Comparing Computational Peritoneal Dialysis Models in Pigs and Patients
Abstract
1. Introduction
2. Methods
2.1. Data Collection
2.2. Model Descriptions
2.3. Model Fitting Procedure
2.3.1. Fitted Models: UGM and UGM-18
- Load subject-specific data: The instilled and drained intraperitoneal volumes were taken from the data. The initial/final intraperitoneal volume was calculated as the sum of the instilled/drained volume and residual volumes. Residual volumes were calculated from the dilution of albumin for the pig data (or total protein if albumin was not available) or total protein for human data using the concentrations in the drained fluid and the new dwell just after instillment of the fresh dialysate. In PETs where no dextran was measured (all human PETs and 1 PET in pigs), the intraperitoneal volume, , was linearly interpolated from the initial and final values. We know this may differ from the actual volumes, but since low glucose concentrations were used experimentally in the pigs, we assumed that a linear interpolation would not introduce many errors. We used the same interpolation for humans, where 3.86% glucose was mostly used, which could lead to an underestimation of the volume at the beginning of the dwell, leading to an underestimation of solute removal. However, for TPM, we used a dynamic fluid model, which we also fitted by using the following parameter: lymphatic flow rate, . The plasma solute concentrations, , values were taken from the data and interpolated between sampling points for pigs and assumed to be constant for humans.
- Initialize optimization parameters and bounds: To run the fitting program, the simulation was started with an initial estimation of the fitting parameter, and the bounds of the parameter were set to encompass the variability of the parameter found in the literature. For example, in UGM in pigs, 11 parameters were fitted (6 MTACs and 5 sieving coefficients (SiCo)—SiCo for glucose in UGM is 0). We would initialize as 10 mL/min and set the bounds of this parameter between 0 and 200 mL/min, which cover the known ranges of urea MTAC (only for TPM, UGM, and UGM-18).
- Minimize the root mean square error: Our aim is to determine which model could predict the dialysate concentration for humans and pigs. In order to compare the predictions for the four/six solutes across the models, we fitted model-specific parameters in each case to compare the normalized solute concentration error, (Equation (3)), between the predicted and measured dialysate concentrations.
- Steps 2–3 were repeated 10 times with different initializations (randomization of MTACs) to find the global minima. The minimization in step 3 is performed with Python 3.9 using Sequential Least Squares Programming (SLSQP). SLSQP is an optimization algorithm that is widely used for solving nonlinear optimization problems with both equality and inequality constraints. It employs a sequence of quadratic programming subproblems, optimizing a function by iteratively approximating it as a quadratic function and adjusting the variables to meet the constraints.
- Calculate the normalized solute-specific concentration error (SSE): We checked the error in concentration determination per solute along with the to assess both the individual solute fits and the overall accuracy of the model.
2.3.2. Fitted Model: TPM
2.3.3. Derived Models: GM and SWM
2.3.4. Derived Model: WM
- Calculation of and SSE
2.4. Ultrafiltration Calculations
3. Results
3.1. Concentration Predictions for Specific Subjects
3.2. Performance of Various Models of Peritoneal Dialysis
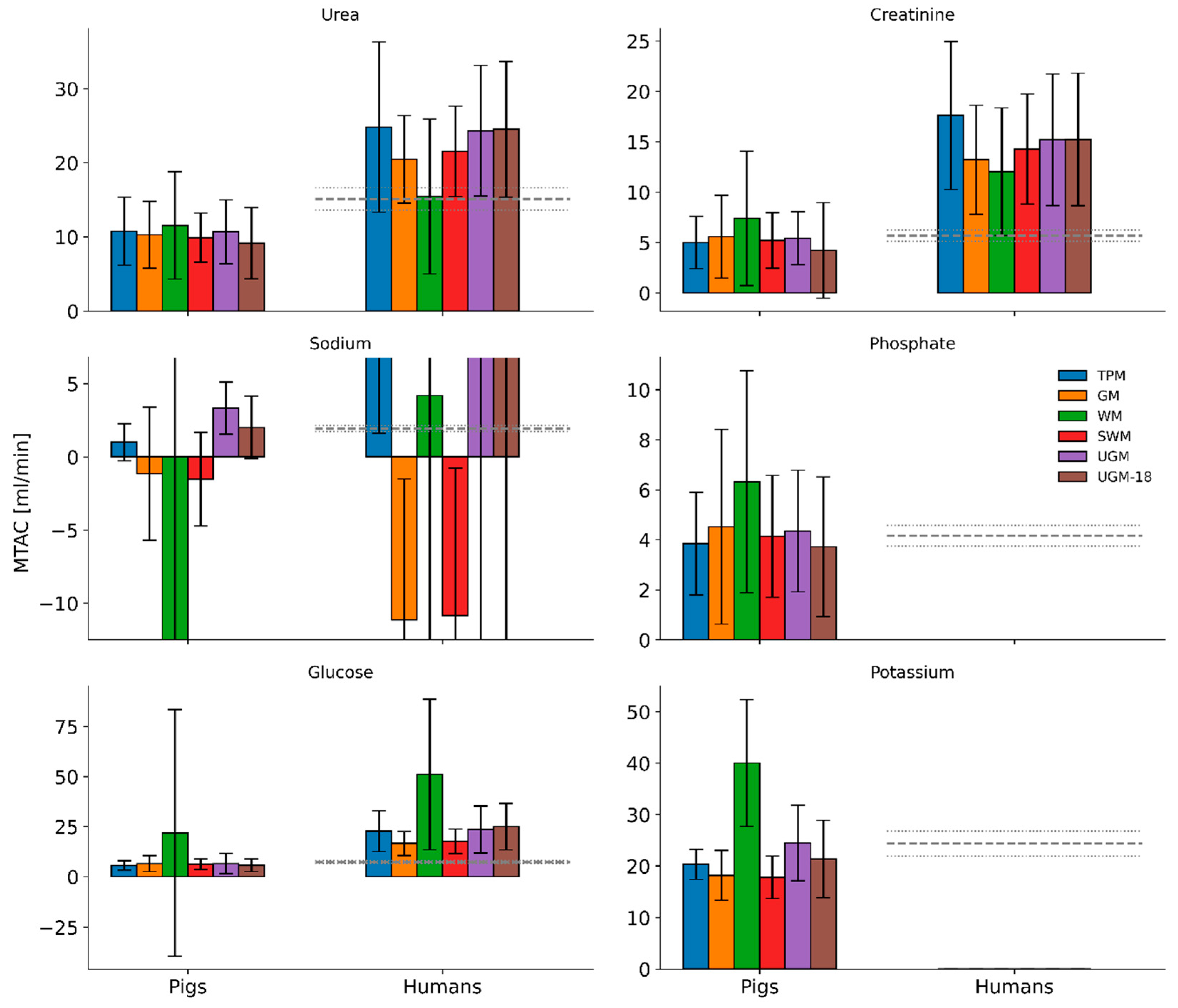
3.3. Population Average of Predicted MTACs in Pigs and Humans
3.4. Prediction of Ultrafiltration Volumes and Lymphatic Flow Rates in Pigs and Humans
4. Discussion
- (a)
- Rigorously check if the models were accurate ( and SSE) and efficient (computational time) overall.
- (b)
- Rigorously check to determine how physical complexity helped in detailing the solute kinetics and how that affected the efficiency.
- (c)
- Apply TPM for the first time to pigs, thereby enabling the analysis of the differences between pigs and humans in conventional PD and peritoneal membrane characteristics.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| HD | Hemodialysis |
| MTAC | Mass transfer area coefficients |
| GM | Garred model |
| PD | Peritoneal dialysis |
| PET | Peritoneal equilibration test |
| SiCo | Sieving coefficient |
| SLSQP | Sequential Least Squares Programming |
| SSE | Solute-specific error |
| SWM | Simplified Waniewski model |
| TPM | Three-pore model |
| UF | Ultrafiltration |
| UGM | Unified Graff model with some pre-determined parameters |
| UGM-18 | Unified Graff model with no pre-determined parameters |
| WM | Waniewski model |
List of Symbols
| [0] | Initial intraperitoneal volume |
| [t] | Final intraperitoneal volume at time t |
| Intraperitoneal volume at intermediate time steps, interpolated linearly | |
| Mean volume used in MTAC calculation | |
| Plasma solute concentrations, interpolated from subject data | |
| Dialysate solute concentrations at different time steps | |
| Predicted dialysate concentrations | |
| Measured dialysate concentrations | |
| Mean plasma solute concentrations | |
| - | Normalized error between the measured and predicted solute concentrations |
| Number of time points | |
| Time points, specifically = 0, 10, 20, 30, 60, 120, 180, and 240 min for pig data and = 0, 20, 120, and 240 min for human data | |
| Fractional hydraulic conductance for ultrasmall pores | |
| Fractional hydraulic conductance for small pores | |
| Fractional hydraulic conductance for large pores | |
| Ultrafiltration coefficient (hydraulic conductance) | |
| or | Fractional value used in derived model calculations (0 for GM, 0.5 for SWM) |
| Weight given to clearance in the treatment score | |
| Weight given to ultrafiltration in the treatment score |
References
- Bonenkamp, A.A.; Sluijs, A.v.E.v.d.; Dekker, F.W.; Struijk, D.G.; de Fijter, C.W.; Vermeeren, Y.M.; van Ittersum, F.J.; Verhaar, M.C.; van Jaarsveld, B.C.; Abrahams, A.C. Technique failure in peritoneal dialysis: Modifiable causes and patient-specific risk factors. Perit. Dial. Int. 2022, 43, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Jaar, B.G.; Plantinga, L.C.; Crews, D.C.; E Fink, N.; Hebah, N.; Coresh, J.; Kliger, A.S.; Powe, N.R. Timing, causes, predictors and prognosis of switching from peritoneal dialysis to hemodialysis: A prospective study. BMC Nephrol. 2009, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, M.K. Phosphate Elimination in Modalities of Hemodialysis and Peritoneal Dialysis. Blood Purif. 2010, 29, 137–144. [Google Scholar] [CrossRef]
- Chuasuwan, A.; Pooripussarakul, S.; Thakkinstian, A.; Ingsathit, A.; Pattanaprateep, O. Comparisons of quality of life between patients underwent peritoneal dialysis and hemodialysis: A systematic review and meta-analysis. Health Qual. Life Outcomes 2020, 18, 191. [Google Scholar] [CrossRef] [PubMed]
- Waniewski, J.; Werynski, A.; Heimbürger, O.; Lindholm, B. Simple Membrane Models for Peritoneal Dialysis Evaluation of Diffusive and Convective Solute Transport. ASAIO J. 1992, 38, 788–796. [Google Scholar] [CrossRef]
- Stachowska-Pietka, J.; Waniewski, J.; Flessner, M.F.; Lindholm, B. Distributed model of peritoneal fluid absorption. Am. J. Physiol.-Heart Circ. Physiol. 2006, 291, H1862–H1874. [Google Scholar] [CrossRef]
- Stachowska-Pietka, J.; Waniewski, J.; Flessner, M.F.; Lindholm, B. Computer simulations of osmotic ultrafiltration and small-solute transport in peritoneal dialysis: A spatially distributed approach. Am. J. Physiol.-Ren. Physiol. 2012, 302, F1331–F1341. [Google Scholar] [CrossRef]
- Stachowska-Pietka, J.; Waniewski, J.; Olszowska, A.; Garcia-Lopez, E.; Wankowicz, Z.; Lindholm, B. Modelling of icodextrin hydrolysis and kinetics during peritoneal dialysis. Sci. Rep. 2023, 13, 6526. [Google Scholar] [CrossRef] [PubMed]
- Waniewski, J.; Stachowska-Pietka, J.; Lindholm, B. On the change of transport parameters with dwell time during peritoneal dialysis. Perit. Dial. Int. 2020, 41, 404–412. [Google Scholar] [CrossRef]
- Öberg, C.M.; Martuseviciene, G. Computer Simulations of Continuous Flow Peritoneal Dialysis Using the 3-Pore Model—A First Experience. Perit. Dial. Int. 2019, 39, 236–242. [Google Scholar] [CrossRef]
- Öberg, C.M.; Rippe, B. Is Adapted APD Theoretically More Efficient than Conventional APD? Perit. Dial. Int. 2017, 37, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Öberg, C.M.; Rippe, B. A distributed two-pore model: Theoretical implications and practical application to the glomerular sieving of Ficoll. Am. J. Physiol.-Ren. Physiol. 2014, 306, F844–F854. [Google Scholar] [CrossRef]
- Öberg, C.M.; Rippe, B. Optimizing Automated Peritoneal Dialysis Using an Extended 3-Pore Model. Kidney Int. Rep. 2017, 2, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Rippe, B. A Three-Pore Model of Peritoneal Transport. Perit. Dial. Int. 1993, 13 (Suppl. S2), 35–38. [Google Scholar] [CrossRef]
- Venturoli, D.; Rippe, B. Transport asymmetry in peritoneal dialysis: Application of a serial heteroporous peritoneal membrane model. Am. J. Physiol.-Ren. Physiol. 2001, 280, F599–F606. [Google Scholar] [CrossRef] [PubMed]
- Vonesh, E.F.; Rippe, B. Net Fluid Absorption under Membrane Transport Models of Peritoneal Dialysis. Blood Purif. 1992, 10, 209–226. [Google Scholar] [CrossRef]
- Kallen, R.J. A Method for Approximating the Efficacy of Peritoneal Dialysis for Uremia. Am. J. Dis. Child. 1966, 111, 156–160. [Google Scholar] [CrossRef]
- Miller, J.H.; Gipstein, R.; Margules, R.; Schwartz, M.; Rubini, M.E. Automated peritoneal dialysis: Analysis of several methods of peritoneal dialysis. ASAIO J. 1966, 12, 98–105. [Google Scholar]
- Henderson, L.W.; Nolph, K.D. Altered permeability of the peritoneal membrane after using hypertonic peritoneal dialysis fluid. J. Clin. Investig. 1969, 48, 992–1001. [Google Scholar] [CrossRef]
- Garred, L.J.; Canaud, B.; Farrell, P.C. A simple kinetic model for assessing peritoneal mass transfer in chronic ambulatory peritoneal dialysis. Asaio J. 1983, 6, 131–137. [Google Scholar]
- Waniewski, J. Mathematical modeling of fluid and solute transport in hemodialysis and peritoneal dialysis. J. Membr. Sci. 2006, 274, 24–37. [Google Scholar] [CrossRef]
- Waniewski, J.; Heimbürger, O.; Werynski, A.; Lindholm, B. Simple Models for Fluid Transport during Peritoneal Dialysis. Int. J. Artif. Organs 1996, 19, 455–466. [Google Scholar] [CrossRef]
- Fugleberg, S.; Graff, J.; Joffe, P.; Løkkegaard, H.; Feldt-Rasmussen, B.; Fogh-Andersen, N.; Nielsen, S.L. Transperitoneal transport of creatinine. A comparison of kinetic models. Clin. Physiol. 1994, 14, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Graff, J.; Fugleberg, S.; Brahm, J.; Fogh-Andersen, N. The transport of phosphate between the plasma and dialysate compartments in peritoneal dialysis is influenced by an electric potential difference. Clin. Physiol. 1996, 16, 291–300. [Google Scholar] [CrossRef]
- Graff, J.; Fugleberg, S.; Brahm, J.; Fogh-Andersen, N. Transperitoneal transport of sodium during hypertonic peritoneal dialysis. Clin. Physiol. 1996, 16, 31–39. [Google Scholar] [CrossRef]
- Graff, J.; Fugleberg, S.; Joffe, P.; Brahm, J.; Fogh-Andersen, N. An evaluation of twelve nested models of transperitoneal transport of urea: The one-compartment assumption is valid. Scand. J. Clin. Lab. Investig. 1995, 55, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Graff, J.; Fugleberg, S.; Joffe, P.; Brahm, J.; Fogh-Andersen, N. Parameter estimation in six numerical models of transperitoneal transport of potassium in patients undergoing peritoneal dialysis. Clin. Physiol. 1995, 15, 185–197. [Google Scholar] [CrossRef]
- Graff, J.; Fugleberg, S.; Joffe, P.; Fogh-Andersen, N. Parameter estimation in six numeric models of transperitoneal transport of glucose. ASAIO J. 1994, 40, 1005–1011. [Google Scholar] [CrossRef]
- Gotch, F.A. Kinetic Modeling of Continuous Flow Peritoneal Dialysis. Semin. Dial. 2001, 14, 378–383. [Google Scholar] [CrossRef]
- Flessner, M.F.; Dedrick, R.L.; Schultz, J.S. A distributed model of peritoneal-plasma transport: Analysis of experimental data in the rat. Am. J. Physiol.-Ren. Physiol. 1985, 248, F413–F424. [Google Scholar] [CrossRef]
- Stachowska-Pietka, J.; Poleszczuk, J.; Flessner, M.F.; Lindholm, B.; Waniewski, J. Alterations of peritoneal transport characteristics in dialysis patients with ultrafiltration failure: Tissue and capillary components. Nephrol. Dial. Transplant. 2019, 34, 864–870. [Google Scholar] [CrossRef]
- van Gelder, M.K.; Vries, J.C.; Simonis, F.; Monninkhof, A.S.; Hazenbrink, D.H.M.; Ligabue, G.; Giovanella, S.; Joles, J.A.; Verhaar, M.C.; Rubio, M.A.B.; et al. Evaluation of a system for sorbent-assisted peritoneal dialysis in a uremic pig model. Physiol. Rep. 2020, 8, e14593. [Google Scholar] [CrossRef] [PubMed]
- Htay, H.; Gow, S.K.; Jayaballa, M.; Oei, E.L.; Chan, C.-M.; Wu, S.-Y.; Foo, M.W. Preliminary safety study of the Automated Wearable Artificial Kidney (AWAK) in Peritoneal Dialysis patients. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2021, 42, 394–402. [Google Scholar] [CrossRef]
- Foo, M.W.; Htay, H.; Brown, E.A.; Chirumarry, S.; Pawlak, M.; Huda, S.N.; Lim, J.T.; Singh, S.; Gori, M.; Venkataraya, S.B.; et al. Thirty Days of Maintenance Peritoneal Dialysis Using a Sorbent-Based Automated Wearable Artificial Kidney (AWAK) PD Device in a Porcine Model: PO0963. J. Am. Soc. Nephrol. 2021, 32, 326. [Google Scholar] [CrossRef]
- Waniewski, J.; Werynski, A.; Heimbürger, O.; Lindholm, B. Simple Models for Description of Small-Solute Transport in Peritoneal Dialysis. Blood Purif. 1991, 9, 129–141. [Google Scholar] [CrossRef]
- van Gelder, M.K.; van Gelder, M.; Simonis, F.; Bolhuis, D.; Rubio, M.A.B.; Gilsanz, G.; Selgas, R.; Donati, G.; Cappelli, G.; Ligabue, G.; et al. Rationale and design of the CORDIAL first-in-human clinical trial: A system for sorbent-assisted continuous flow peritoneal dialysis. Steps Towards A Wearable Artif. Kidney 2022, 39. [Google Scholar] [CrossRef]
- Borrelli, S.; La Milia, V.; De Nicola, L.; Cabiddu, G.; Russo, R.; Provenzano, M.; Minutolo, R.; Conte, G.; Garofalo, C.; On behalf of Study group Peritoneal Dialysis of Italian Society of Nephrology. Sodium removal by peritoneal dialysis: A systematic review and meta-analysis. J. Nephrol. 2019, 32, 231–239. [Google Scholar] [CrossRef]
- Devuyst, O.; Yool, A.J. Aquaporin-1: New Developments and Perspectives for Peritoneal Dialysis. Perit. Dial. Int. 2010, 30, 135–141. [Google Scholar] [CrossRef]
- Kraft, D. A Software Package for Sequential Quadratic Programming. Forschungsbericht- Deutsche Forschungs- und Versuchsanstalt fur Luft- und Raumfahrt. 1988. Available online: https://degenerateconic.com/uploads/2018/03/DFVLR_FB_88_28.pdf (accessed on 25 June 2025).
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Heimbürger, O.; Hegbrant, J.; Martus, G.; Wilkie, M.; De Leon, C.; Carlsson, O.; Johansson, A.-C. Effects of Steady Glucose Concentration Peritoneal Dialysis on Ultrafiltration Volume and Sodium Removal: A Pilot Crossover Trial. Clin. J. Am. Soc. Nephrol. 2024, 19, 224–232. [Google Scholar] [CrossRef]
- Wolf, M.B. Mechanisms of Peritoneal Acid-Base Kinetics During Peritoneal Dialysis: A Mathematical Model Study. ASAIO J. 2021, 67, 809–816. [Google Scholar] [CrossRef]
- Carlsson, O.; Nielsen, S.; Zakaria, E.-R.; Rippe, B. In vivo inhibition of transcellular water channels (aquaporin-1) during acute peritoneal dialysis in rats. Am. J. Physiol.-Heart Circ. Physiol. 1996, 271, H2254–H2262. [Google Scholar] [CrossRef]
- Akonur, A.; Holmes, C.J.; Leypoldt, J.K. Predicting the peritoneal absorption of icodextrin in rats and humans including the effect of α–amylase activity in dialysate. Perit. Dial. Int. 2015, 35, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Rippe, B.; Carlsson, O. Role Oftranscellularwater Channels in Peritoneal Dialysis. Perit. Dial. Int. 1999, 19 (Suppl. S2), 95–101. [Google Scholar] [CrossRef]
- Rippe, A.; Rippe, C.; SwÄRd, K.; Rippe, B. Disproportionally low clearance of macromolecules from the plasma to the peritoneal cavity in a mouse model of peritoneal dialysis. Nephrol. Dial. Transplant. 2007, 22, 88–95. [Google Scholar] [CrossRef][Green Version]
- De Vries, J.C.; van Gelder, M.K.; Monninkhof, A.S.; Ahmed, S.; Hazenbrink, D.H.M.; Nguyen, T.Q.; de Kort, G.A.P.; Vonken, E.-J.P.A.; Vaessen, K.R.D.; Joles, J.A.; et al. A Uremic Pig Model for Peritoneal Dialysis. Toxins 2022, 14, 635. [Google Scholar] [CrossRef]
- Popovich, R.P.; Moncrief, J.W.; Pyle, W.K. Transport Kinetics, in Peritoneal Dialysis; Kluwer Academic Publishers Springer: Dordrecht, The Netherlands, 1989; pp. 96–116. [Google Scholar]
- Drake, R.; Davis, E. A corrected equation for the calculation of reflection coefficients. Microvascular Research 1978, 15, 259. [Google Scholar] [CrossRef]
- Li, P.K.-T.; Chow, K.M.; Cho, Y.; Fan, S.; Figueiredo, A.E.; Harris, T.; Kanjanabuch, T.; Kim, Y.-L.; Madero, M.; Malyszko, J.; et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2022, 42, 110–153. [Google Scholar] [CrossRef]
- Vanholder, R.; Glorieux, G.; Eloot, S. Once upon a time in dialysis: The last days of Kt/V? Kidney Int. 2015, 88, 460–465. [Google Scholar] [CrossRef]






| Model | Ref | Notes | Number of Fitted Parameters | Clinical Inputs for This Study |
|---|---|---|---|---|
| No Fluid Model + Linear Solute Model | ||||
| Garred model (GM) | [20] | Default model | 0 | Initial and final dialysate and plasma concentration, Instilled and drained volume |
| Linear Fluid Model + Non-linear Solute Model | ||||
| Graff (UGM) | [23,24,25,26,27,28] | Graff and Fugleberg et al. (1994–1996) established a series of models to fit measured concentrations of sodium, potassium, creatinine, phosphate, glucose, and urea. For the comparison, we only use the best-performing model for the solute and set some parameters according to previous work (see Table S1). | 7 (humans) 11 (pigs) | Dialysate and plasma concentration, Instilled and drained volume |
| Waniewski model (WM) | [5] | Default model | 6 (humans) 12 (pigs) | Dialysate and plasma concentration, Instilled and drained volume |
| Dynamic Fluid Model + Non-linear Solute Model | ||||
| Three-pore model (TPM) | [10] | The three-pore model, originally the two-pore model by Rippe [14], has been developed for continuous flow PD. Here, we have tweaked the model to resemble a typical PD dwell. | 5 (humans) 7 (pigs) | Dialysate and plasma concentration, Instilled and drained volume, Residual volume |
| Variations | ||||
| Fullfit Unified Graff Model UGM-18 | - | For this variation of the Graff model, we did not restrict any of the parameters to the previously fitted values. | 9 (humans) 18 (pigs) | Dialysate and plasma concentration, Instilled and drained volume |
| Simplified Waniewski Model (SWM) | [35] | For this variation, we used the Garred model with f set to 0.5 instead of 0. | 0 | Initial and final dialysate and plasma concentration, Instilled and drained volume |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swapnasrita, S.; de Vries, J.C.; Stachowska-Piętka, J.; Öberg, C.M.; Gerritsen, K.G.F.; Carlier, A. Comparing Computational Peritoneal Dialysis Models in Pigs and Patients. Toxins 2025, 17, 329. https://doi.org/10.3390/toxins17070329
Swapnasrita S, de Vries JC, Stachowska-Piętka J, Öberg CM, Gerritsen KGF, Carlier A. Comparing Computational Peritoneal Dialysis Models in Pigs and Patients. Toxins. 2025; 17(7):329. https://doi.org/10.3390/toxins17070329
Chicago/Turabian StyleSwapnasrita, Sangita, Joost C. de Vries, Joanna Stachowska-Piętka, Carl M Öberg, Karin G. F. Gerritsen, and Aurélie Carlier. 2025. "Comparing Computational Peritoneal Dialysis Models in Pigs and Patients" Toxins 17, no. 7: 329. https://doi.org/10.3390/toxins17070329
APA StyleSwapnasrita, S., de Vries, J. C., Stachowska-Piętka, J., Öberg, C. M., Gerritsen, K. G. F., & Carlier, A. (2025). Comparing Computational Peritoneal Dialysis Models in Pigs and Patients. Toxins, 17(7), 329. https://doi.org/10.3390/toxins17070329






