Associations Between Uraemic Toxins and Gut Microbiota in Adults Initiating Peritoneal Dialysis
Abstract
1. Introduction
2. Results
2.1. Participant Characteristics
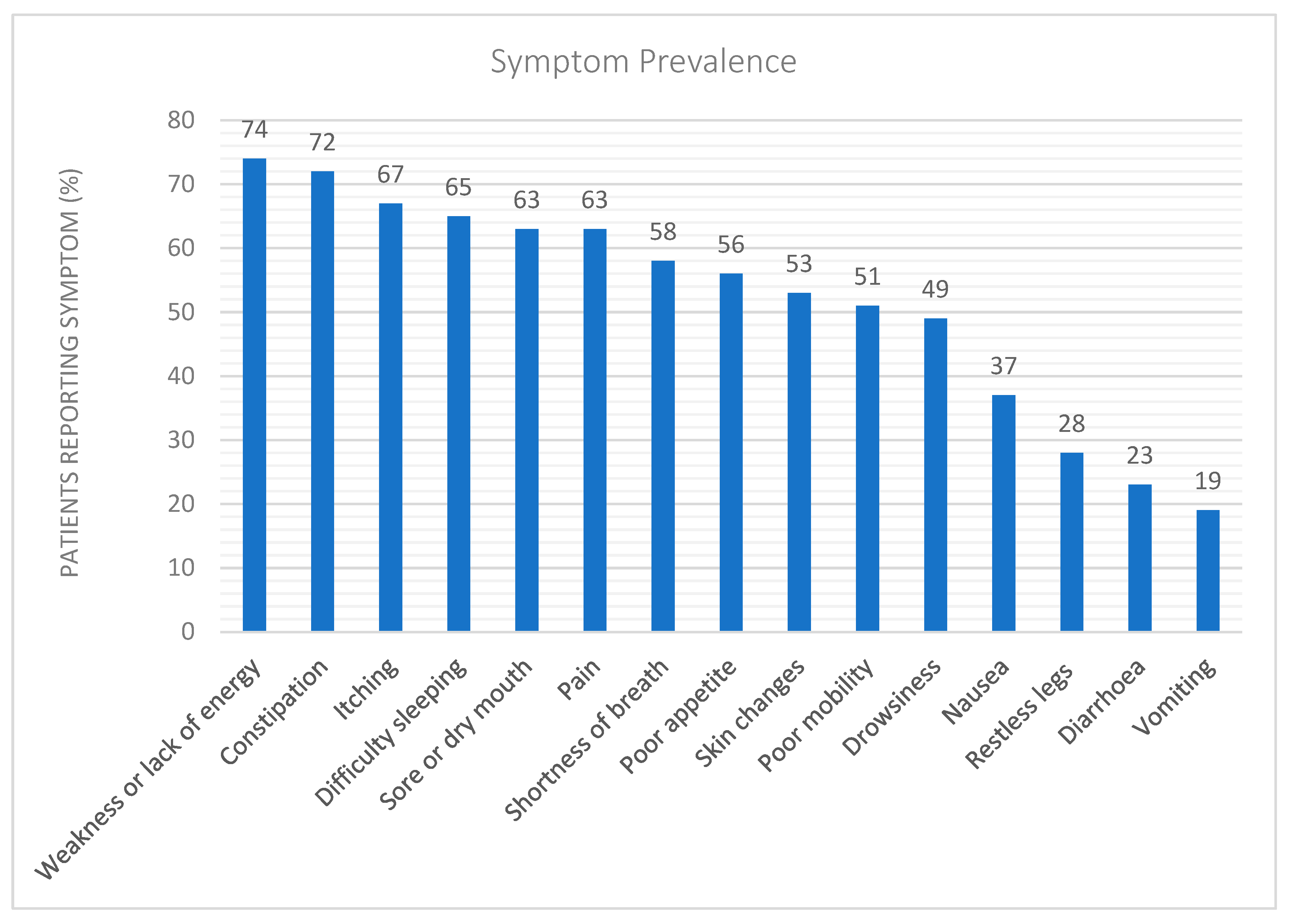
2.2. Uraemic Symptoms
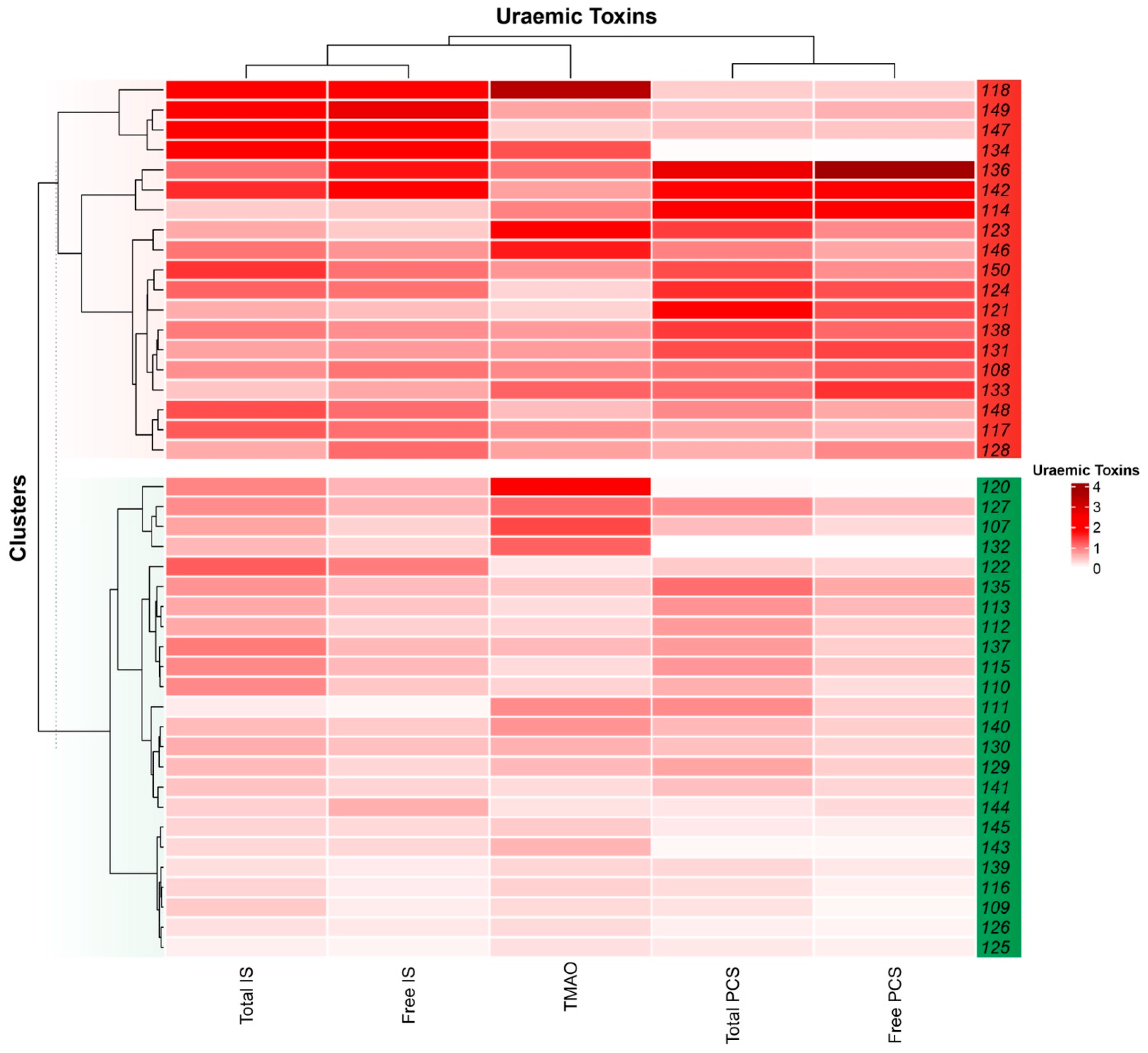
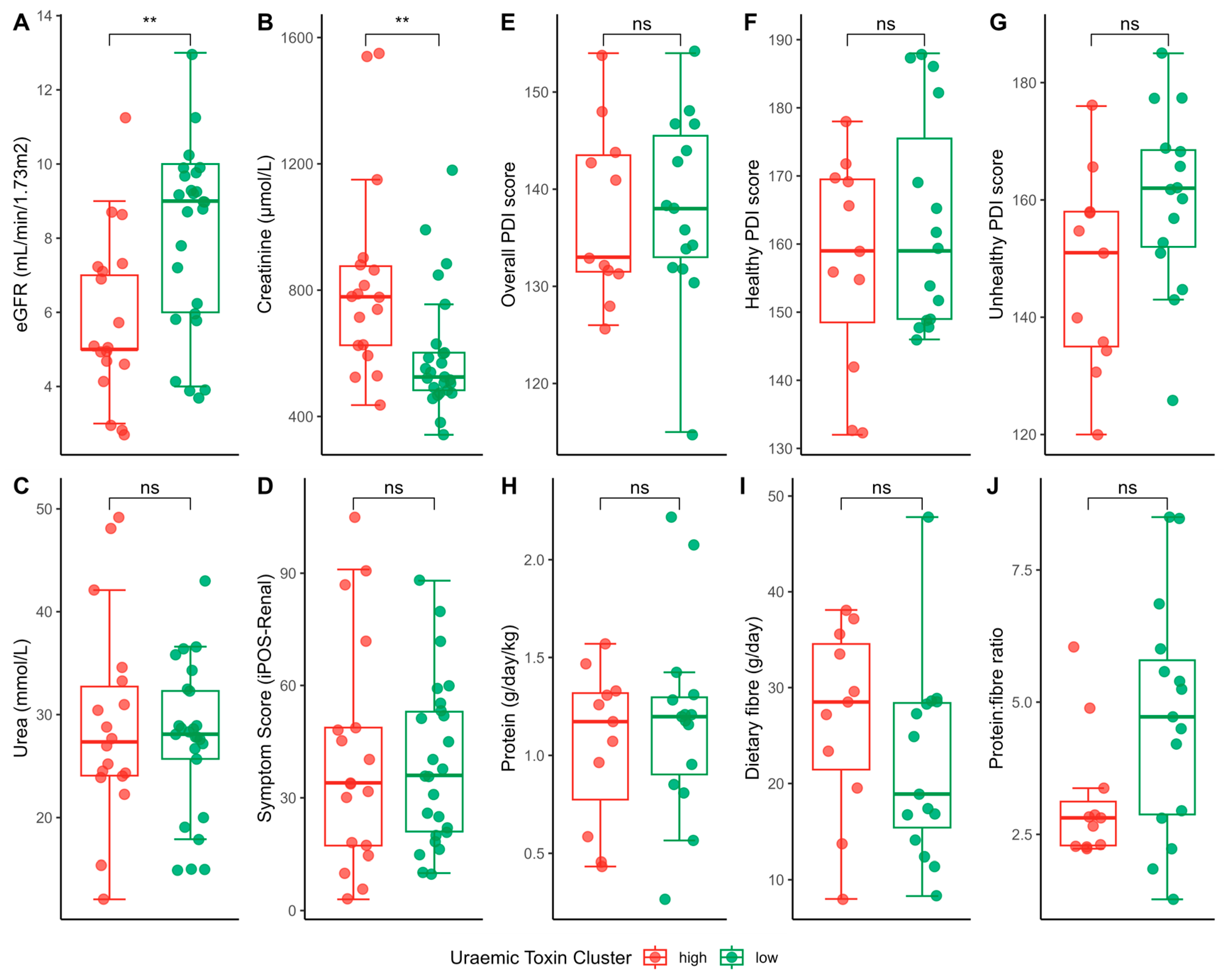
2.3. Uraemic Toxin Phenotype
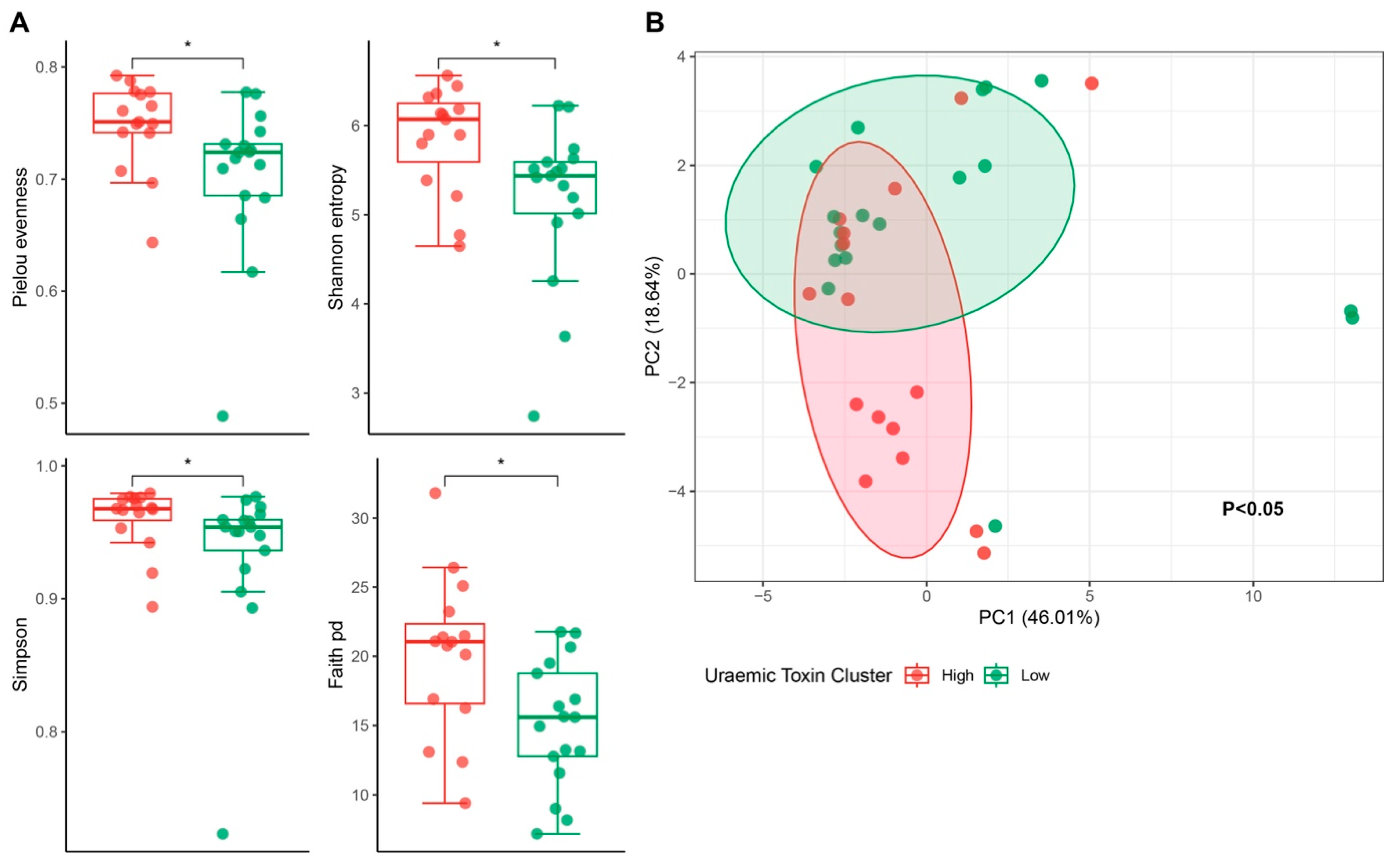
2.4. Uraemic Toxin Phenotype and the Gut Microbiota
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Sample and Data Collection
4.3. Sample and Diet Analysis
4.3.1. Microbial DNA Analysis
4.3.2. Uraemic Toxin Analysis
4.3.3. Dietary Analysis
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CKD | Chronic kidney disease |
| PCS | P-cresyl sulfate |
| IS | Indoxyl sulfate |
| TMAO | Trimethyl-amine-N-oxide |
| PD | Peritoneal dialysis |
| SD | Standard deviation |
| eGFR | Estimated glomerular filtration rate |
| ADPCKD | Autosomal dominant polycystic kidney disease |
| IQR | Interquartile range |
| RR | Reference range |
| BMI | Body mass index |
| PDI | Plant-based diet index |
| PERMANOVA | Permutational multivariate analysis of variance |
| hPDI | healthful PDI |
| uPDI | unhealthful PDI |
| UPLC | Ultra Performance Liquid chromatography |
| PCA | Principal Component Analysis |
Appendix A
Supplementary Methods
References
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global prevalence of chronic kidney disease—A systematic review and meta-analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed]
- Al Khodor, S.; Shatat, I.F. Gut microbiome and kidney disease: A bidirectional relationship. Pediatr. Nephrol. 2017, 32, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Almutary, H.; Bonner, A.; Douglas, C. Symptom burden in chronic kidney disease: A review of recent literature. J. Ren. Care 2013, 39, 140–150. [Google Scholar] [CrossRef]
- Meyer, T.W.; Hostetter, T.H. Uremia. N. Engl. J. Med. 2007, 357, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Corradi, V.; Caprara, C.; Barzon, E.; Mattarollo, C.; Zanetti, F.; Ferrari, F.; Husain-Syed, F.; Giavarina, D.; Ronco, C.; Zanella, M. A possible role of P-Cresyl Sulfate and Indoxyl Sulfate as biomarkers in the prediction of renal function according to the GFR (G) categories. Integr. Med. Nephrol. Androl. 2024, 11, e24-00002. [Google Scholar] [CrossRef]
- Armani, R.G.; Ramezani, A.; Yasir, A.; Sharama, S.; Canziani, M.E.F.; Raj, D.S. Gut microbiome in chronic kidney disease. Curr. Hypertens. Rep. 2017, 19, 29. [Google Scholar] [CrossRef]
- Briskey, D.; Tucker, P.; Johnson, D.W.; Coombes, J.S. The role of the gastrointestinal tract and microbiota on uremic toxins and chronic kidney disease development. Clin. Exp. Nephrol. 2017, 21, 7–15. [Google Scholar] [CrossRef]
- Onal, E.M.; Afsar, B.; Covic, A.; Vaziri, N.D.; Kanbay, M. Gut microbiota and inflammation in chronic kidney disease and their roles in the development of cardiovascular disease. Hypertens. Res. 2019, 42, 123–140. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.-H.; Andersen, G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef]
- Anders, H.-J.; Andersen, K.; Stecher, B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013, 83, 1010–1016. [Google Scholar] [CrossRef]
- Hobby, G.P.; Karaduta, O.; Dusio, G.F.; Singh, M.; Zybailov, B.L.; Arthur, J.M. Chronic kidney disease and the gut microbiome. Am. J. Physiol. Ren. Physiol. 2019, 316, F1211–F1217. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, A.; Massy, Z.A.; Meijers, B.; Evenepoel, P.; Vanholder, R.; Raj, D.S. Role of the gut microbiome in uremia: A potential therapeutic target. Am. J. Kidney Dis. 2016, 67, 483–498. [Google Scholar] [CrossRef]
- Massy, Z.A.; Chesnaye, N.C.; Larabi, I.A.; Dekker, F.W.; Evans, M.; Caskey, F.J.; Torino, C.; Porto, G.; Szymczak, M.; Drechsler, C. The relationship between uremic toxins and symptoms in older men and women with advanced chronic kidney disease. Clin. Kidney J. 2022, 15, 798–807. [Google Scholar] [CrossRef]
- Wang, C.-P.; Lu, Y.-C.; Tsai, I.-T.; Tang, W.-H.; Hsu, C.-C.; Hung, W.-C.; Yu, T.-H.; Chen, S.-C.; Chung, F.-M.; Lee, Y.-J. Increased levels of total p-cresylsulfate are associated with pruritus in patients with chronic kidney disease. Dermatology 2016, 232, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.-W.; Hsu, K.-H.; Lee, C.-C.; Sun, C.-Y.; Hsu, H.-J.; Tsai, C.-J.; Tzen, C.-Y.; Wang, Y.-C.; Lin, C.-Y.; Wu, M.-S. p-cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol. Dial. Transpl. 2011, 26, 938–947. [Google Scholar] [CrossRef]
- Bammens, B.; Evenepoel, P.; Keuleers, H.; Verbeke, K.; Vanrenterghem, Y. Free serum concentrations of the protein-bound retention solute p-cresyl predict mortality in hemodialysis patients. Kidney Int. 2006, 69, 1081–1087. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef]
- Evenepoel, P.; Poesen, R.; Meijers, B. The gut-kidney axis. Pediatr. Nephrol. 2017, 32, 2005–2014. [Google Scholar] [CrossRef] [PubMed]
- Dhondt, A.; Vanholder, R.; Van Biesen, W.; Lameire, N. The removal of uremic toxins. Kidney Int. 2000, 58 (Suppl. S76), S47–S59. [Google Scholar] [CrossRef]
- Leeming, E.R.; Louca, P.; Gibson, R.; Menni, C.; Spector, T.D.; Le Roy, C.I. The complexities of the diet-microbiome relationship: Advances and perspectives. Genome Med. 2021, 13, 10. [Google Scholar] [CrossRef]
- Pretorius, C.J.; McWhinney, B.C.; Sipinkoski, B.; Johnson, L.A.; Rossi, M.; Campbell, K.L.; Ungerer, J.P.J. Reference ranges and biological variation of free and total serum indoxyl- and p-cresyl sulphate measured with rapid UPLC fluorescence detection method. Clin. Chim. Acta 2013, 419, 122–136. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council; Australian Government Department of Health and Ageing; New Zealand Ministry of Health. Nutrient Reference Values for Australia and New Zealand; National Health and Medical Research Council: Canberra, Australia, 2006.
- Gessner, A.; di Giuseppe, R.; Koch, M.; Fromm, M.F.; Lieb, W.; Maas, R. Trimethylamine-N-oxide (TMAO) determined by LC-MS/MS: Distribution and correlates in the population-based PopGen cohort. Clin. Chem. Lab. Med. 2020, 58, 733–740. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council. Australian Dietary Guidelines Summary; National Health and Medical Research Council: Canberra, Australia, 2013.
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Kriss, M.; Hazleton, K.Z.; Nusbacher, N.M.; Martin, C.G.; Lozupone, C.A. Low diversity gut microbiota dysbiosis: Drivers, functional implications and recovery. Curr. Opin. Microbiol. 2018, 44, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- Stanford, J.; Charlton, K.; Stefoska-Needham, A.; Zheng, H.; Bird, L.; Borst, A.; Fuller, A.; Lambert, K. Associations among plant-based diet quality, uremic toxins, and gut microbiota profile in adults undergoing haemodialysis therapy. J Ren Nutr 2021, 31, 177–188. [Google Scholar] [CrossRef]
- Gryp, T.; Vanholder, R.; Vaneechoutte, M. p-Cresyl Sulfate. Toxins 2017, 9, 52. [Google Scholar] [CrossRef]
- Hu, X.; Ouyang, S.; Xie, Y.; Gong, Z.; Du, J. Characterizing the gut microbiota in patients with chronic kidney disease. Postgrad. Med. 2020, 132, 495–505. [Google Scholar] [CrossRef]
- Liu, W.; Huang, J.; Liu, T.; Hu, Y.; Shi, K.; Zhou, Y.; Zhang, N. Changes in gut microbial community upon chronic kidney disease. PLoS ONE 2023, 18, e0283389. [Google Scholar] [CrossRef]
- Jiang, S.; Xie, S.; Lv, D.; Wang, P.; He, H.; Zhang, T.; Zhou, Y.; Lin, Q.; Zhou, H.; Jiang, J. Alteration of the gut microbiota in Chinese population with chronic kidney disease. Sci. Rep. 2017, 7, 2870. [Google Scholar] [CrossRef]
- Li, X.-J.; Shan, Q.-Y.; Wu, X.; Miao, H.; Zhao, Y.-Y. Gut microbiota regulates oxidative stress and inflammation: A double-edged sword in renal fibrosis. Cell. Mol. Life Sci. 2024, 81, 480. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Liu, F.; Wang, Y.-N.; Yu, X.-Y.; Zhuang, S.; Guo, Y.; Vaziri, N.D.; Ma, S.-X.; Su, W.; Shang, Y.-Q.; et al. Targeting Lactobacillus johnsonii to reverse chronic kidney disease. Signal Transduct. Target. Ther. 2024, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Jager, K.J.; Ocak, G.; Drechsler, C.; Caskey, F.J.; Evans, M.; Postorino, M.; Dekker, F.W.; Wanner, C. The EQUAL study: A European study in chronic kidney disease stage 4 patients. Nephrol. Dial. Transpl. Transplant. 2012, 27 (Suppl. S3), iii27–iii31. [Google Scholar] [CrossRef]
- Świerczyńska-Mróz, K.; Nowicka-Suszko, D.; Fleszar, M.G.; Fortuna, P.; Krajewski, P.K.; Krajewska, M.; Białynicki-Birula, R.; Szepietowski, J.C. Serum level of protein-bound uraemic toxins in haemodialysis patients with chronic kidney disease-associated pruritus: Myths and facts. J. Clin. Med. 2023, 12, 2310. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, P.; Żebrowska-Różańska, P.; Kujawa, D.; Łaczmański, Ł.; Samochowiec, J.; Jabłoński, M.; Plichta, P.; Piotrowski, P.; Bielawski, T.; Misiak, B. Gut microbiota alterations in schizophrenia might be related to stress exposure: Findings from the machine learning analysis. Psychoneuroendocrinology 2023, 155, 106335. [Google Scholar] [CrossRef]
- Borkent, J.; Ioannou, M.; Laman, J.D.; Haarman, B.C.M.; Sommer, I.E.C. Role of the gut microbiome in three major psychiatric disorders. Psychol. Med. 2022, 52, 1222–1242. [Google Scholar] [CrossRef]
- Chen, L.; Shi, J.; Ma, X.; Shi, D.; Qu, H. Effects of microbiota-driven therapy on circulating indoxyl sulfate and P-cresyl sulfate in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Adv. Nutr. 2022, 13, 1267–1278. [Google Scholar] [CrossRef]
- Yang, H.-L.; Feng, P.; Xu, Y.; Hou, Y.-Y.; Ojo, O.; Wang, X.-H. The role of dietary fibre supplementation in regulating uremic toxins in patients with chronic kidney disease: A meta-analysis of randomized controlled trials. J. Ren. Nutr. 2021, 31, 438–447. [Google Scholar] [CrossRef]
- Liu, C.; Yang, L.; Wei, W.; Fu, P. Efficacy of probiotics/synbiotics supplementation in patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Front. Nutr. 2024, 11, 1434613. [Google Scholar] [CrossRef]
- Wu, M.; Cai, X.; Lin, J.; Zhang, X.; Scott, E.M.; Li, X. Association between fibre intake and indoxyl sulphate/P-cresyl sulphate in patients with chronic kidney disease: Meta-analysis and systematic review of experimental studies. Clin. Nutr. 2019, 38, 2016–2022. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Mirrahimi, A.; Sievenpiper, J.L.; Jenkins, D.J.A.; Darling, P.B. Dietary fibre effects in chronic kidney disease: A systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr. 2015, 69, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Johnson, D.W.; Xu, H.; Carrero, J.J.; Pascoe, E.; French, C.; Campbell, K.L. Dietary protein-fibre ratio associates with circulating levels of indoxyl sulfate and p-cresyl sulfate in chronic kidney disease patients. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 860–865. [Google Scholar] [CrossRef]
- Satija, A.; Bhupathiraju, S.N.; Spiegelman, D.; Chiuve, S.E.; Manson, J.E.; Willett, W.; Rexrode, K.M.; Rimm, E.B.; Hu, F.B. Healthful and unhealthful plant-based diets and the risk of coronary heart disease in U.S. adults. J. Am. Coll. Cardiol. 2017, 70, 411–422. [Google Scholar] [CrossRef]
- McFarlane, C.; Krishnasamy, R.; Stanton, T.; Savill, E.; Snelson, M.; Mihala, G.; Morrison, M.; Johnson, D.W.; Campbell, K.L. Diet quality and protein-bound uraemic toxins: Investigation of novel risk factors and the role of microbiome in chronic kidney disease. J. Ren. Nutr. 2022, 32, 542–551. [Google Scholar] [CrossRef] [PubMed]
- DNA/RNA Shield Fecal Collection Kit. Available online: https://zymoresearch.eu/products/dna-rna-shield-fecal-collection-kit?srsltid=AfmBOoqEf1OBPyx3nchAipzlFpvGcP856_Xh5gU0yUz1jOu8jgBNun7b (accessed on 5 June 2025).
- Czaja-Stolc, S.; Potrykus, M.; Ruszkowski, J.; Styburski, D.; Dębska-Ślizień, A.; Małgorzewicz, S. The associations between nutrition and circulating gut microbiota-derived uremic toxins in patients undergoing kidney replacement therapy: An observational, cross-sectional study. Clin. Nutr. ESPEN 2025, 65, 105–114. [Google Scholar] [CrossRef]
- Puig-Castellví, F.; Pacheco-Tapia, R.; Deslande, M.; Jia, M.; Andrikopoulos, P.; Chechi, K.; Bonnefond, A.; Froguel, P.; Dumas, M.-E. Advances in the integration of metabolomics and metagenomics for human gut microbiome and their clinical applications. Trends Anal. Chem. 2023, 167, 117248. [Google Scholar] [CrossRef]
- Krukowski, H.; Valkenburg, S.; Madella, A.-M.; Garssen, J.; van Bergenhenegouwen, J.; Overbeek, S.A.; Huys, G.R.B.; Raes, J.; Glorieux, G. Gut microbiome studies in CKD: Opportunities, pitfalls and therapeutic potential. Nat. Rev. Nephrol. 2023, 19, 87–101. [Google Scholar] [CrossRef]
- Stanford, J.; McMahon, S.; Lambert, K.; Charlton, K.E.; Stefoska-Needham, A. Expansion of an Australian food composition database to estimate plant and animal intakes. Br. J. Nutr. 2023, 130, 1950–1960. [Google Scholar] [CrossRef]
- Stanford, J.; Stefoska-Needham, A.; Lambert, K.; Batterham, M.J.; Charlton, K. Association between plant-based diet quality and chronic kidney disease in Australian adults. Public Health Nutr. 2024, 27, e142. [Google Scholar] [CrossRef]
- Lambert, K.; Ryan, M.; Flanagan, J.; Broinowski, G.; Nicdao, M.; Stanford, J.; Chau, K. Dietary patterns, dietary adequacy and nutrient intake in adults commencing peritoneal dialysis: Outcomes from a longitudinal cohort study. Nutrients 2024, 16, 663. [Google Scholar] [CrossRef]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Community Ecology Package, version 2.6-4; CRAN: Vienna, Austria, 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 3 January 2025).

| Baseline Demographic Data | ||
|---|---|---|
| N | % | |
| Sex | ||
| Male | 30 | 70 |
| Female | 13 | 30 |
| Age (yrs) | ||
| 21–40 | 5 | 11.6 |
| 41–60 | 12 | 27.9 |
| 61–80 | 26 | 60.4 |
| Primary Renal Disease | ||
| Diabetes mellitus | 22 | 51.2 |
| Hypertension | 12 | 27.9 |
| ADPCKD | 3 | 7.0 |
| Glomerulonephritis | 10 | 23.3 |
| Other | 8 | 18.6 |
| Multiple | 10 | 23.3 |
| Comorbidities | ||
| Hypertension | 35 | 81.4 |
| Ischemic Heart Disease | 14 | 32.6 |
| Diabetes mellitus | 25 | 58.1 |
| Hypercholesterolaemia | 18 | 41.9 |
| Gout | 13 | 30.2 |
| Antibiotics in the Previous 12 Months | ||
| Yes | 38 | 88.4 |
| No | 5 | 11.6 |
| Cephalosporin | 37 | 86.0 |
| Penicillin | 14 | 32.6 |
| Sulfamethoxazole-Trimethoprim | 4 | 9.3 |
| Ciprofloxacin | 3 | 7.0 |
| Doxycycline | 3 | 7.0 |
| Metronidazole | 3 | 7.0 |
| Vancomycin | 2 | 4.7 |
| Regular Medications | ||
| Total (median, IQR) | 11 | 8–15 |
| Proton pump inhibitor | 17 | 38.6 |
| Calcium-based phosphate binder | 27 | 62.8 |
| Non-calcium phosphate binder | 11 | 25 |
| Erythropoietin | 25 | 58.1 |
| Serum Biochemistry at Commencement of PD | ||
| Creatinine (Median, IQR) | 599 | 512–802 |
| Urea (Median, IQR) | 28 | 24–32 |
| Serum Uraemic Toxin Concentration (Median, IQR) | ||
| TMAO (RR: 1.28–19.67 µmol/L) [23] | 48.0 | 22.8–73.3 |
| Total IS (RR: 0.70–6.30 µmol/L) [21] | 49.0 | 26.5–71.5 |
| Free IS (RR: 0.0–0.19 µmol/L) [21] | 3.9 | 1.3–6.6 |
| Total PCS (RR: 0.0–38.4 µmol/L) [21] | 97.0 | 39.5–154.5 |
| Free PCS (RR: 0.14–2.44 µmol/L) [21] | 3.1 | 0.8–5.5 |
| Weight (kg) | 78 | 67–91 |
| BMI (kg/m2) | 27 | 23–31 |
| Median (IQR) | Reference Range [22,24] | |
|---|---|---|
| Macronutrients | ||
| Energy (kJ/day) | 8017 (6404–9421) | |
| Energy (kJ/kg/day) | 112 (80–127) | 9500–12,100 |
| Protein (g/day) | 83 (64–103) | 125–159 |
| Protein (g/kg/day) | 1.2 (0.9–1.3) | 52–64 |
| Fat (g/day) | 79 (50–92) | 0.7–0.8 |
| Saturated fat (g/day) | 22 (17–38) | |
| Carbohydrate (g/day) | 180 (148–260) | |
| Micronutrients | ||
| Phosphorus (mg) | 1237 (1075–1585) | <1000 |
| Potassium (mg) | 2703 (2388–3469) | 2800–3800 |
| Sodium (mg) | 1724 (1120–2440) | <2300 |
| Calcium (mg) | 638 (408–925) | 1000–1300 |
| Zinc (mg) | 9 (6–12) | 8–14 |
| Vitamin B6 (mg) | 1.4 (1.0–2) | 1.5–1.7 |
| Vitamin C (mg) | 88 (44–136) | 45 |
| Fibre (g) | 25 (17–29) | 25–35 |
| % kJ from fibre | 2.2 (1.7–2.8) | |
| % kJ from carbohydrate | 43 (39–49) | 45–65 |
| Core Food Groups | ||
| Grain serves | 6 (4–8) | 4–6 |
| Fruit serves | 0.9 (0.5–1.8) | 2 |
| Vegetables serves | 4 (2–5) | 5.0–5.5 |
| Dairy serves | 1.1 (0.6–1.5) | ≤1 |
| Meat/alternative serves | 2.5 (1.6–2.9) | 2.0–2.5 |
| Alcohol standard serves | 0 (0–0) | <2 |
| Added sugar (g) | 15 (4–34) | <36 |
| Diet Quality Indices (Range of possible scores: PDI: 46–230; Healthy PDI: 53–265; Unhealthy PDI: 51–225) | ||
| Plant-Based Diet Index | 138 (10) | |
| Healthy PDI | 160 (14) | |
| Unhealthy PDI | 155 (16) | |
| Genera | Log2 Fold Change | SE | p-Value | Adjusted p-Value |
|---|---|---|---|---|
| Catenibacterium | 9.43 | 2.08 | <0.01 | <0.01 |
| Prevotella | 6.20 | 1.46 | <0.01 | <0.01 |
| Clostridia | 7.81 | 2.25 | <0.01 | 0.02 |
| Lachnospiraceae UCG-004 | 4.05 | 1.22 | <0.01 | 0.03 |
| Christensenellaceae R-7 | 3.43 | 1.14 | <0.01 | 0.07 |
| Ruminococcus gnavus | −3.83 | 1.35 | <0.01 | 0.09 |
| Eubacterium coprostanoligenes | 3.32 | 1.22 | 0.01 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
James, P.; Stanford, J.; Dixit, O.V.A.; Nicdao, M.A.; McWhinney, B.; Sud, K.; Ryan, M.; Read, S.; Ahlenstiel, G.; Lambert, K.; et al. Associations Between Uraemic Toxins and Gut Microbiota in Adults Initiating Peritoneal Dialysis. Toxins 2025, 17, 334. https://doi.org/10.3390/toxins17070334
James P, Stanford J, Dixit OVA, Nicdao MA, McWhinney B, Sud K, Ryan M, Read S, Ahlenstiel G, Lambert K, et al. Associations Between Uraemic Toxins and Gut Microbiota in Adults Initiating Peritoneal Dialysis. Toxins. 2025; 17(7):334. https://doi.org/10.3390/toxins17070334
Chicago/Turabian StyleJames, Philippa, Jordan Stanford, Ojas V. A. Dixit, Mary Ann Nicdao, Brett McWhinney, Kamal Sud, Michele Ryan, Scott Read, Golo Ahlenstiel, Kelly Lambert, and et al. 2025. "Associations Between Uraemic Toxins and Gut Microbiota in Adults Initiating Peritoneal Dialysis" Toxins 17, no. 7: 334. https://doi.org/10.3390/toxins17070334
APA StyleJames, P., Stanford, J., Dixit, O. V. A., Nicdao, M. A., McWhinney, B., Sud, K., Ryan, M., Read, S., Ahlenstiel, G., Lambert, K., O’Brien, C., & Chau, K. (2025). Associations Between Uraemic Toxins and Gut Microbiota in Adults Initiating Peritoneal Dialysis. Toxins, 17(7), 334. https://doi.org/10.3390/toxins17070334





