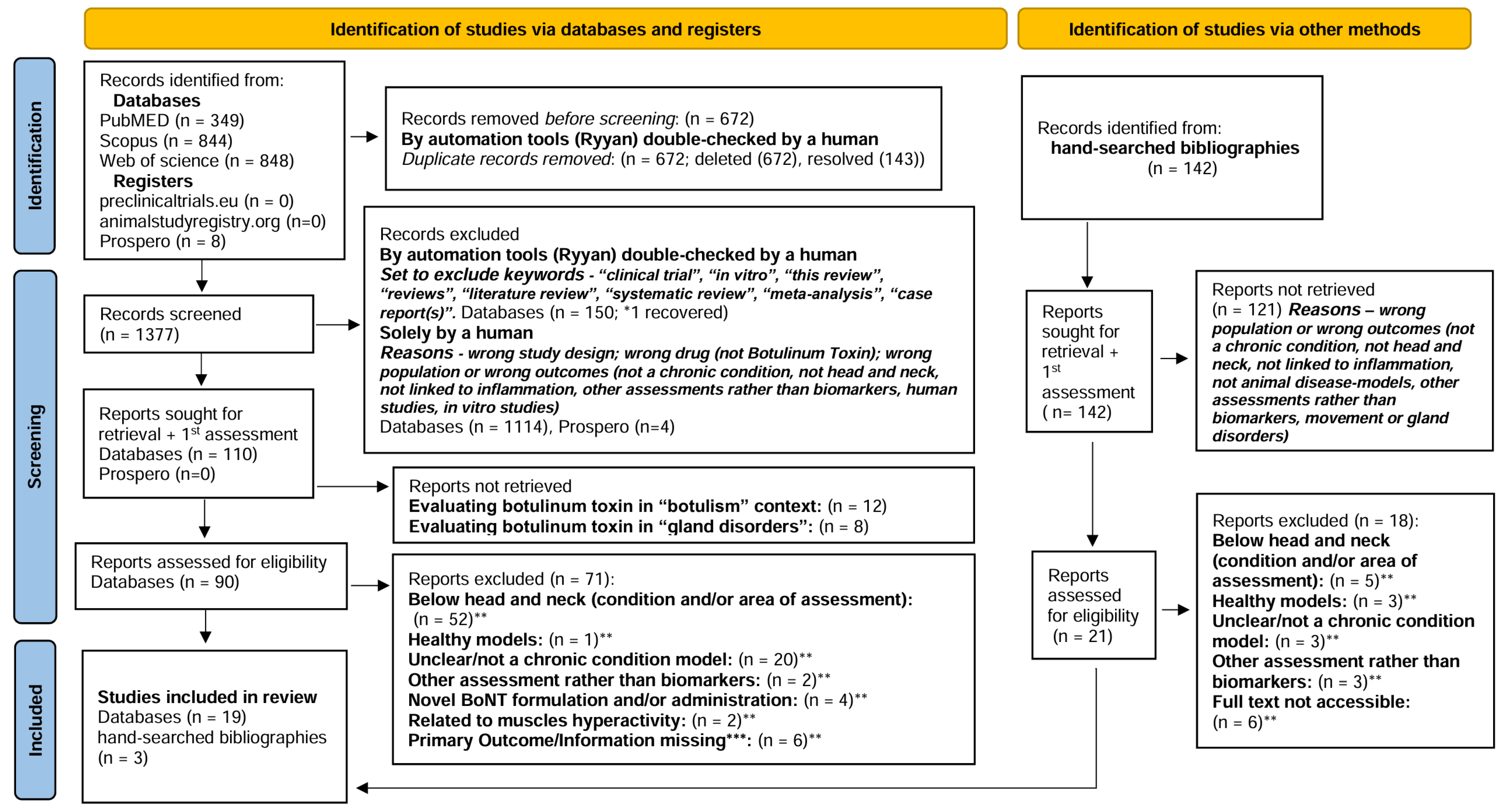
5.3. Selection Process
5.4. Data Collection
5.5. Study Characteristics, Risk of Bias, and Certainty Assessment
5.6. Effect Measures and Synthesis Methods
5.7. Registration and Protocol
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BoNT | Botulinum toxin |
| SNAP25 | Synaptosomal-associated protein-25 |
| CGRP | Calcitonin gene related peptide |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SYRCLE | Systematic Review Centre for Laboratory animal Experimentation |
| GRADE | Grading of Recommendations Assessment, Development and Evaluation |
| CI | Confidence interval |
| TMJ | Temporomandibular joint |
| MDD | Major depressive disorder |
| TG | Trigeminal ganglia |
| TNC | Trigeminal nucleus caudalis |
| SNpc | Substantia nigra pars compacta |
| IL-1β | Interleukin 1 beta |
| TNF-α | Tumor necrotic factor alfa |
| SP | Substance P |
| CX3CR1 | Microglial purinergic CX3 chemokine receptor 1 |
| TGF-β1 | Transforming growth factor beta |
| α-SMA | α- smooth muscle actin |
| TRPA | Transient receptor potential ankyrin |
| IBA-1 | Ionized calcium-binding adaptor molecule 1 |
| VGAT | Inhibitory presynaptic marker vesicular GABA transporter |
| MMP-13 | Matrix metallopeptidase 13 |
| BDNF | Brain derived neurotrophic factor |
| p-ERK | Phosphorylated extracellular signal-regulated kinase |
| p-CREB | cAMP response element binding protein |
| IgE | Immunoglobulin E |
| GFAP | Astroglia marker—glial fibrillary acidic protein |
| IB4 | Isolectin B4-binding |
| HIF-1α | Hypoxia-inducible factor |
| PSD95 | Postsynaptic density-95 |
| VGlut2 | Presynaptic marker vesicular glutamate transporter 2 |
| ATF3 | Activating transcription factor 3 |
| TRPM | Transient receptor potential melastatin |
| TRPV | Protein expression of transient receptor potential vanilloid |
| PIH | Persistent inflammatory hypernociception |
| clSNAP25 | Cleaved synaptosomal-associated protein-25 |
| SNARE | N-ethylmaleimide-sensitive-factor attachment receptor |
| IoNC | Infraorbital nerve constriction |
| CFA | Complete Freund’s Adjuvant |
| AEW | Acetone–diethylether–water |
References
- Hajj, R.; Haddad, C. The Anti-Nociceptive/Anti-Inflammatory Actions of Botulinum Toxin A for the Treatment of Chronic Pain: A Literature Review. Curr. Res. Dent. 2021, 12, 62–70. [Google Scholar] [CrossRef]
- Geoghegan, L.; Rodrigues, R.; Harrison, C.J.; Rodrigues, J.N. The Use of Botulinum Toxin in the Management of Hidradenitis Suppurativa: A Systematic Review. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4660. [Google Scholar] [CrossRef] [PubMed]
- Drugs@FDA: FDA-Approved Drugs. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/103000s5236lbl.pdf (accessed on 14 January 2025).
- Li, Y.; Liu, T.; Luo, W. Botulinum Neurotoxin Therapy for Depression: Therapeutic Mechanisms and Future Perspective. Front. Psychiatry 2021, 12, 584416. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for Neuropathic Pain in Adults: A Systematic Review and Meta-Analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Matak, I.; Bölcskei, K.; Bach-Rojecky, L.; Helyes, Z. Mechanisms of Botulinum Toxin Type A Action on Pain. Toxins 2019, 11, 459. [Google Scholar] [CrossRef]
- Novo Pereira, I.; Durão, S.; Hassan, H.; Braga, A.C.; Mariz Almeida, A.; Manso, A.C.; Faria-Almeida, R.; De la Torre Canales, G. Botulinum Toxin Effects on Biochemical Biomarkers Related to Inflammation-Associated Head and Neck Chronic Conditions: A Systematic Review of Clinical Research. J. Neural. Transm. 2025. [Google Scholar] [CrossRef]
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and Other Tools) Resource; Food and Drug Administration (US): Silver Spring, MD, USA, 2016. [Google Scholar]
- Smith, S.; Dworkin, R.; Turk, D.; Baron, R.; Polydefkis, M.; Tracey, I.; Borsook, D.; Edwards, R.; Harris, R.; Wager, T.; et al. The Potential Role of Sensory Testing, Skin Biopsy, and Functional Brain Imaging as Biomarkers in Chronic Pain Clinical Trials: IMMPACT Considerations. J. Pain 2017, 18, 757–777. [Google Scholar] [CrossRef]
- Bodaghi, A.; Fattahi, N.; Ramazani, A. Biomarkers: Promising and Valuable Tools towards Diagnosis, Prognosis and Treatment of Covid-19 and Other Diseases. Heliyon 2023, 9, e13323. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Kashfi, K.; Ghasemi, A. Importance of Systematic Reviews and Meta-Analyses of Animal Studies: Challenges for Animal-to-Human Translation. J. Am. Assoc. Lab. Anim. Sci. 2020, 59, 469–477. [Google Scholar] [CrossRef]
- Sandercock, P.; Roberts, I. Systematic Reviews of Animal Experiments. Lancet 2002, 360, 586. [Google Scholar] [CrossRef]
- Makawi, D.; Korany, N.S.; Taha, N.S.; Abbass, M.M.S. Regenerative Potential of Botox Combined and Uncombined with Platelet-Rich Plasma in Treating Induced Osteoarthritis of Temporomandibular Joint in Albino Rats. Egypt. J. Histol. 2022, 45, 145–161. [Google Scholar] [CrossRef]
- Chen, W.-J.; Niu, J.-Q.; Chen, Y.-T.; Deng, W.-J.; Xu, Y.-Y.; Liu, J.; Luo, W.-F.; Liu, T. Unilateral Facial Injection of Botulinum Neurotoxin A Attenuates Bilateral Trigeminal Neuropathic Pain and Anxiety-like Behaviors through Inhibition of TLR2-Mediated Neuroinflammation in Mice. J. Headache Pain 2021, 22, 38. [Google Scholar] [CrossRef]
- Lora, V.R.M.M.; Clemente-Napimoga, J.T.; Abdalla, H.B.; Macedo, C.G.; de la Torre Canales, G.; Barbosa, C.M.R. Botulinum Toxin Type A Reduces Inflammatory Hypernociception Induced by Arthritis in the Temporomadibular Joint of Rats. Toxicon 2017, 129, 52–57. [Google Scholar] [CrossRef]
- Manuel Muñoz-Lora, V.R.; Abdalla, H.B.; Del Bel Cury, A.A.; Clemente-Napimoga, J.T. Modulatory Effect of Botulinum Toxin Type A on the Microglial P2X7/CatS/FKN Activated-Pathway in Antigen-Induced Arthritis of the Temporomandibular Joint of Rats. Toxicon 2020, 187, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Son, J.Y.; Ju, J.S.; Kim, Y.M.; Ahn, D.K. Cellular Mechanisms Mediating the Antinociceptive Effect of Botulinum Toxin A in a Rodent Model of Trigeminal Irritation by a Foreign Body. J. Pain 2022, 23, 2070–2079. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yin, Q.; Li, Q.; Huo, A.-R.; Shen, T.-T.; Cao, J.-Q.; Liu, C.-F.; Liu, T.; Luo, W.-F.; Cong, Q.-F. Botulinum Neurotoxin A Ameliorates Depressive-like Behavior in a Reserpine-Induced Parkinson’s Disease Mouse Model via Suppressing Hippocampal Microglial Engulfment and Neuroinflammation. Acta Pharmacol. Sin. 2023, 44, 1322–1336. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.-F.; Zhang, Y.; Zhao, P.; Yan, W.-J.; Kong, X.-P.; Fan, L.-L.; Hou, Y.-P. Botulinum Toxin Type a Therapy in Migraine: Preclinical and Clinical Trials. Iran. Red Crescent Med. J. 2013, 15, e7704. [Google Scholar] [CrossRef]
- Lacković, Z.; Filipović, B.; Matak, I.; Helyes, Z. Activity of Botulinum Toxin Type A in Cranial Dura: Implications for Treatment of Migraine and Other Headaches. Br. J. Pharmacol. 2016, 173, 279–291. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Liu, X.; Su, C.-J.; Zhang, Q.-L.; Wang, Z.-H.; Cao, L.-F.; Guo, X.-Y.; Huang, Y.; Luo, W.; et al. Antidepressant-Like Action of Single Facial Injection of Botulinum Neurotoxin A Is Associated with Augmented 5-HT Levels and BDNF/ERK/CREB Pathways in Mouse Brain. Neurosci. Bull. 2019, 35, 661–672. [Google Scholar] [CrossRef]
- Muñoz-Lora, V.R.M.; Dugonjić Okroša, A.; Matak, I.; Del Bel Cury, A.A.; Kalinichev, M.; Lacković, Z. Antinociceptive Actions of Botulinum Toxin A1 on Immunogenic Hypersensitivity in Temporomandibular Joint of Rats. Toxins 2022, 14, 161. [Google Scholar] [CrossRef]
- Wu, C.; Xie, N.; Lian, Y.; Xu, H.; Chen, C.; Zheng, Y.; Chen, Y.; Zhang, H. Central Antinociceptive Activity of Peripherally Applied Botulinum Toxin Type A in Lab Rat Model of Trigeminal Neuralgia. Springerplus 2016, 5, 431. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Chen, H.; Xu, X.; Sun, D.; Cai, H.; Wang, L.; Tang, Q.; Hao, Y.; Cao, S.; Hu, X. Neurocircuitry Underlying the Antidepressant Effect of Retrograde Facial Botulinum Toxin in Mice. Cell Biosci. 2023, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, D.; Li, X.; Li, X.-J. BTXA Could Induce Fibroblast Apoptosis and Inhibit the Expression of α-SMA and Myosin II in Scar Tissue of Rabbit Ears. Biotechnol. Bioproc E 2020, 25, 699–706. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, G.-W.; Kim, M.-J.; Yang, K.-Y.; Kim, S.-T.; Bae, Y.-C.; Ahn, D.-K. Antinociceptive Effects of Transcytosed Botulinum Neurotoxin Type A on Trigeminal Nociception in Rats. Korean J. Physiol. Pharmacol. 2015, 19, 349–355. [Google Scholar] [CrossRef]
- Han, S.B.; Kim, H.; Cho, S.H.; Chung, J.H.; Kim, H.S. Protective Effect of Botulinum Toxin Type A Against Atopic Dermatitis-Like Skin Lesions in NC/Nga Mice. Dermatol. Surg. 2017, 43 (Suppl. 3), S312–S321. [Google Scholar] [CrossRef]
- Filipović, B.; Matak, I.; Bach-Rojecky, L.; Lacković, Z. Central Action of Peripherally Applied Botulinum Toxin Type A on Pain and Dural Protein Extravasation in Rat Model of Trigeminal Neuropathy. PLoS ONE 2012, 7, e29803. [Google Scholar] [CrossRef]
- Kitamura, Y.; Matsuka, Y.; Spigelman, I.; Ishihara, Y.; Yamamoto, Y.; Sonoyama, W.; Kamioka, H.; Yamashiro, T.; Kuboki, T.; Oguma, K. Botulinum Toxin Type a (150 kDa) Decreases Exaggerated Neurotransmitter Release from Trigeminal Ganglion Neurons and Relieves Neuropathy Behaviors Induced by Infraorbital Nerve Constriction. Neuroscience 2009, 159, 1422–1429. [Google Scholar] [CrossRef]
- Xiong, J.; Li, X.; Xu, G.; Wang, Y.; Wen, H. Effectiveness of Fractional Carbon Dioxide Laser Combined with Botulinum Toxin Type A in a Rabbit Ear Model with the Underlying Mechanism. J. Cosmet. Dermatol. 2023, 22, 2225–2232. [Google Scholar] [CrossRef]
- Yang, K.Y.; Kim, M.J.; Ju, J.S.; Park, S.K.; Lee, C.G.; Kim, S.T.; Bae, Y.C.; Ahn, D.K. Antinociceptive Effects of Botulinum Toxin Type A on Trigeminal Neuropathic Pain. J. Dent. Res. 2016, 95, 1183–1190. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, Q.; Lian, Y.; Chen, Y. Botulinum Toxin Type A Reduces the Expression of Transient Receptor Potential Melastatin 3 and Transient Receptor Potential Vanilloid Type 4 in the Trigeminal Subnucleus Caudalis of a Rat Model of Trigeminal Neuralgia. Neuroreport 2019, 30, 735–740. [Google Scholar] [CrossRef]
- Cao, L.-F.; Si, M.; Huang, Y.; Chen, L.-H.; Peng, X.-Y.; Qin, Y.-Q.; Liu, T.-T.; Zhou, Y.; Liu, T.; Luo, W.-F. Long-Term Anti-Itch Effect of Botulinum Neurotoxin A Is Associated with Downregulation of TRPV1 and TRPA1 in the Dorsal Root Ganglia in Mice. Neuroreport 2017, 28, 518–526. [Google Scholar] [CrossRef]
- Yesudhas, A.; Radhakrishnan, R.K.; Sukesh, A.; Ravichandran, S.; Manickam, N.; Kandasamy, M. BOTOX® Counteracts the Innate Anxiety-Related Behaviours in Correlation with Increased Activities of Key Antioxidant Enzymes in the Hippocampus of Ageing Experimental Mice. Biochem. Biophys. Res. Commun. 2021, 569, 54–60. [Google Scholar] [CrossRef]
- Baral, H.; Sekiguchi, A.; Uchiyama, A.; Nisaa Amalia, S.; Yamazaki, S.; Inoue, Y.; Yokoyama, Y.; Ogino, S.; Torii, R.; Hosoi, M.; et al. Inhibition of Skin Fibrosis in Systemic Sclerosis by Botulinum Toxin B via the Suppression of Oxidative Stress. J. Dermatol. 2021, 48, 1052–1061. [Google Scholar] [CrossRef]
- Choi, J.E.; Werbel, T.; Wang, Z.; Wu, C.C.; Yaksh, T.L.; Di Nardo, A. Botulinum Toxin Blocks Mast Cells and Prevents Rosacea like Inflammation. J. Dermatol. Sci. 2019, 93, 58–64. [Google Scholar] [CrossRef]
- Ward, N.L.; Kavlick, K.D.; Diaconu, D.; Dawes, S.M.; Michaels, K.A.; Gilbert, E. Botulinum Neurotoxin A Decreases Infiltrating Cutaneous Lymphocytes and Improves Acanthosis in the KC-Tie2 Mouse Model. J. Investig. Dermatol. 2012, 132, 1927–1930. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ye, Y.; Wang, L.; Zhou, W.; Chu, X.; Li, T. Botulinum Toxin Type a Combined with Transcranial Direct Current Stimulation Reverses the Chronic Pain Induced by Osteoarthritis in Rats. Toxicon 2022, 212, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Amalia, S.N.; Uchiyama, A.; Baral, H.; Inoue, Y.; Yamazaki, S.; Fujiwara, C.; Sekiguchi, A.; Yokoyama, Y.; Ogino, S.; Torii, R.; et al. Suppression of Neuropeptide by Botulinum Toxin Improves Imiquimod-Induced Psoriasis-like Dermatitis via the Regulation of Neuroimmune System. J. Dermatol. Sci. 2021, 101, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-Q.; Li, X.-J.; Weng, X.-J. Effect of BTXA on Inhibiting Hypertrophic Scar Formation in a Rabbit Ear Model. Aesthetic Plast. Surg. 2017, 41, 721–728. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Cutrona, C.; Marchet, F.; Costanzo, M.; De Bartolo, M.I.; Leodori, G.; Ferrazzano, G.; Conte, A.; Fabbrini, G.; Berardelli, A.; Belvisi, D. Exploring the Central Mechanisms of Botulinum Toxin in Parkinson’s Disease: A Systematic Review from Animal Models to Human Evidence. Toxins 2023, 16, 9. [Google Scholar] [CrossRef]
- Ham, H.J.; Yeo, I.J.; Jeon, S.H.; Lim, J.H.; Yoo, S.S.; Son, D.J.; Jang, S.-S.; Lee, H.; Shin, S.-J.; Han, S.B.; et al. Botulinum Toxin A Ameliorates Neuroinflammation in the MPTP and 6-OHDA-Induced Parkinson’s Disease Models. Biomol. Ther. 2022, 30, 90–97. [Google Scholar] [CrossRef]
- Gfrerer, L.; Xu, W.; Austen, W.; Ashina, S.; Melo-Carrillo, A.; Longhi, M.S.; Adams, A.M.; Houle, T.; Brin, M.F.; Burstein, R. OnabotulinumtoxinA Alters Inflammatory Gene Expression and Immune Cells in Chronic Headache Patients. Brain 2022, 145, 2436–2449. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- GRADE Book. Available online: https://book.gradepro.org/ (accessed on 17 January 2025).
- Wei, D.; Tang, K.; Wang, Q.; Estill, J.; Yao, L.; Wang, X.; Chen, Y.; Yang, K. The Use of GRADE Approach in Systematic Reviews of Animal Studies. J. Evid. Based Med. 2016, 9, 98–104. [Google Scholar] [CrossRef]
- Pereira, I.N.; Durão, S.; De la Torre Canales, G.; Braga, A.C.; Almeida, A.M.; Hassan, H.; Manso, A.C.; Almeida, R.F. Biomarkers in Preclinical Research on Botulinum Toxin Effects on Inflammation-Associated Chronic Conditions. PROSPERO 2023 CRD42023432411. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023432411 (accessed on 1 January 2024).
| Author | Sample | Group/Sample | Biological Sampling | Chronic Condition | Species | Age | Sex | Weight | Follow-Up |
|---|---|---|---|---|---|---|---|---|---|
| Makawi, 2022 [13] | n = 42 | 4/(n = 14) × 3 + (n = 3) | TMJ tissues | TMJ osteoarthritis monosodium iodoacetate | Wister albino rats | 3–4 months | Male | (180–200 g) | 2, 4 weeks |
| Chen, 2021 [14] | n = 48 | 4/(n = 12) × 2 + (n = 18) + (n = 6) | TNC | TN IoNC and anxiety-like behaviors | C57BL/6 mice | 6–8 weeks | Male | (20 g) | 5 days |
| Muñoz-Lora, 2017 [15] | ? | 5/? | Peri-articular tissues from TMJ and TG | PIH systemic immunization—mBSA/PBS+CFA and TMJ rheumatoid arthritis mBSA + formalin (0.5%) | Wistar rats | ? | Male | (250–500 g) | 24 h or 14 days |
| Muñoz-Lora, 2020 [16] | n = 40 | 5/(n = 8) × 5 | Trigeminal subnucleus caudalis | PIH systemic immunization—mBSA/PBS+CFA and TMJ rheumatoid arthritis mBSA + formalin (0.5%) | Wistar rats | ? | Male | (300–400 g) | 24 h, 7 or 14 days |
| Cho, 2022 [17] | n = 236 | 5/(n = 6) × 5 | TG | TN compression of the trigeminal nerve root | Sprague Dawley rats | ? | Male | (250–280 g) | 2 days |
| Li, 2023 [18] | ? | 4/(n = 3, 5–6) (n = 3) protein, %, fluorescence intensity, volume (n = 5–6) mRNA | Brain—Substantia nigra pars compacta and hippocampus | Depression reserpine chronic administration in Parkinson’s disease | Parkinson disease model-ICR mice | 6–8 weeks | Male | (30 g) | ? |
| Shao, 2013 [19] | n = 32 | 4/(n = 8) × 4 | Jugular plasma and medulla oblongata—containing caudal trigeminal nucleus | Migraine NTG | Sprague Dawley rats | ? | Female | (250–300 g) | 24 h |
| Lacković, 2016 [20] | n = 105 | 3/(n = 6) × 3—CGRP 5/(n = 5–9) × 5—dura 3/(n = 5–8) × 3—dura 3/(n = 5—cell profiles (n = 4)—SNAP-25 | Dura mater, TNC, TG, Cerebrospinal fluid sampling | Trigeminal pain—temporomandibular disorders (inflammatory pain) CFA | Wistar rats | 3–3.5 months | Male | (300–350 g) | 4 days |
| Li, 2019 [21] | ? | 5/(n = 6)—(naive) protein 4/(n = 5–6)—(naive) mRNA 7/(n = 6) —Tx protein 6/(n = 6) —Tx mRNA 3/(n = 6–7)—naive 6/(n = 6–7)—Tx | Hippocampus, hypothalamus, prefrontal cortex, amygdala (Brain) | Depression SRS | ICR mice | 6–8 weeks | Male | (20–25 g) | 1, 7, 14 days |
| Muñoz-Lora, 2022 [22] | n = 40 | 6/(n = 5)—c-fos 8/(n = 5)—CGRP, GFAP ?/(n = 3)—SNAP25 | TNC | PIH systemic immunization—mBSA/PBS+CFA and TMJ rheumatoid arthritis mBSA + formalin (0.5%) | Sprague Dawley rats | 6–8 weeks | Male | (300–400 g) | day 14 |
| Wu, 2016 [23] | ? | 4/(n = 6)—SNAP-25 7/(n = 6)—TRPs | Brainstem Vc region (caudal subnucleus of the spinal trigeminal nucleus) | TN IoNC | Sprague Dawley rats | ? | Male | (220–300 g) | 7 days |
| Ni, 2023 [24] | ? | 6/(n = 4 cells from 3 mice) × 3; (n = 4 or 5 or 6 brain sections from 3 mice) × 3—SNAP25 3/(n = 3 mice)—c-fos | Brain | Major depressive disorder chronic restraint stress | pathogen-free C57BL/6J mice | 8 weeks | Male | (25 g) | 24 h after day 23 to 27 |
| Wang, 2020 [25] | n = 18 | 4/(n = 36) ears 6 wound/per 36 ears (n = 216) wounds | Scar tissue (ear) | Hypertrophic scar lesion/wound | New Zealand big-ear albino rabbits | ? (mature) | ? | (2.5~3.5 kg) | day 28 and day 60 |
| Kim, 2015 [26] | ? | 4/(n = 5) × 4 | medullary dorsal horn | Trigeminal nociception (orofacial inflammatory pain) formalin; (chronic pain/inflammation); CFA; NMDA | Sprague Dawley rats | ? | Male | (230–280 g) | ? |
| Han, 2017 [27] | n = 42 | 6/(n = 6) × 4, (n = 9) × 2 | Rostral dorsal Skin and Serum—retro orbital plexus | Atopic Dermatitis NC/Nga + contact sensitizer (TNCB) | NC/Nga mice | 6 weeks | Female | ? | day 14 after 1st challenge |
| Filipović, 2012 [28] | n = 200 | 4/(n = 20) | Cranial dura Plasma protein complexes | TN IoNC (with/without formalin) | Wistar rats | ? | Male | (300–350 g) | 3 days |
| Kitamura 2009 [29] | ? | 4/(n = 11), (n = 9), (n = 8) × 2—IB4+ 4/(n = 10), (n = 9), (n = 8) × 2—IB4− | TG sensory neurons | TN IoNC | Sprague Dawley rats | ? (adult) | Male | (200–250 g) | 11 days |
| Xiong, 2023 [30] | n = 24 | 4/(n = 6) × 4 | Ear tissue—scar-related | Hypertrophic scar lesion/wound | New Zealand white rabbits | 6 months | Female | (3.0~3.3 kg) | 5 weeks |
| Yang, 2016 [31] | ? | 3/(n = 5) × 3 | nerve-injured TG (mandibular (V3) division & boundary area) | Trigeminal neuropathic pain malpositioned dental implants | Sprague Dawley rats | ? | Male | (220 and 240 g) | 6 days |
| Zhang, 2019 [32] | ? | 4/(n = ?) | trigeminal spinal subnucleus caudalis | TN IoNC | Sprague Dawley rats | ? (adult) | Male | (200–250 g) | 7 days |
| Cao, 2017 [33] | n = 525 | 6?/(n = 6–7) per group? | Dorsal root ganglia | Chronic dry skin itch models AEW | CD1 (ICR) mice | 6–8 weeks | Male | ? | 30 min, 1, 3, 7, 14 days |
| Yesudhas 2021 [34] | n = 12 | 2/(n = 6) × 2 | Hippocampus (brain) tissues—total protein isolates | Anxiety and ageing | ageing model-BALB/c mice | 7–8 months | Male | ? | 30 days |
| Biomarkers | CIS | Author Year | Biological Sampling | Biomarker vs. BoNT | Biomarker vs. CIS | H&N Clinical Trials? |
|---|---|---|---|---|---|---|
| 6 STUDIES | ||||||
| IL-1β PRO-INFLAMMATORY CYTOKINE (ASSOCIATED TO PERIPHERAL INFLAMMATION AND NEUROINFLAMMATION) | TN | Chen, 2021 [14] | TNC | Peripherally administered BoNT induces modulation of neuronal and non-neuronal components in the nociceptive pathways. It may inhibit the overexpression of microglia-derived pro-inflammatory factors (e.g., IL-1β) and neuroinflammation. | Interplay of immune–neuronal–non-neuronal cells is crucial for the development and maintenance of chronic pain states, characterized by sensitization of the CNS. Microglia cells are essential in central sensitization and plasticity, stimulating the neuronal release of inflammatory mediators in response to injury. Neuroinflammation may be involved in the pathophysiology of TN and depression in PD. IL-1β is a mediator of inflammation with a key role in neuroinflammation and inflammatory diseases (e.g, important marker to monitor the progression of OA and TMJ arthritis, once IL-1β increased production leads to cartilage and connective tissue degradation). | √ |
| PHI and TMJ arthritis | Muñoz-Lora, 2020 [16] | |||||
| Depression—PD | Li, 2023 [18] | Hippocampus | ||||
| TMJ OA | Makawi, 2022 [13] | TMJ tissues | Peripherally administered BoNT may decrease peripheral pro-inflammatory cell infiltrate (at the injection site and sensory ganglia), potentially: 1. BoNT is taken up by sensory nerve terminals inhibiting peripheral release of neuropeptides, neurotransmitters, and transcription factors that reduce the inflammatory chemotaxis—neurogenic inflammation; 2. altered activation of glia in sensory ganglia via direct regulation of glia or mediated by regulation of sensory neurons through the prevention of neuropeptides and neurotransmitters release, 3. direct effect on immune cells at the injection site—anti-inflammatory effects. | |||
| PIH and TMJ arthritis | Muñoz-Lora, 2017 [15] | Peri-articular tissues from TMJ and TG | ||||
| TN | Cho, 2022 [17] | TG | ||||
| 5 STUDIES | ||||||
| TNF-α PRO-INFLAMMATORY CYTOKINE (ASSOCIATED TO PERIPHERAL INFLAMMATION AND NEUROINFLAMMATION) | TN | Chen, 2021 [14] | TNC | Peripherally delivered BoNT is transsynaptically or transcytotically transported from primary afferents to glia within the CNS or postsynaptic neurons and induce central changes like reduced activation of microglia and microglia-derived pro-inflammatory factors (e.g., TNF-α) and neuroinflammation. | TNF-α mediates inflammatory diseases and plays a key role in neuroinflammation and neuropathic pain processes. It can activate other cytokines (e.g., IL-1). Neurotrophic factors and neuroinflammation are involved in the pathophysiology of depression in PD. TNF-α has been associated with cognition, depression, and disability. TNF-α high levels in the synovial fluid of TMJs has been related to TMJ disorders, joint inflammation, pain, and connective tissue destruction. | √ |
| PHI and TMJ arthritis | Muñoz-Lora, 2020 [16] | |||||
| Depression—PD | Li, 2023 [18] | Hippocampus | ||||
| PIH and TMJ arthritis | Muñoz-Lora, 2017 [15] | Peri-articular tissues from TMJ and TG | BoNT may decrease peripheral pro-inflammatory cell infiltrate in the periphery, which may reduce local neurogenic inflammation. | |||
| TN | Cho, 2022 [17] | TG | ||||
| SNAP25 PROTEIN/COMPONENT OF THE SNARE COMPLEX Cleaved SNAP-25—MARKER OF BoNT PROTEOLYTIC ACTIVITY AND PRESENCE OF ACTIVE BoNT (ASSOCIATED TO NEUROPLASTICITY—synaptic plasticity and neurotransmission) | Depression | Li, 2019 [21] | Hippocampus | BoNT cleaves SNAP25, blocking the release of neurotransmitters and neuropeptides with effects in the PNS and CNS. Central effects of BoNT may be induced by sensory input from the PNS, retrograde axonal transport to upper sensory regions (sensory ganglia and central terminals) or direct injection into the CNS (may have receptors and targets for BoNT). Changes in the CNS (e.g., reduced phosphorylation of glutamate receptors in second-order neurons, decreased microglia-activation, contralateral localization, and cortical reorganization), may be related to alterations in primary afferents or directly mediated by transsynaptic, transcytosis, or bloodstream transport. BoNT antinociceptive activity on the PIH may be related to central effects. In depression, one of the prevailing theories include modulation of neuroinflammation. | SNAP25—essential in cellular processes: exocytosis and neurotransmitter release, secretory vesicle extravasation, synaptic messaging, ion channel opening (mostly calcium channels), intercellular signalling, and has also been involved in promoting normal vesicle fusion and coordinating lysosomal trafficking. SNAP25 may be involved in regulating short-term plasticity at synapses. Low SNAP25 levels have been linked with depression and may be implicated in the pathophysiology of MDD. TMJ rheumatoid arthritis is a chronic inflammatory condition associated with a variety of systemic and local proinflammatory mediators (e.g., neurotransmitters). The related inflammation can cause pain, swelling, and limited movement in the TMJ. | √ |
| PIH and TMJ arthritis | Muñoz-Lora, 2022 [22] | TNC | ||||
| TMDs trigeminal inflammatory pain | Lacković, 2016 [20] | Cranial dura | ||||
| MDD | Ni, 2023 [24] | Brain—hindbrain sections | ||||
| TN | Wu, 2016 [23] | Brainstem Vc region (caudal subnucleus of the spinal trigeminal nucleus) | ||||
| 4 STUDIES | ||||||
| c-Fos CELL ACTIVATION MARKER (mostly used for NEURONAL ACTIVITY, but can also be expressed in GLIA CELLS) | TN | Chen, 2021 [14] | TNC | Following BoNT administration, c-Fos expression may represent the activation of central pathways. BoNT is retrogradely transported to upper regions and reduces the expression of ion channels or cytokines from sensory ganglia, neuropeptides and neurotransmitter release from primary afferent central terminals, which may result in reduced central sensitization in the dorsal horn of the spinal cord or TNuc. | In the context of TMJ rheumatoid arthritis, TN, MDD, c-Fos expression has been used as a correlate of neuronal activity to map central pathways involved in sensory processing, including nociception, or to confirm whether trigeminal neurons participate in antinociception. | Used in animals (mostly postmortem) and cultured human cells |
| PHI and TMJ arthritis | Muñoz-Lora, 2022 [22] | |||||
| MDD | Ni, 2023 [24] | Brain—hindbrain sections (vlPAG) | ||||
| TN | Kim, 2015 [26] | Medullary dorsal horn | ||||
| CGRP NEUROPEPTIDE (CAN ACT AS EXCITATORY NEUROTRANSMITTER) | Migraine | Shao, 2013 [19] | Jugular plasma and medulla oblongata—containing caudal trigeminal nucleus | BoNT may block secretion of CGRP and other neuropeptides from nerve terminals: 1. peripheral terminals of primary afferents at the injection site, 2. central terminals of primary afferents, likely owning to their release-dependence on the SNARE complex. The effect of BoNT may involve the suppression of neuropeptide release, which has a central role in neurogenic inflammation and neuroinflammation-associated conditions. | CGRP—regulatory neuropeptide is strongly involved in the pathology of migraines and deemed a key mediator of trigeminal sensitization. In trigeminal neuralgia, high levels of CGRP suggest a link between CGRP and the condition. Many other conditions (e.g., chronic pain, neuropathic disorders, musculoskeletal pain) depend on the release of excitatory neurotransmitters. Higher release of CGRP is involved in inflammatory conditions. | √ |
| PIH and TMJ arthritis | Muñoz-Lora, 2022 [22] | TNC | ||||
| PIH and TMJ arthritis | Muñoz-Lora, 2017 [15] | Peri-articular tissues (TMJ) & TG | ||||
| Trigeminal inflammatory pain—TMDs | Lacković, 2016 [20] | Dura mater, TNC, TG, CSF | ||||
| 2 STUDIES | ||||||
| SP NEUROPEPTIDE AND NEUROTRANSMITTER | Migraine | Shao, 2013 [19] | Jugular plasma and medulla oblongata—containing caudal trigeminal nucleus | BoNT may block the release of SP and other neuropeptides in both CNS and PNS, likely owing to their release-dependence on the SNARE complex. The effect of BoNT may involve the inhibition of the neuropeptide and neurotransmitter release, being essential in neurogenic inflammation and neuroinflammation-associated conditions. | SP is synthesized in the dorsal root ganglia sensory neurons and transported both centrally and peripherally, playing a pivotal role in pain neurotransmission. Higher release of SP is involved in inflammatory conditions. | √ |
| PIH and TMJ arthritis | Muñoz-Lora, 2017 [15] | Peri-articular tissues (TMJ) & TG | ||||
| IL-6 PRO- and ANTI-INFLAMMATORY CYTOKINE (ASSOCIATED TO PERIPHERAL INFLAMMATION AND NEUROINFLAMMATION) | TN | Cho, 2022 [17] | TG | BoNT may decrease peripheral pro-inflammatory cell infiltrate (potentially by inhibiting peripheral neuropeptides, neurotransmitters, and transcription factors that reduce the inflammatory chemotaxis or anti-inflammatory effect on site). | IL-6 has a crucial role in neuropathic pain development. Glia activation has been implicated in the development of central sensitization, which contributes to the pathogenesis of neuropathic pain. Activated microglia by peripheral nerve injury induces proliferation and activation of microglia in the CNS, and can release pro-inflammatory mediators (e.g., IL 6), which contributes to the development and continuity of neuropathic pain. | √ |
| Chen, 2021 [14] | TNC | Microglia activation in the CNS may be suppressed by BoNT, through inhibiting the overexpression of microglia-derived pro-inflammatory factors and neuroinflammation. | ||||
| CX3CR1 CHEMOKINE RECEPTOR (NEUROPLASTICITY-RELATED) | PIH and TMJ arthritis | Muñoz-Lora, 2020 [16] | TNC | BoNT may have effects on microglia-activated pathways through the reduction of microglia modulators, leading to the reduction of pro-inflammatory cytokines, thus inhibiting microglia-mediated neuroinflammation. BoNT undergoes binding to nerve terminals, thus cleaving SNAREs. By interacting with SNAP25, BoNT blocks the release of neurotransmitters and neuropeptides by vesicle-regulated exocytosis, which may affect neuronal functions as well as alleviate the activation of microglia (potentially via interaction with receptors on microglia). | CX3CR1 is selectively expressed in microglia. The receptor is involved in processes that stimulate the phosphorylation of diverse microglial intra-cellular mechanisms to prompt cell adhesion, migration, and release of pro-inflammatory cytokines, and hence triggering sensory neurons and running pain signals to upper centres. Microglia activation in the hippocampus was reported in depression in PD. | In vitro and model of human-into-mice grafts. Reports of phase 1 trials targeting CX3CR1-blockers in cancer pain and anti- CX3CR1 nanobody in kidney disease. |
| Depression—PD | Li, 2023 [18] | Hippocampus | ||||
| Dural Protein Extravasation INDICATOR OF NEUROGENIC INFLAMMATION AND TRIGEMINAL ACTIVATION | TN | Filipović, 2012 [28] | Cranial dura Plasma protein complexes | Dural protein extravasation is a relevant aspect of trigeminal pain and can be influenced by the central and peripheral actions of BoNT. BoNT antinociception effect seems to rely on axonal transport through sensory neurons beyond the ganglion, very probably into the CNS. | Dural plasma protein extravasation is often associated with migraine, other headache and pain conditions in the trigeminal region. Plasma protein extravasation in cranial dura is a helpful marker of trigeminal activation, commonly used in preclinical testing of migraine medication. | √ Often employed to explore migraine pathophysiology, but not in BoNT context |
| Trigeminal inflammatory pain—TMDs | Lacković, 2016 [20] | Cranial dura tissue, brainstem (TNC) | ||||
| Collagen COL- I AND COL- III—INDICATORS OF COLLAGEN DEPOSITION | HS | Xiong, 2023 [30] | Scar tissue (ear) | BoNT improves the appearance of hypertrophic scars (HSs) possibly by inhibiting their growth, throughly interfering with the cell cycle and regulating the TGFβ signalling pathways (e.g., TGF- β1/Smad, ERK, and JNK). BoNT may improve HSs by inhibiting the expression of α-SMA and myosin II, which may block the contraction of fibroblasts. | TGF-β1 high expression is among the key factors precipitating the formation of HS. TGF-β1 may activate several cellular pathways to participate in scar formation. It is a relevant regulator of inflammation, tissue repair and fibrosis. α-SMA is essential in the conversion of fibroblasts into myofibroblasts. Thus, α-SMA high expression suggests enhanced fibroblast fibrosis and overproduction of collagen. The atypical rise in collagen content in the extracellular matrix will determine the characteristics of HS, which is mostly associated with increased expression of COL- I. | Experiments on cultured cells derived from humans with the condition |
| Wang, 2020 [25] | ||||||
| α-SMAS and Myosin II Proteins PRO-FIBROTIC AND COLLAGEN-RELATED PROTEINS—MARKER FOR FIBROBLASTS-MYOFIBROBLASTS TRANSFORMATION | Xiong, 2023 [30] | |||||
| Wang, 2020 [25] | ||||||
| TGF-β1 PRO-FIBROSIS CYTOKINE (PERIPHERAL INFLAMMATION-RELATED) | Xiong, 2023 [30] | |||||
| Wang, 2020 [25] | ||||||
| TRPA-1 NOCICEPTORS NONSELECTIVE CATION CHANNEL PROTEINS (REGULATION OF ION CHANNELS) | TN | Wu, 2016 [23] | Brainstem Vc region (caudal subnucleus of the spinal trigeminal nucleus) | Peripherally applied BoNT has potentially peripheral and central antinociceptive effects. BoNT plays a key role in the modulation of expression of certain receptors or ion channels in nociceptor cell membranes, potentially reducing the excitability of pain-transmitting nerve cells. | TRPs have been implicated in the pathogenesis of pain sensation production and hyperalgesia, and are involved in the perception of pain, inflammation, and detecting noxious stimuli. Neuronal excitation, release of inflammatory neuropeptides, and resulting pain hypersensitivity, require TRPA1 channels. These are activated by the liberation of inflammatory agents from neuronal/non-neuronal cells (central and peripheral glial cells—e.g., Schwann cell TRPA1) around injury. | TRPA1 antagonists have been developed and tested in phase -I and -II clinical trials for diseases with prominent pain, but not in BoNT context. |
| Chronic dry skin itch | Cao, 2017 [33] | Dorsal root ganglia | TRPA1—key regulator of neuropeptide release and neurogenic inflammation. | |||
| Biomarker (CIS) | Author Year | Unit Measure | BoNT Key Effect (Outcome) | Summary | Overall GRADE | |
|---|---|---|---|---|---|---|
| Biological Sampling | ||||||
| 6 STUDIES | ||||||
| IL-1β | TNC | |||||
| TN | Chen, 2021 [14] | (mRNA expression) |
| 1 RCT (n = 48), mean ± SEM (T1) 5 days after BoNT (↓) |  | |
| PHI and TMJ arthritis | Muñoz-Lora, 2020 [16] | (pg/mL tissue) |
| 1 RCT (n = 40) mean ± SD (T1) 24 h after BoNT (↓) (T2) 7 days after BoNT (NS) (T3) 14 days after BoNT (NS) | ||
| TMJ tissues | ||||||
| TMJ OA | Makawi, 2022 [13] | (pg/mL tissue; mRNA; protein expression) |
| 1RCT (n = 42), mean (m), SD (T1) 2 weeks after BoNT (↓) (T2) 4 weeks after BoNT (↓) |  | |
| Peri-articular tissues from TMJ and TG | ||||||
| PIH and TMJ arthritis | Muñoz-Lora, 2017 [15] | (pg/mL) |
| 1 ? (n = ?) ? (T1) 24 h after BoNT (↓) (T2) 14 days after BoNT (↓) |  | |
| TG | ||||||
| TN | Cho, 2022 [17] | (pg/mL tissue) |
| 1 RCT (n = 236) mean ± SEM (T1) 2 days after BoNT (↓) |  | |
| Hippocampus | ||||||
| Depression—PD | Li, 2023 [18] | (mRNA; protein expression) |
| 1 ? (n = ?) mean ± SEM (T1) ? (↓) |  | |
| 5 STUDIES | ||||||
| TNF-α | TNC | |||||
| TN | Chen, 2021 [14] | (mRNA; protein expression) |
| 1 RCT (n = 48), mean ± SEM (T1) 5 days after BoNT (↓) |  | |
| PHI and TMJ arthritis | Muñoz-Lora, 2020 [16] | (pg/mL tissue) |
| 1 RCT (n = 40) mean ± SD (T1) 24 h after BoNT (NS) (T2) 7 days after BoNT (NS) (T3) 14 days after BoNT (↓) |  | |
| Peri-articular tissues from TMJ and TG | ||||||
| PIH and TMJ arthritis | Muñoz-Lora, 2017 [15] | (pg/mL) |
| 1 ? (n = ?) ? (T1) 24 h after BoNT (NS) (T2) 14 days after BoNT (NS) |  | |
| TG | ||||||
| TN | Cho, 2022 [17] | (pg/mL tissue) |
| 1 RCT (n = 236) mean ± SEM (T1) 2 days after BoNT (↓) |  | |
| Hippocampus | ||||||
| Depression—PD | Li, 2023 [18] | mRNA; protein expression |
| 1 ? (n = ?) mean ± SEM (T1) ? (↓) |  | |
| SNAP25 | Hippocampus | |||||
| Depression | Li, 2019 [21] | protein expression |
| 1 nRT (n = ?) mean ± SEM (T1) 1 h, 1, 3, 7 days (SRS) 16, 18, 22, 29 days (BoNT) (NS) |  | |
| TNC | ||||||
| PIH and TMJ arthritis | Muñoz-Lora, 2022 [22] | (cSNAP-25 staining/positive fibres) |
| 1 RCT (n = 40) mean ± SEM (T1) 14 days after OnaBoNT (7 U) (+) ipl. |  | |
| Cranial dura | ||||||
| TMDs trigeminal inflammatory pain | Lacković, 2016 [20] | (presence clSNAP25—fibers containing SNAP-25 co-expressed for CGRP. |
| 1 RCT (n = 105) mean ± SEM (T1) 4 days after BoNT (+) ipl. |  | |
| Brain—hindbrain sections | ||||||
| MDD | Ni, 2023 [24] | (positive signal) (% positive signal area) |
| 1 RCT (n = ?) mean ± SEM (T1) 10 days after BoNT (+) IFN ipl. (T2) 4 weeks after BoNT (+) IFN ipl. (T3) 7 weeks after BoNT (+) IFN ipl. |  | |
| Brainstem Vc region (caudal subnucleus of the spinal trigeminal nucleus) | ||||||
| TN | Wu, 2016 [23] | (by β-actin) |
| 1 ? (n = ?) mean ± SD (T1) 7 days after BoNT (↑) |  | |
| 4 STUDIES | ||||||
| TNC | ||||||
| c-Fos | TN | Chen, 2021 [14] | (mRNA; protein expression) |
| 1 RCT (n = 48), mean ± SEM (T1) 5 days after BoNT (↓) |  |
| PHI and TMJ arthritis | Muñoz-Lora, 2022. [22] | levels of c-Fos-positive nuclei |
| 1 RCT (n = 40) mean ± SEM (T1) 14 days after OnaBoNT (7 U, 14 U) (↓) ipl & cl. |  | |
| Brain—hindbrain sections (Ventrolateral periaqueductal gray) | ||||||
| MDD | Ni, 2023 [24] | (positive signal/neurons) (% positive signal area) |
| 1 RCT (n = ?) mean ± SEM (T1) 24 h after day 23 to 27 (↓) |  | |
| Medullary dorsal horn | ||||||
| TN | Kim, 2015 [26] | (expression—number of neurons—Scale bar, 100 µm) |
| 1 ? (n = ?) means ± SEM (T1) ? (↓) |  | |
| CGRP | Jugular plasma and medulla oblongata—containing caudal trigeminal nucleus | |||||
| Migraine | Shao, 2013 [19] | (pg/mL) |
| 1 ? (n = 32) means ± SEM (T1) 24 h after BoNT (↓) |  | |
| TNC | ||||||
| PIH and TMJ arthritis | Muñoz-Lora, 2022 [22] | (pg/mL) area (µm2) |
| 1 RCT (n = 40) mean ± SEM (T1) 14 days after BoNT (7 U/14 U) (NS) |  | |
| Peri-articular tissues (TMJ) and TG | ||||||
| PIH and TMJ arthritis | Muñoz-Lora, 2017 [15] | (ng/mL) |
| 1 ? (n = ?) ? (T1) 24 h after BoNT (↓) (T2) 14 days after BoNT (↓) |  | |
| Dura mater, TNC, TG, CSF | ||||||
| Trigeminal inflammatory pain—TMDs | Lacković, 2016 [20] | Concentration (fmol mg−1 or fmol mL−1) |
| 1 RCT (n = 105) mean ± SEM (T1) 4-days after BoNT (↓) cranial dura |  | |
| 2 STUDIES | ||||||
| SP | Jugular plasma and medulla oblongata—containing caudal trigeminal nucleus | |||||
| Migraine | Shao, 2013 [19] | (pg/mL) |
| 1 ? (n = 32) means ± SEM (T1) 24 h after BoNT (↓) |  | |
| Peri-articular tissues (TMJ) & TG | ||||||
| PIH and TMJ arthritis | Muñoz-Lora, 2017 [15] | (ng/mL) |
| 1 ? (n = ?) ? (T1) 24 h after BoNT (↓) (T2) 14 days after BoNT (↓) |  | |
| IL-6 | TG | |||||
| TN | Cho, 2022 [17] | pg/mLtissue |
| 1 RCT (n = 236) mean ± SEM (T1) 2 days after BoNT (↓) |  | |
| TNC | ||||||
| TN | Chen, 2021 [14] | (mRNA; protein expression) |
| 1 RCT (n = 48), mean ± SEM (T1) 5 days after BoNT (↓) |  | |
| CX3CR1 | TNC | |||||
| PIH and TMJ arthritis | Muñoz-Lora, 2020 [16] | (OD, protein level) |
| 1 RCT (n = 40) mean ± SD (T1) 24 h after BoNT (NS) (T2) 7 days after BoNT (NS) (T3) 14 days after BoNT (NS) |  | |
| Hippocampus | ||||||
| Depression—PD | Li, 2023 [18] | (mRNA expression levels) |
| 1 ? (n = ?) mean ± SEM (T1) ? (↓) |  | |
| Dural Protein Extravasation | Cranial dura Plasma protein complexes | |||||
| TN | Filipović, 2012 [28] | (ng of Evans blue per mg of dural tissue) |
| 1 nRT (n = 200) mean ± SEM (T1) 3 days after BoNT (↓) ipl. and cl. |  | |
| Cranial dura tissue, brainstem (TNC) | ||||||
| Trigeminal inflammatory pain—TMDs | Lacković, 2016 [20] | ng (mg tissue)−1 |
| 1 RCT (n = 105) mean ± SEM (T1) 4 days after BoNT (↓) ipl. |  | |
| Collagen | Scar tissue (ear) | |||||
| HS | Xiong, 2023 [30] | protein concentration and expression |
| 1 RCT (n = 24) mean ± SD (T1) 5 weeks after BoNT (↓) |  | |
| Wang, 2020 [25] |
| 1 RCT (n = 18) mean ± SD (T1) 28 days after BoNT (↓) |  | |||
| α-SMAS and Myosin II Proteins | Xiong, 2023 [30] | proteins, optical density (OD) |
| 1 RCT (n = 24) mean ± SD (T1) 5 weeks after BoNT (↓) |  | |
| Wang, 2020 [25] |
| 1 RCT (n = 18) mean ± SD (T1) 28 days after BoNT (↓) |  | |||
| TGF-β1 | Xiong, 2023 [30] | Protein concentration and expression |
| 1 RCT (n = 24) mean ± SD (T1) 5 weeks after BoNT (↓) |  | |
| Wang, 2020 [25] |
| 1 RCT (n = 18) mean ± SD (T1) 28 days after BoNT (↓) |  | |||
| TRPA-1 | Brainstem Vc region (caudal subnucleus of the spinal trigeminal nucleus) | |||||
| TN | Wu, 2016 [23] | protein expression |
| 1 ? (n = ?) mean ± SD (T1) 7 days after BoNT (↓) |  | |
| Dorsal root ganglia | ||||||
| TN | Cao, 2017 [33] | protein expression |
| 1 ? (n = 525) mean ± SD (T1) 3 days BoNT (↓) (T2) 7 days after BoNT (↓) |  | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novo Pereira, I.; De la Torre Canales, G.; Durão, S.; Shado, R.; Braga, A.C.; Almeida, A.M.; Hassan, H.; Manso, A.C.; Faria-Almeida, R. Botulinum Toxin Effects on Biochemical Biomarkers Related to Inflammation-Associated Head and Neck Chronic Conditions: A Systematic Review of Preclinical Research. Toxins 2025, 17, 377. https://doi.org/10.3390/toxins17080377
Novo Pereira I, De la Torre Canales G, Durão S, Shado R, Braga AC, Almeida AM, Hassan H, Manso AC, Faria-Almeida R. Botulinum Toxin Effects on Biochemical Biomarkers Related to Inflammation-Associated Head and Neck Chronic Conditions: A Systematic Review of Preclinical Research. Toxins. 2025; 17(8):377. https://doi.org/10.3390/toxins17080377
Chicago/Turabian StyleNovo Pereira, Ines, Giancarlo De la Torre Canales, Sara Durão, Rawand Shado, Ana Cristina Braga, André Mariz Almeida, Haidar Hassan, Ana Cristina Manso, and Ricardo Faria-Almeida. 2025. "Botulinum Toxin Effects on Biochemical Biomarkers Related to Inflammation-Associated Head and Neck Chronic Conditions: A Systematic Review of Preclinical Research" Toxins 17, no. 8: 377. https://doi.org/10.3390/toxins17080377
APA StyleNovo Pereira, I., De la Torre Canales, G., Durão, S., Shado, R., Braga, A. C., Almeida, A. M., Hassan, H., Manso, A. C., & Faria-Almeida, R. (2025). Botulinum Toxin Effects on Biochemical Biomarkers Related to Inflammation-Associated Head and Neck Chronic Conditions: A Systematic Review of Preclinical Research. Toxins, 17(8), 377. https://doi.org/10.3390/toxins17080377








