Pre-Harvest Aflatoxin Contamination in Crops and Climate Change Factors: A European Overview
Abstract
1. Introduction
2. Results
2.1. Literature Retrieval
2.2. Geographical Location and Crops of Included Studies
2.3. AF Occurrence and Levels
2.3.1. Contamination Rate
2.3.2. Contamination Level and Uncompliant Samples
2.3.3. Mycotoxin Co-Contamination
2.4. Environmental Risk Factors for Contamination
2.4.1. Climate Conditions
Weather Parameters
Data Sources and Collection Methods
2.4.2. Agronomic Factors
2.5. Methodological Characteristics of Studies
2.5.1. Sampling
Origin
Timing
Procedure
2.5.2. Analysis
Number of Samples
Analytical Methods
Detection Limits
3. Discussion
3.1. Scope of the Current Research
3.1.1. Geographical Location and Period Covered
3.1.2. Crops
3.2. Pre-Harvest AF Contamination in Europe
3.3. Understanding Risk Factors for Pre-Harvest AF Contamination
3.3.1. Climate Conditions
3.3.2. Agronomic Factors
3.4. Methodological Shortcomings
3.4.1. Weather Parameters
3.4.2. Sampling
3.4.3. Analysis
4. Conclusions
4.1. Practical Implications and Future Research Directions
- -
- Geographic expansion of monitoring efforts to include underrepresented and climate-vulnerable regions across Europe.
- -
- Long-term, high-resolution monitoring to capture seasonal variability and long-term trends in climate–AF dynamics.
- -
- Standardized data collection protocols for environmental, crop, and contamination variables, including harmonized sampling methods and clear reporting of AF detection techniques (e.g., LOD/LOQ).
- -
- Broader crop coverage, with inclusion of feed crops and resistant hybrids to support more comprehensive risk assessments.
- -
- Integration of agronomic factors such as sowing and harvest dates, soil characteristics, and field management practices.
- -
- Adoption of a minimal dataset for AF monitoring, including specific variables on climate, crop phenology, sampling, and AF types and levels. See Table S4.
- -
- Development of centralized and accessible databases with standardized metadata and transparent documentation to enable cross-study comparisons and inform evidence-based decision-making.
4.2. Final Remarks
5. Methods
5.1. Search Strategy
5.2. Selection Criteria
- Study type: Only original research articles were included. We excluded reviews, editorials, concept papers, and book chapters.
- Study design: We included case studies and field experiments. Articles based on data modeling, laboratory experiments, or biocontrol trials were excluded.
- Geography: Only studies reporting data from Europe were included.
- Topic: We included studies addressing climate-related changes and pre-harvest AF contamination in crops. Specifically, we included: (a) studies reporting in-field or pre-harvest contamination; (b) studies analysing AFs (i.e., AFB1, AFB2, AFG1, and AFG2); and (c) studies investigating climate-related environmental factors (e.g., weather conditions, weather events, and climate patterns). We excluded: (a) studies not addressing either climate change or AFs; (b) studies focused on the health effects of AFs (e.g., toxicology, disease burden); (c) studies analyzing AF occurrence in non-crop samples (e.g., milk, human or animal samples, air, soil); (d) studies reporting mycotoxins other than AFs; (e) studies using artificial inoculation of aflatoxigenic fungi; (f) studies focused solely on post-harvest contamination (e.g., stored, packaged, or marketed products) or environmental factors specific to storage; (g) studies addressing mitigation strategies, analytical methods, or prevention/control measures without considering the role of climate-related environmental factors.
- Results: We included studies that reported quantitative AF occurrence data. Studies reporting only aflatoxigenic fungal colony counts without AF measurements were excluded.
- Language and availability: Only studies published in English with full-text availability were included.
5.3. Data Extraction
- Country: The country where the study was conducted.
- Period: The time frame during which data were collected.
- Environmental conditions: (a) Weather parameters—variables such as temperature, humidity, and rainfall; (b) Data sources—whether the data were primary or secondary; (c) Data collection methods—how environmental data were gathered (e.g., via in-situ measurements, meteorological stations, models, or other databases); (d) Agronomic factors—crop-related conditions such as soil type, irrigation, and farming practices.
- Crop: The specific crop analyzed for AF contamination.
- Sampling: (a) The type(s) of AFs analyzed; (b) The number of samples tested; (c) Analytical methods or techniques used for AF detection (e.g., HPLC, ELISA); (d) Detection limits—the minimum detectable (LOD) and quantifiable (LOQ) concentrations of AFs.
- Contamination: (a) Contamination rate—the percentage of contaminated samples; (b) Contamination level—the measured AF concentrations; (c) Non-compliant samples—the percentage of samples exceeding EU regulatory limits (EU Regulation EC 466/2006) [84].
- Additional information: Any relevant details not covered in the categories above (e.g., policy context, interventions, statistical approaches).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Glossary
| AF | aflatoxin |
| BEA | Beauvericin |
| CC | climate change |
| CPA | Cyclopiazonic Acid |
| CIT | Citrinin |
| DON | deoxynivalenol |
| EA | ergot alkaloids |
| EFSA | European Food Safety Authority |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| ENN | enniatin |
| FFS | food and feed safety |
| FUM | fumonisin |
| GDD | growing degree days |
| HPLC | High-performance liquid chromatography |
| IARC | International Agency for Research on Cancer |
| LC MS/MS | Liquid chromatography mass spectrometry |
| LOD | limit of detection |
| LOQ | limit of quantification |
| OTA | ochratoxin A |
| ZEA | zearalenone |
References
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific Opinion on Ergot alkaloids in food and feed (CONTAM Panel). EFSA J. 2012, 10, 2798. [Google Scholar]
- EFSA. International frameworks dealing with human risk assessment of combined exposure to multiple chemicals. European Food Safety Authority. EFSA J. 2013, 11, 3313. [Google Scholar]
- EFSA. Scientific Opinion on the risks to human and animal health related to the presence of beauvericin and enniatins in food and feed (CONTAM Panel). EFSA J. 2014, 12, 3802. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain; Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.-C.; et al. Scientific opinion—Risk assessment of aflatoxins in food. EFSA J. 2020, 18, e06040. [Google Scholar] [CrossRef]
- Hof, H. Mycotoxins in milk for human nutrition: Cow, sheep and human breast milk. GMS Infect. Dis. 2016, 4, Doc03. [Google Scholar] [CrossRef]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. A Review of Human Carcinogens: Chemical Agents and Related Occupations; International Agency for Research on Cancer: Lyon, France, 2012; Volume 100F. [Google Scholar]
- Ngindu, A.; Kenya, P.R.; Ocheng, D.M.; Omondi, T.N.; Ngare, W.; Gatei, D.; Johnson, B.K.; Ngira, J.A.; Nandwa, H.; Jansen, A.J.; et al. Outbreak of Acute Hepatitis Caused By Aflatoxin Poisoning in Kenya. Lancet 1982, 319, 1346–1348. [Google Scholar] [CrossRef]
- Lewis, L.; Onsongo, M.; Njapau, H.; Schurz-Rogers, H.; Luber, G.; Kieszak, S.; Nyamongo, J.; Backer, L.; Dahiye, A.M.; Misore, A.; et al. Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in eastern and central Kenya. Environ. Health Perspect. 2005, 113, 1763–1767. [Google Scholar] [CrossRef]
- EFSA. Opinion of the Scientific Panel on Contaminants in the Food Chain (CONTAM Panel) related to aflatoxin B1 as undesirable substance in animal feed. European Food Safety Authority. EFSA J. 2004, 2, 39. [Google Scholar] [CrossRef]
- Zingales, V.; Taroncher, M.; Martino, P.A.; Ruiz, M.J.; Caloni, F. Climate Change and Effects on Molds and Mycotoxins. Toxins 2022, 14, 445. [Google Scholar] [CrossRef]
- Klich, M.A. Aspergillus flavus: The major producer of aflatoxin. Mol. Plant Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Ojiambo, P.S.; Battilani, P.; Cary, J.W.; Blum, B.H.; Carbone, I. Cultural and genetic approaches to manage aflatoxin contamination: Recent insights provide opportunities for improved control. Phytopathology 2018, 108, 1024–1037. [Google Scholar] [CrossRef] [PubMed]
- CAST. Council for Agricultural Science and Technology—Task Force Report; CAST: Glendale, CA, USA, 2003. [Google Scholar]
- Pitt, J.I.; Taniwaki, M.H.; Cole, M.B. Mycotoxin production in major crops as influenced by growing, harvesting, storage and processing, with emphasis on the achievement of Food Safety Objectives. Food Control 2013, 32, 205–215. [Google Scholar] [CrossRef]
- Wu, F.; Mitchell, N.J. How climate change and regulations can affect the economics of mycotoxins. World Mycotoxin J. 2016, 9, 653–663. [Google Scholar] [CrossRef]
- Pitt, J.I.; Miller, J.D. A Concise History of Mycotoxin Research. J. Agric. Food Chem. 2017, 65, 7021–7033. [Google Scholar] [CrossRef] [PubMed]
- European Commission. RASFF—Food and Feed Safety Alerts. 2022. Available online: https://ec.europa.eu/food/safety/rasff_en (accessed on 16 February 2022).
- Van Der Fels-Klerx, H.J.; Liu, C.; Battilani, P. Modelling climate change impacts on mycotoxin contamination. World Mycotoxin J. 2016, 9, 717–726. [Google Scholar] [CrossRef]
- Magan, N.; Medina, A. Integrating gene expression, ecology and mycotoxin production by Fusarium and Aspergillus species in relation to interacting environmental factors. World Mycotoxin J. 2016, 9, 673–684. [Google Scholar] [CrossRef]
- Medina, A.; Rodriguez, A.; Magan, N. Effect of climate change on Aspergillus flavus and aflatoxin B1 production. Front. Microbiol. 2014, 5, 348. [Google Scholar] [CrossRef]
- Miraglia, M.; Marvin, H.J.P.; Kleter, G.A.; Battilani, P.; Brera, C.; Coni, E.; Cubadda, F.; Croci, L.; De Santis, B.; Dekkers, S.; et al. Climate change and food safety: An emerging issue with special focus on Europe. Food Chem. Toxicol. 2009, 47, 1009–1021. [Google Scholar] [CrossRef]
- Battilani, P.; Toscano, P.; Van Der Fels-Klerx, H.J.; Moretti, A.; Leggieri, M.C.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B 1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef]
- Paterson, R.R.; Lima, N. Thermophilic fungi to dominate aflatoxigenic/mycotoxigenic fungi on food under global warming. Int. J. Environ. Res. Public Health 2017, 14, 199. [Google Scholar] [CrossRef] [PubMed]
- Piva, G.; Battilani, P.; Pietri, A. Emerging issues in Southern Europe: Aflatoxins in Italy. In The Mycotoxin Factbook, Food and Feed Topics; Barug, D., Bhatnagar, D., van Egmond, H.P., van der Kamp, J.W., van Osenbruggen, W.A., Visconti, A., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2006; pp. 139–153. [Google Scholar] [CrossRef]
- Tabuc, C.; Marin, D.; Guerre, P.; Sesan, T.; Bailly, J.D. Molds and mycotoxin content of cereals in southeastern romania. J. Food Prot. 2009, 72, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Dobolyi, C.; Sebok, F.; Varga, J.; Kocsubé, S.; Szigeti, G.; Baranyi, N.; Szécsi, A.; Tóth, B.; Varga, M.; Kriszt, B.; et al. Occurrence of aflatoxin producing Aspergillus flavus isolates in maize kernel in Hungary. Acta Aliment. 2013, 42, 451–459. [Google Scholar] [CrossRef]
- Pleadin, J.; Vulić, A.; Perši, N.; Škrivanko, M.; Capek, B.; Cvetnić, Ž. Aflatoxin B1 occurrence in maize sampled from Croatian farms and feed factories during 2013. Food Control 2014, 40, 286–291. [Google Scholar] [CrossRef]
- Leggieri, M.C.; Bertuzzi, T.; Pietri, A.; Battilani, P. Mycotoxin occurrence in maize produced in Northern Italy over the years 2009–2011: Focus on the role of crop related factors. Phytopathol. Mediterr. 2015, 54, 212–221. [Google Scholar] [CrossRef]
- Dimitrieska-Stojković, E.; Stojanovska-Dimzoska, B.; Ilievska, G.; Uzunov, R.; Stojković, G.; Hajrulai-Musliu, Z.; Jankuloski, D. Assessment of aflatoxin contamination in raw milk and feed in Macedonia during 2013. Food Control 2016, 59, 201–206. [Google Scholar] [CrossRef]
- Van Asselt, E.D.; van der Fels-Klerx, H.J.; Marvin, H.J.P.; van Bokhorst-van de Veen, H.; Groot, M.N. Overview of Food Safety Hazards in the European Dairy Supply Chain. Compr. Rev. Food Sci. Food Saf. 2017, 16, 59–75. [Google Scholar] [CrossRef]
- Assunção, R.; Martins, C.; Viegas, S.; Viegas, C.; Jakobsen, L.S.; Pires, S.; Alvito, P. Climate change and the health impact of aflatoxins exposure in Portugal–an overview. Food Addit. Contam.—Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 1610–1621. [Google Scholar] [CrossRef]
- Udovicki, B.; Audenaert, K.; De Saeger, S.; Rajkovic, A. Overview on the mycotoxins incidence in serbia in the period 2004–2016. Toxins 2018, 10, 279. [Google Scholar] [CrossRef]
- Focker, M.; van Eupen, M.; Verweij, P.; Liu, C.; van Haren, C.; van der Fels-Klerx, H.J. Effects of Climate Change on Areas Suitable for Maize Cultivation and Aflatoxin Contamination in Europe. Toxins 2023, 15, 599. [Google Scholar] [CrossRef]
- Herrera, M.; Cavero, J.; Franco-Luesma, S.; Álvaro-Fuentes, J.; Ariño, A.; Lorán, S. Mycotoxins and Crop Yield in Maize as Affected by Irrigation Management and Tillage Practices. Agronomy 2023, 13, 798. [Google Scholar] [CrossRef]
- Dövényi-Nagy, T.; Rácz, C.; Molnár, K.; Bakó, K.; Szláma, Z.; Jóźwiak, Á.; Farkas, Z.; Pócsi, I.; Dobos, A.C. Pre-Harvest Modelling and Mitigation of Aflatoxins in Maize in a Changing Climatic Environment-A Review. Toxins 2020, 12, 768. [Google Scholar] [CrossRef] [PubMed]
- Marroquín-Cardona, A.G.; Johnson, N.M.; Phillips, T.D.; Hayes, A.W. Mycotoxins in a changing global environment—A review. Food Chem. Toxicol. 2014, 69, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Kos, J.; Anić, M.; Radić, B.; Zadravec, M.; Janić Hajnal, E.; Pleadin, J. Climate Change—A Global Threat Resulting in Increasing Mycotoxin Occurrence. Foods 2023, 12, 2704. [Google Scholar] [CrossRef]
- Valencia-Quintana, R.; Milić, M.; Jakšić, D.; Klarić, M.Š.; Tenorio-Arvide, M.G.; Pérez-Flores, G.A.; Bonassi, S.; Sánchez-Alarcón, J. Environment changes, aflatoxins, and health issues, a review. Int. J. Environ. Res. Public Health 2020, 17, 7850. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food REPEALINGREGULATION(EC) No. 1881/2006. Off. J. Eur. Union 2023, 119, 103–157. [Google Scholar]
- Battilani, P.; Camardo Leggieri, M.; Rossi, V.; Giorni, P. AFLA-maize, a mechanistic model for Aspergillus flavus infection and aflatoxin B1 contamination in maize. Comput. Electron. Agric. 2013, 94, 38–46. [Google Scholar] [CrossRef]
- Liu, N.; Liu, C.; Dudaš, T.N.; Loc, M.Č.; Bagi, F.F.; Van Der Fels-Klerx, H.J. Improved aflatoxins and fumonisins forecasting models for maize (PREMA and PREFUM), using Combined Mechanistic and Bayesian Network Modeling—Serbia as a case study. Front. Microbiol. 2021, 12, 643604. [Google Scholar] [CrossRef]
- Inglis, A.; Parnell, A.C.; Subramani, N.; Doohan, F.M. Machine Learning Applied to the Detection of Mycotoxin in Food: A Systematic Review. Toxins 2024, 16, 268. [Google Scholar] [CrossRef]
- Cotty, P.J.; Jaime-Garcia, R. Influences of climate on aflatoxin producing fungi and aflatoxin contamination. Int. J. Food Microbiol. 2007, 119, 109–115. [Google Scholar] [CrossRef]
- Fountain, J.C.; Scully, B.T.; Ni, X.; Kemerait, R.C.; Lee, R.D.; Chen, Z.Y.; Guo, B. Environmental influences on maize-Aspergillus flavus interactions and aflatoxin production. Front. Microbiol. 2014, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Casu, A.; Camardo Leggieri, M.; Toscano, P.; Battilani, P. Changing climate, shifting mycotoxins: A comprehensive review of climate change impact on mycotoxin contamination. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13323. [Google Scholar] [CrossRef] [PubMed]
- Bunny, S.M.; Umar, A.; Bhatti, H.S.; Honey, S.F. Aflatoxin risk in the era of climatic change-a comprehensive review. CABI Agric. Biosci. 2024, 5, 105. [Google Scholar] [CrossRef]
- Gallo, G.; Lo Bianco, M.; Bognanni, R.; Saimbene, G. Mycotoxins in Durum Wheat Grain: Hygienic-Health Quality of Sicilian Production. J. Food Sci. 2008, 73, T42–T47. [Google Scholar] [CrossRef]
- Buyukunal, S.; Kahraman, T.; Ciftcioglu, G.R. Occurrence of AF, AFB1, OTA in Rice Commercialized in Eastern Turkey. Pol. J. Environ. Stud. 2010, 19, 907–912. [Google Scholar]
- Van Asselt, E.D.; Azambuja, W.; Moretti, A.; Kastelein, P.; De Rijk, T.C.; Stratakou, I.; Van Der Fels-Klerx, H.J. A Dutch field survey on fungal infection and mycotoxin concentrations in maize. Food Addit. Contam. Part A 2012, 29, 1556–1565. [Google Scholar] [CrossRef]
- Pietri, A.; Battilani, P.; Gualla, A.; Bertuzzi, T. Mycotoxin levels in maize produced in northern Italy in 2008 as influenced by growing location and FAO class of hybrid. World Mycotoxin J. 2012, 5, 409–418. [Google Scholar] [CrossRef]
- Tóth, B.; Török, O.; Kótai, É.; Varga, M.; Toldiné Tóth, É.; Pálfi, X.; Háfra, E.; Varga, J.; Téren, J.; Mesterházy, Á. Role of Aspergilli and Penicillia in mycotoxin contamination of maize in Hungary. Acta Agron. Hung. 2012, 60, 143–149. [Google Scholar] [CrossRef]
- Kos, J.; Mastilović, J.; Hajnal, E.J.; Šarić, B. Natural occurrence of aflatoxins in maize harvested in Serbia during 2009–2012. Food Control 2013, 34, 31–34. [Google Scholar] [CrossRef]
- Alkadri, D.; Rubert, J.; Prodi, A.; Pisi, A.; Mañes, J.; Soler, C. Natural co-occurrence of mycotoxins in wheat grains from Italy and Syria. Food Chem. 2014, 157, 111–118. [Google Scholar] [CrossRef]
- Pleadin, J.; Vulić, A.; Perši, N.; Škrivanko, M.; Capek, B.; Cvetnić, Ž. Annual and regional variations of aflatoxin B1 levels seen in grains and feed coming from Croatian dairy farms over a 5-year period. Food Control 2015, 47, 221–225. [Google Scholar] [CrossRef]
- Janić Hajnal, E.; Kos, J.; Krulj, J.; Krstović, S.; Jajić, I.; Pezo, L.; Šarić, B.; Nedeljković, N. Aflatoxins contamination of maize in Serbia: The impact of weather conditions in 2015. Food Addit. Contam.—Part A Chem. Anal. Control Expo. Risk Assess. 2017, 34, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Kos, J.; Janić Hajnal, E.; Šarić, B.; Jovanov, P.; Mandić, A.; Đuragić, O.; Kokić, B. Aflatoxins in maize harvested in the Republic of Serbia over the period 2012–2016. Food Addit. Contam. Part B 2018, 11, 246–255. [Google Scholar] [CrossRef]
- Bailly, S.; El Mahgubi, A.; Carvajal-Campos, A.; Lorber, S.; Puel, O.; Oswald, I.P.; Bailly, J.D.; Orlando, B. Occurrence and identification of aspergillus section flavi in the context of the emergence of aflatoxins in french maize. Toxins 2018, 10, 525. [Google Scholar] [CrossRef]
- Keriene, I.; Mankeviciene, A.; Cesnuleviciene, R. Risk factors for mycotoxin contamination of buckwheat grain and its products. World Mycotoxin J. 2018, 11, 519–530. [Google Scholar] [CrossRef]
- Kos, J.; Janić Hajnal, E.; Malachová, A.; Steiner, D.; Stranska, M.; Krska, R.; Poschmaier, B.; Sulyok, M. Mycotoxins in maize harvested in Republic of Serbia in the period 2012–2015. Part 1: Regulated mycotoxins and its derivatives. Food Chem. 2020, 312, 126034. [Google Scholar] [CrossRef]
- Leggieri, M.C.; Lanubile, A.; Dall’Asta, C.; Pietri, A.; Battilani, P. The impact of seasonal weather variation on mycotoxins: Maize crop in 2014 in northern Italy as a case study. World Mycotoxin J. 2020, 13, 25–36. [Google Scholar] [CrossRef]
- Kifer, D.; Sulyok, M.; Jakšić, D.; Krska, R.; Šegvić Klarić, M. Fungi and their metabolites in grain from individual households in Croatia. Food Addit. Contam. Part B 2021, 14, 98–109. [Google Scholar] [CrossRef]
- Nikolic, M.; Srdic, J.; Savic, I.; Zilic, S.; Stevanovic, M.; Kandic, V.; Stankovic, S. The occurrence of mycotoxins in sweet maize hybrids. Genetika 2021, 53, 1311–1320. [Google Scholar] [CrossRef]
- Ferrari, L.; Fumagalli, F.; Rizzi, N.; Grandi, E.; Vailati, S.; Manoni, M.; Ottoboni, M.; Cheli, F.; Pinotti, L. An Eight-Year Survey on Aflatoxin B1 Indicates High Feed Safety in Animal Feed and Forages in Northern Italy. Toxins 2022, 14, 763. [Google Scholar] [CrossRef]
- Mesterházy, Á.; Szieberth, D.; Szabó, B.; Berényi, A.; Tóth, B. Mycotoxin contamination of maize (Zea mays L.) samples in Hungary, 2012–2017. Cereal Res. Commun. 2022, 50, 1065–1073. [Google Scholar] [CrossRef]
- Kovač, M.; Bulaić, M.; Nevistić, A.; Rot, T.; Babić, J.; Panjičko, M.; Kovač, T.; Šarkanj, B. Regulated Mycotoxin Occurrence and Co-Occurrence in Croatian Cereals. Toxins 2022, 14, 112. [Google Scholar] [CrossRef] [PubMed]
- Molnár, K.; Rácz, C.; Dövényi-Nagy, T.; Bakó, K.; Pusztahelyi, T.; Kovács, S.; Adácsi, C.; Pócsi, I.; Dobos, A. The Effect of Environmental Factors on Mould Counts and AFB1 Toxin Production by Aspergillus flavus in Maize. Toxins 2023, 15, 227. [Google Scholar] [CrossRef] [PubMed]
- Pleadin, J.; Kos, J.; Radić, B.; Vulić, A.; Kudumija, N.; Radović, R.; Janić Hajnal, E.; Mandić, A.; Anić, M. Aflatoxins in Maize from Serbia and Croatia: Implications of Climate Change. Foods 2023, 12, 548. [Google Scholar] [CrossRef]
- Misihairabgwi, J.M.; Ezekiel, C.N.; Sulyok, M.; Shephard, G.S.; Krska, R. Mycotoxin contamination of foods in Southern Africa: A 10-year review (2007–2016). Crit. Rev. Food Sci. Nutr. 2017, 59, 43–58. [Google Scholar] [CrossRef]
- Nleya, N.; Adetunji, M.C.; Mwanza, M. Current status of mycotoxin contamination of food commodities in Zimbabwe. Toxins 2018, 10, 89. [Google Scholar] [CrossRef]
- Adhikari, M.; Isaac, E.L.; Paterson, R.R.; Maslin, M.A. A review of potential impacts of climate change on coffee cultivation and mycotoxigenic fungi. Microorganisms 2020, 8, 1625. [Google Scholar] [CrossRef]
- Pickova, D.; Ostry, V.; Malir, J.; Toman, J.; Malir, F. A Review on Mycotoxins and Microfungi in Spices in the Light of the Last Five Years. Toxins 2020, 12, 789. [Google Scholar] [CrossRef]
- Frazzoli, C.; Gherardi, P.; Saxena, N.; Belluzzi, G.; Mantovani, A. The hotspot for (global) one health in primary food production: Aflatoxin M1 in dairy products. Front. Public Health 2017, 4, 294. [Google Scholar] [CrossRef]
- Moretti, A.; Pascale, M.; Logrieco, A.F. Mycotoxin risks under a climate change scenario in Europe. Trends Food Sci. Technol. 2019, 84, 38–40. [Google Scholar] [CrossRef]
- Gasperini, A.M.; Rodriguez-Sixtos, A.; Verheecke-Vaessen, C.; Garcia-Cela, E.; Medina, A.; Magan, N. Resilience of Biocontrol for Aflatoxin Minimization Strategies: Climate Change Abiotic Factors May Affect Control in Non-GM and GM-Maize Cultivars. Front. Microbiol. 2019, 10, 2525. [Google Scholar] [CrossRef] [PubMed]
- Pócsi, I.; Giacometti, F.; Ambrus, Á.; Logrieco, A.F. Editorial: Aspergillus-Derived Mycotoxins in the Feed and Food Chain. Front. Microbiol. 2020, 11, 606108. [Google Scholar] [CrossRef] [PubMed]
- Jallow, A.; Xie, H.; Tang, X.; Qi, Z.; Li, P. Worldwide aflatoxin contamination of agricultural products and foods: From occurrence to control. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2332–2381. [Google Scholar] [CrossRef]
- Fouché, T.; Claassens, S.; Maboeta, M. Aflatoxins in the soil ecosystem: An overview of its occurrence, fate, effects and future perspectives. Mycotoxin Res. 2020, 36, 303–309. [Google Scholar] [CrossRef]
- Hanano, A.; Almousally, I.; Shaban, M. Exposure of Aspergillus flavus nrrl 3357 to the environmental toxin, 2,3,7,8-tetrachlorinated dibenzo-p-dioxin, results in a hyper aflatoxicogenic phenotype: A possible role for caleosin/peroxygenase (afpxg). Front. Microbiol. 2019, 10, 2338. [Google Scholar] [CrossRef]
- Kovač, T.; Borišev, I.; Kovač, M.; Lončarić, A.; Čačić Kenjerić, F.; Djordjevic, A.; Strelec, I.; Ezekiel, C.N.; Sulyok, M.; Krska, R.; et al. Impact of fullerol C60(OH)24 nanoparticles on the production of emerging toxins by Aspergillus flavus. Sci. Rep. 2020, 10, 725. [Google Scholar] [CrossRef]
- Kovač, T.; Marček, T.; Šarkanj, B.; Borišev, I.; Ižaković, M.; Jukić, K.; Lončarić, A.; Krska, T.; Sulyok, M.; Krska, R. Fullerol C60(OH)24 nanoparticles and drought impact on wheat (Triticum aestivum L.) during growth and infection with Aspergillus flavus. J. Fungi 2021, 7, 236. [Google Scholar] [CrossRef]
- Omar, S.S.; Haddad, M.A.; Parisi, S. Validation of HPLC and Enzyme-Linked Immunosorbent Assay (ELISA) Techniques for Detection and Quantification of Aflatoxins in Different Food Samples. Foods 2020, 9, 661. [Google Scholar] [CrossRef]
- Cancelliere, R.; Di Tinno, A.; Cataldo, A.; Bellucci, S.; Kumbhat, S.; Micheli, L. Nafion-based label-free immunosensor as a reliable warning system: The case of AFB1 detection in cattle feed. Microchem. J. 2023, 191, 108868. [Google Scholar] [CrossRef]
- Commission Regulation (EC). No. 1881/2006. 19 December 2006. Setting Maximum Levels for Certain Contaminants in Foodstuffs (Text with EEA Relevance). 2006, Volume 364. Available online: http://data.europa.eu/eli/reg/2006/1881/oj/eng (accessed on 25 April 2025).


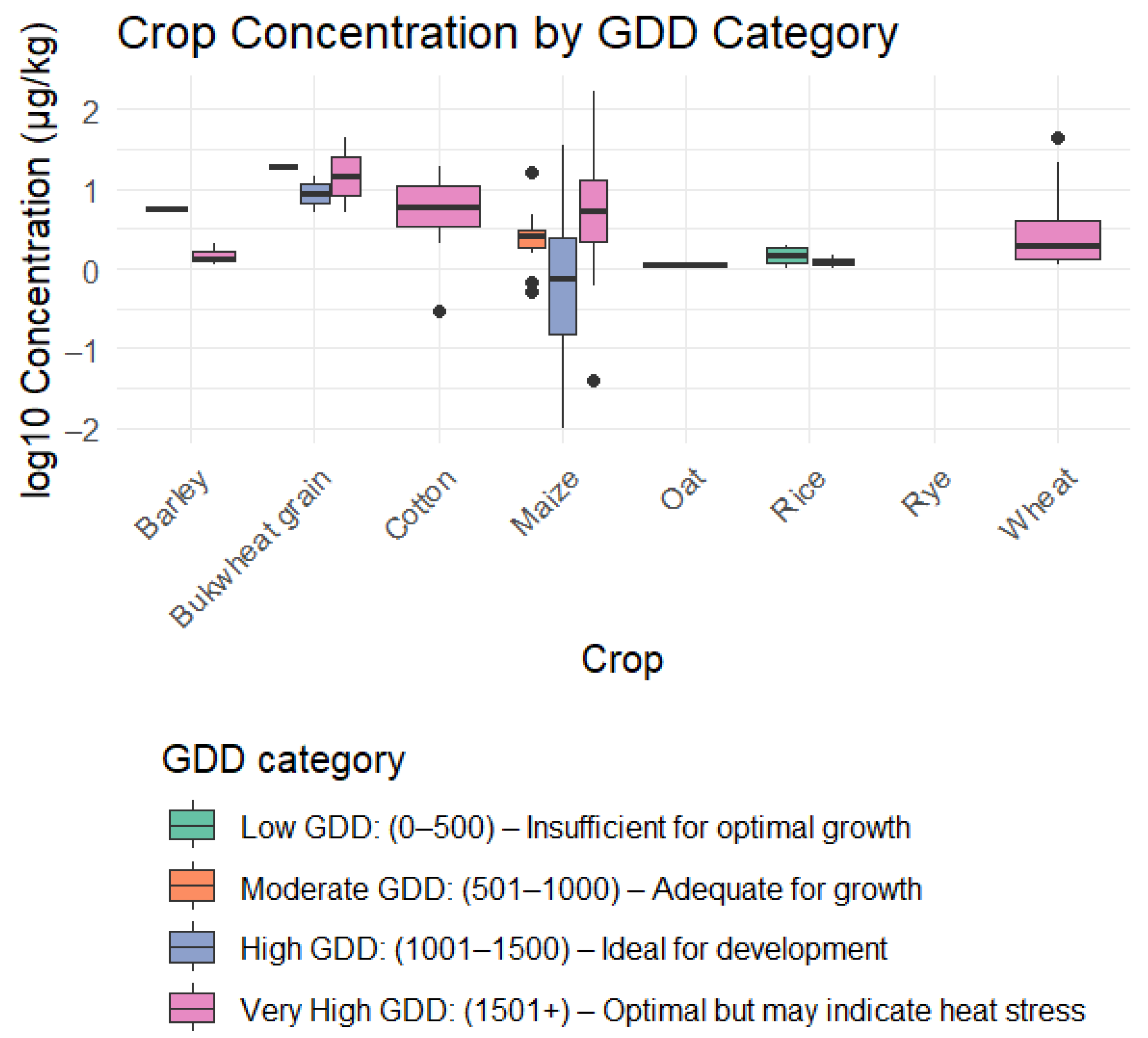
| Article | Crop | Weather Parameters | Contamination | ||
|---|---|---|---|---|---|
| Toxin | Rate 1 | Level 2 | |||
| Gallo et al., 2008 (Italy) [48] | Wheat | 2006: May was less rainy and much warmer than usual. June registered a thermal rise and low air humidity levels. | AF | 10 (100) | 1.1 (0.4–1.9) |
| Buyukunal et al., 2010 (Turkey) * [49] | Rice | Winter: −7 °C, 70.3% Relative Humidity | AF | 25 (100) | 1.9 (1.1–3.3) |
| Spring: 7.1 °C, 66.7% Relative Humidity | AF | 25 (32) | 1.7 (<LOD-3.0) | ||
| Summer: 21.9 °C, 47.4% Relative Humidity | AF | 25 (28) | 1.5 (<LOD-3.0) | ||
| Autumn: 9.8 °C, 57.8% Relative Humidity | AF | 25 (100) | 1.8 (0.1–3.2) | ||
| All year | AF | 100 (65) | 1.7 (<LOD-3.2) | ||
| Asselt et al., 2011 (The Netherlands) [50] | Maize | Growing degree-days (GDD) as the accumulation of the average daily temperature subtracted from a base temperature of 6 °C, below which maize seeds do not germinate. | AF | 42 (0) | <LOD |
| Pietri et al., 2012 (Italy) [51] | Maize | LR Summer: 22 °C, 41.93 mm | AFB1 | 66 (36) | 28.9 (<LOD-1254.1) |
| VR Summer: 22.6 °C, 50.6 mm | AFB1 | 34 (79) | 2.4 (<LOD-29.9) | ||
| P Summer: 20.4 °C, 60.7 mm | AFB1 | 4 (0) | <LOD | ||
| FV Summer: 21.3 °C, 122.1 mm | AFB1 | 33 (18) | 0.2 (<LOD-3.44) | ||
| E Summer: 23.1 °C, 23.9 mm | AFB1 | 60 (8) | 28.9 (<LOD-1254.1) | ||
| Tóth et al., 2012 (Hungary) [52] | Maize | 2010 Summer: Rainy | AF | NA (0) | <LOD |
| 2011 Summer: Dry and hot | AF | NA (0) | <LOD | ||
| Kos et al., 2013 (Serbia) [53] | Maize | 2012 maize growing season (April–September): extremely hot and dry conditions and drought. N Tmax 30 °C = 68 and 35 °C = 19/Number days precipitation = 39 | AF | 2009–2011: 60 (0) 2012: 200 (0) | 15% of samples had a range of 1–10. 24% from 10 to 50 and 29.5% from 50 to 90 |
| Pleadin et al., 2014 (Croatia) [28] | Maize | 2012: was extremely warm (>98%) and dry (<2%), characterized by a very low average rainfall. | AFB1 | NC: 460 (40) CC: 97 (28) EC: 633 (38) Total: 633 (38) | NC: 165 (1.2–2072) CC: 34 (1.1–1728) EC: 44 (1.3–945) Total: 81 (1.1–2072) |
| Alkadri et al., 2014 (Italy) [54] | Wheat | The northern Italian areas have warm, humid summers, with occasional rains compared with the southern part, which is hot and dry. | AF | 46 (0) | <LOD |
| Pleadin et al., 2015 (Croatia) [55] | Maize-M Wheat-W Barley-B, Oat-O | 2009, 2011 and 2013: the period of maize planting, growing, and harvesting (April–September) was very to (rarely) extremely warm with normal to scarce precipitation. 2010: was equally warm, but wet to highly wet. 2012: the maize growth and harvesting period (May, August) had warm weather and the lack of precipitation. | AFB1 | 2009–2013 M: 972 (31) W: 201 (7) B: 147 (6) O: 136 (5) | 2009–2013 M: 38.5 ± 75.7 W: 1.6 ± 1.7 B: 1.5 ± 1.2 O: 1.2 ± 0.8 |
| Leggieri et al., 2015 (Italy) * [29] | Maize | 2009: Average T in Celsius: 24.1 (21–26)/Humidity, in %: 65 (60–72)/Rain in days: 2.8 (1–6)/Rain in mm: 25.8 (4–59) | AFB1 | 46 (96) | 34.7 ± 115 |
| 2010: Average T in Celsius: 23.5 (22–26)/Humidity in %: 67.6 (59–75)/Rain in days: 4.8 (3–8)/Rain in mm: 76.3 (9–131) | AFB1 | 48 (77) | 15.9 ± 42.9 | ||
| 2011: Average T in Celsius: 23.2 (21–25)/Humidity in %: 66 (56–73)/Rain in days: 3.7 (0–7)/Rain in mm: 33.8 (0–92) | AFB1 | 46 (59) | 9.8 ± 48.2 | ||
| Janić Hajnal et al., 2017 (Serbia) * [56] | Maize | NWB 2015 (April–September): N Tmax > 25 °C = 96 and >35 °C = 12/sum Precipitation (mm) = 466 | AF | 32 (34) | 6.7 (1.3–28.1) |
| NNB 2015 (April–September): N Tmax > 25 °C = 104 and >35 °C = 23/sum Precipitation (mm) = 292 | AF | 25 (64) | 9.4 (1.4–33.8) | ||
| NSB 2015 (April–September): N Tmax > 25 °C = 105 and >35 °C = 26/sum Precipitation (mm) = 359 (0–92) | AF | 90 (64) | 11.6 (1.3–91.4) | ||
| CS 2015 (April–September): N Tmax > 25 °C = 110 and >35 °C = 25/sum Precipitation (mm) = 312 | AF | 33 (91) | 18.5 (1.4–86.3) | ||
| All regions 2015: one of the hottest and driest summers in the last ten years in Serbia. | AF | 180 (57) | 12.7 (1.3–91.4) | ||
| Kos et al., 2018 (Serbia) [57] | Maize | 2012 (April–September): Extreme drought/N Tmax > 30 °C = 63 and >35 °C = 18/sum P (mm) = 270 | AF | 600 (72) | 37.4 (1.0–111.2) |
| 2013 (April–September): Dry and hot/N Tmax > 30 °C = 37 and >35 °C = 8/sumP (mm) = 326 | AF | 600 (25) | 13.4 (1.2–65.2) | ||
| 2014 (April–September): Rainiest year/N Tmax > 30 °C = 14 and >35 °C = 0/sumP (mm) = 780 | AF | 600 (0) | <LOD | ||
| 2015 (April–September): Dry and hot/N Tmax > 30 °C = 53 and >35 °C = 14/sumP (mm) = 313 | AF | 600 (37) | 9.9 (1.1–76.2) | ||
| 2016 (April–September): Moderate weather/N Tmax > 30 °C = 25 and >35 °C = 1/sumP (mm) = 485 | AF | 600 (5) | 3.1 (1.3–6.9) | ||
| Bailly et al., 2018 (France) * [58] | Maize | 2015: hot and dry climatic conditions during summer (maize flowering period). | AF | 118 (6) | 20.2 (0.3–70) |
| Keriene et al., 2018 (Lithuania) [59] | Buckwheat grain | 2013: Temperature and amount of rainfall in July–August were close to the long-term average. | AFB1 | BBCH85: 12 (0) | BBCH85:<LOD |
| 2014: The amount of rainfall that fell in August (162.1 mm) was 70% higher than the long-term average. | AFB1 | BBCH85: 12 (100) BBCH8: 24 (100) | BBCH85: 14.9 (3.2–25.5) BBCH8: 5.1 (2.2–8.4) | ||
| 2015: spring was cold and dry. In June, only 14.1 mm of rainfall (5 times less than the long-term average). In July, rainfall amounted to 75.9 mm. In August, it was dry again. | AFB1 | BBCH77: 12 (100) BBCH85: 12 (100) BBCH89: 24 (100) | BBCH77: 43 (1.7–71.6) BBCH85: 19.1 (5.2–33.5) BBCH89: 4.9 (2.9–12.8) | ||
| Kos et al., 2020 (Serbia) * [60] | Maize | 2012 growing season: was characterized by the highest air temperatures and the lowest amount of precipitation compared to the other years investigated and the long-term average. | AFB1 | 51 (94) | 44 (0.6–205) |
| 2013 growing season: hot and dry weather conditions were dominant during most of the maize growing season. | AFB1 | 51 (33) | 8 (0.5–48) | ||
| 2014 growing season: was characterized by extreme high amount of precipitation. | AFB1 | 51 (0) | <LOD | ||
| 2015 growing season: Hot and dry weather conditions were recorded. | AFB1 | 51 (90) | 8 (0.4–41) | ||
| Leggieri et al., 2020 (Italy) [61] | Maize | COL: particularly low AI with the most arid conditions | AFB1 | 9 (44) | 0.275 (0.1–0.4) |
| LU: maximum monthly rain. Highest mean AI | AFB1 | 2 (50) | 34 (NA) | ||
| MI: highest mean AI with the widest variability | AFB1 | 8 (88) | 18.1 (0.4–93.8) | ||
| PE: mean AI close to 0 | AFB1 | 5 (80) | 3.3 (0.2–12.3) | ||
| ME: mean AI close to 0 | AFB1 | 2 (50) | 30.4 | ||
| FE: mean AI close to 0 | AFB1 | 8 (0) | <LOD | ||
| COP: mean AI close to 0 | AFB1 | 2 (50) | 3.7 | ||
| LU: mean AI close to 0 | AFB1 | 5 (40) | 2.2 (0.7–3.7) | ||
| MM: particularly low AI with the most arid conditions | AFB1 | 10 (30) | 0.67 (0.5–0.8) | ||
| Kifer et al., 2021 (Croatia) * [62] | Maize, Wheat Triticale, Oat, Barley | Gornji Stupnik-GS (control village): The yearly total precipitation was between 853.8 and 888.5 mm | AFB1 | 20 (0) | <LOD <LOD |
| Gunja-G (flooded village): The yearly total precipitation was between 642.7 and 785.5 mm | AFB1 | 20 (5) | 8.2 (NA) | ||
| Nikolic et al., 2021 (Serbia) * [63] | Maize hybrid (PK1,3,4,5,6) | 2019–2020: The mean monthly temperatures (˃20 °C), total monthly rainfall (>35 mm) and mean monthly relative humidity (RH) (˃50%) at the flowering stage (June) and the milk stage (July) were suitable for fungal maize colonization. | AF | For each year 2019—2020 for ZP & KR PK6:4 (0) | 2019—ZP: PK6: 3.0 (NA) 2019—KR: PK6: 4.6 (NA) 2020—ZP: PK6: 1.6 (NA) 2020—KR: PK6: < LOD |
| Ferrari et al., 2022 (Italy) [64] | Cotton (C), Maize flour (M) | In the present study, it was not possible to combine contamination levels with regional trends and climate patterns. According to Locatelli et al. (2022), 2015 and 2018 are the years in which the highest temperatures of the last 10 years were recorded in Po Valley. The year 2015 especially showed the harshest conditions, with high temperatures (23.46 °C), which were counterbalanced by low rainfall (155.62 mm) with respect to 2018 (209.83 mm). | AFB1 | For each year from 2013 to 2020 C: 480 (NA) M: 5278 (NA) | 2013: C: 4 (2–6); M: 3 (1.5–5) 2014: C: 5 (3–7.5); M: 3.5 (1.9–5.1) 2015: C: 14 (11.9–16.5); M: 15.6 (14–17) 2016: C:2.1 (0–4.5); M: 12.2 (11–14) 2017: C: 6.9 (4.8–9); M: 6.2 (4.8–8) 2018: C: 18.9 (16.5–21); M: 3.5 (2–5.2) 2019: C: 10 (7.8–12); M: 3.6 (2–5.2) 2020: C: 0.3 (0–2.8); M: 3 (1–4.9) |
| Mesterházy et al., 2022 (Hungary) [65] | Maize | The weather conditions are warmer and drier in southern counties. | AF | 2013: 2009 (NA) 2014: 4743 (NA) 2015: 5713 (NA) 2016: 2010 (NA) 2017: 2107 (NA) | 2013: 3.4 (NA) 2014: 1.1 (NA) 2015: 0.3 (NA) 2016: 0.3 (NA) 2017: 1.2 (NA) |
| Kovač et al., 2022 (Croatia) * [66] | Maize (M), Wheat (W), Barley (B), Rye (R). Oats (O) | 2016: May–June, normal to very warm temperature, normal to wet precipitation. August, normal precipitation and temperature. October, low temperatures and high amounts of precipitation | AF | M:61 (0) W:57 (0) B: 2 (0) | M: <LOD W: <LOD B: 5.5 (1.2–9.7) |
| 2017: May–June, normal to very low temperature, normal to wet precipitation. July—August, extremely high temperatures and drought in the southern regions. October, below-average precipitation. | AFB1 AF AF AF AF | M:23 (9) W:47 (2) B: 7 (0) R: 6 (0) O: 6 (0) | M: 0.5 (NA) W: NA B: <LOD R: <LOD O: <LOD | ||
| Molnár et al., 2023 (Hungary) [67] | Maize hybrid (FAO 370–390) | 2020: The rainiest growing season with +53.4 mm; the temperature never reached 35 °C. | AFB1 | NA | Non irrigated: 0.2 (0.0–1.3) Irrigated: 0.04 (0.0–0.2) |
| 2021: Extremely dry. Tmax > 35 °C = 3 in R4 during the growing season. | |||||
| 2022: The entire growing season was the most severe drought in the area for decades. | |||||
| Pleadin et al., 2023 (Croatia) [68] | Maize | 2018 April–September: N Tmax > 30 °C = 35 and >35 °C = 0/Number days precipitation = 45/SumP (mm) = 442 | AFB1 | 110 (14) | 6.2 (1.6–75.1) |
| 2019 April–September: N Tmax > 30 °C = 43 and >35 °C = 1/Number days precipitation = 53/SumP (mm) = 615 | AFB1 | 109 (16) | 2.5 (1.5–26.9) | ||
| 2020 April–September: N Tmax > 30 °C = 31 and >35 °C = 1/Number days precipitation = 47/SumP (mm) = 462 | AFB1 | 103 (19) | 1.6 (1.5–3.3) | ||
| 2021 April–September: N Tmax > 30 °C = 42 and >35 °C = 8/Number days precipitation = 42/SumP (mm) = 379 | AFB1 | 111 (40) | 34.1 (1.5–422.2) | ||
| Pleadin et al., 2023 (Serbia) * [68] | Maize | 2018 April–September: N Tmax > 30 °C = 42 and >35 °C = 0/Number days precipitation = 54/SumP (mm) = 382 | AF | 100 (8) | 8.1 (NA) |
| 2019 April–September: N Tmax > 30 °C = 42 and >35 °C = 0/Number days precipitation = 54/SumP (mm) = 382 | AF | 100 (11) | 3 (0.6–10.9) | ||
| 2020 April–September: N Tmax > 30 °C = 42 and >35 °C = 0/Number days precipitation = 54/SumP(mm) = 382 | AF | 100 (5) | 2.1 (1.1–3) | ||
| 2021 April–September: N Tmax > 30 °C = 42 and >35 °C = 0/Number days precipitation = 54/SumP (mm) = 382 | AF | 100 (84) | 38.8 (0.5–246.3) | ||
| Key Challenge | Future Research Direction | Actionable Recommendations |
|---|---|---|
| Geographic gaps remain | Conduct studies in underrepresented and climate-vulnerable European regions to improve risk mapping and develop localized mitigation strategies. | Expand geographic coverage of AF monitoring studies across diverse agroclimatic zones, especially in currently underrepresented regions. |
| Short study timelines limit insight | Implement long-term, high-resolution monitoring with frequent sampling intervals to capture seasonal dynamics and long-term climate trends. | Design multi-year studies with regular (e.g., weekly or monthly) sampling to assess temporal patterns and climate anomaly impacts. |
| Inconsistent and non-standardized data collection | Develop harmonized protocols for collecting and reporting environmental and contamination data to improve cross-study comparability. | Adopt standardized metrics for temperature, humidity, rainfall, and AF levels; ensure clear documentation of methods and data sources (see below). |
| Narrow crop coverage in studies | Expand research to include a broader range of crops, including feed crops and resistant hybrid varieties, across varying regions. | Include multiple crop types in AF risk assessments; evaluate the performance of resistant hybrids under diverse environmental conditions. |
| Lack of integration of agronomic and sampling details | Incorporate key agronomic variables and sampling protocols into study designs to strengthen model accuracy and field relevance. | Systematically collect data on sowing/harvest dates, soil conditions, irrigation, and farming practices; align with regulatory sampling standards. |
| Lack of consensus on essential monitoring variables | Establish a minimal dataset framework encompassing key climate, crop, sampling, and contamination variables for consistent monitoring. | Include in all studies (see Table S4):
|
| Inconsistent field study designs hinder comparability | Define best practices for field study designs, including duration, crop selection, and sampling frequency, to improve consistency and data utility. | Follow standardized guidelines for long-term, high-frequency sampling; incorporate both food and feed crops, including resistant varieties. |
| Fragmented and inaccessible data limit policy application | Promote the development of centralized, open-access databases with standardized metadata and transparent documentation to support integrative analysis. | Ensure data are stored in centralized repositories with clear metadata standards and open access for researchers, policymakers, and stakeholders. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bereziartua, A.; Huss, A.; Kers, J.G.; Smit, L.A.M.; Vermeulen, R.; Figueiredo, D.M. Pre-Harvest Aflatoxin Contamination in Crops and Climate Change Factors: A European Overview. Toxins 2025, 17, 344. https://doi.org/10.3390/toxins17070344
Bereziartua A, Huss A, Kers JG, Smit LAM, Vermeulen R, Figueiredo DM. Pre-Harvest Aflatoxin Contamination in Crops and Climate Change Factors: A European Overview. Toxins. 2025; 17(7):344. https://doi.org/10.3390/toxins17070344
Chicago/Turabian StyleBereziartua, Ainhoa, Anke Huss, Jannigje G. Kers, Lidwien A. M. Smit, Roel Vermeulen, and Daniel Martins Figueiredo. 2025. "Pre-Harvest Aflatoxin Contamination in Crops and Climate Change Factors: A European Overview" Toxins 17, no. 7: 344. https://doi.org/10.3390/toxins17070344
APA StyleBereziartua, A., Huss, A., Kers, J. G., Smit, L. A. M., Vermeulen, R., & Figueiredo, D. M. (2025). Pre-Harvest Aflatoxin Contamination in Crops and Climate Change Factors: A European Overview. Toxins, 17(7), 344. https://doi.org/10.3390/toxins17070344






