Antibacterial Activity of Jelleine-I, a Peptide Isolated from Royal Jelly of Apis mellifera, Against Colistin-Resistant Klebsiella pneumoniae
Abstract
1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Reagents
4.2. Microorganisms
4.3. Antibacterial Activity
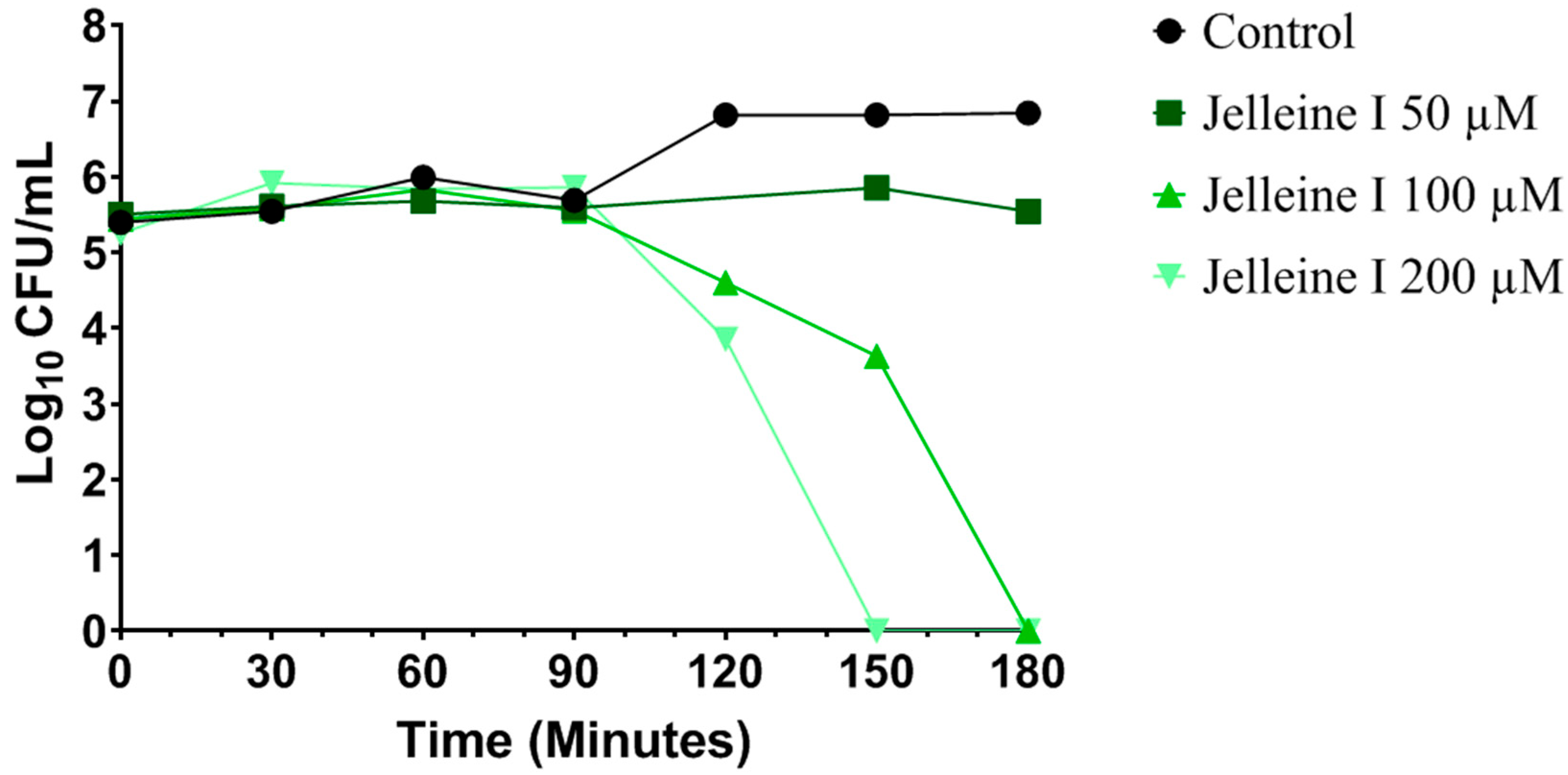
4.4. Time-Kill Curve
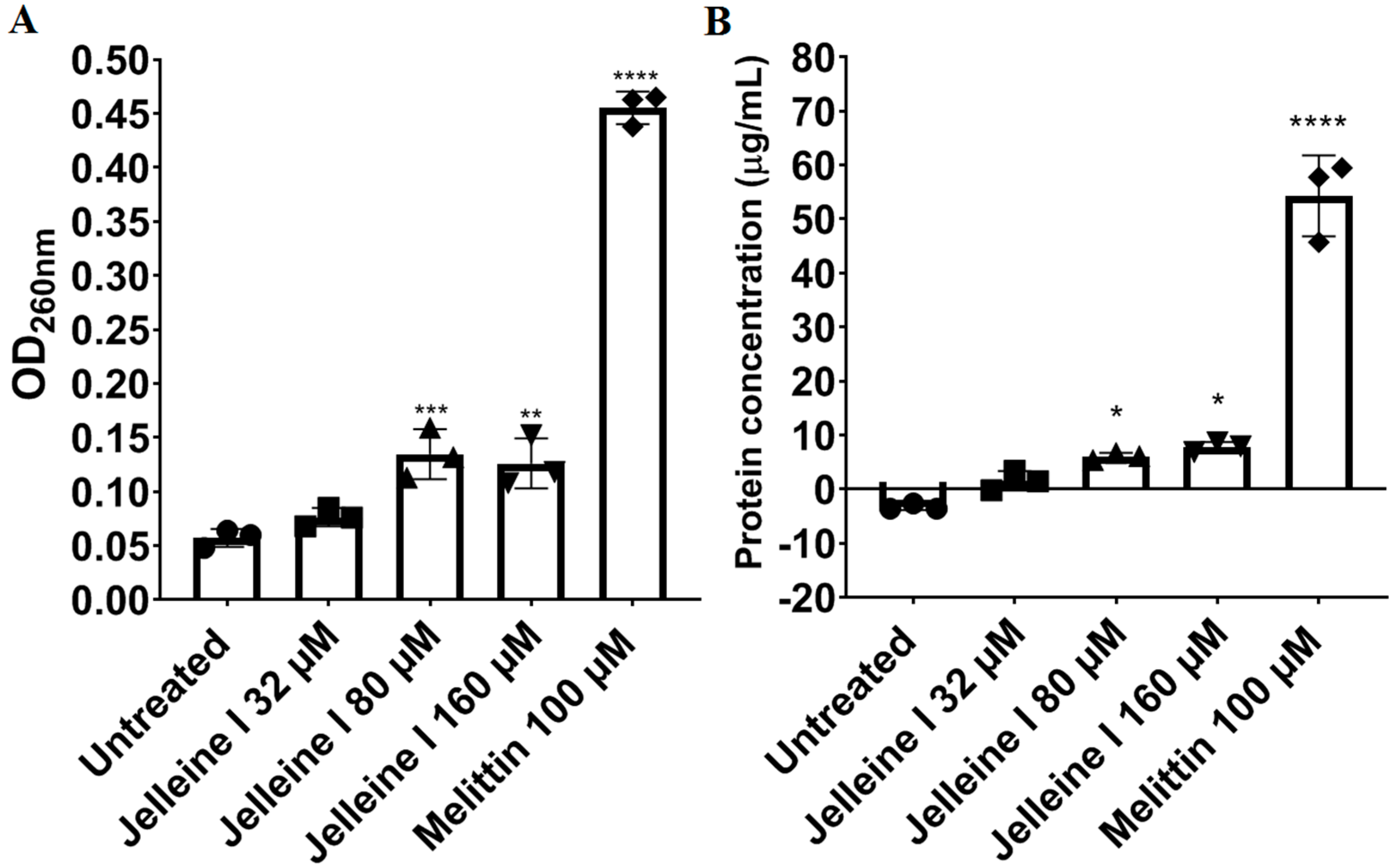
4.5. Release of Bacterial DNA/RNA
4.6. Release of Protein
4.7. Binding to Divalent Cations
4.8. Induction of Oxidative Stress
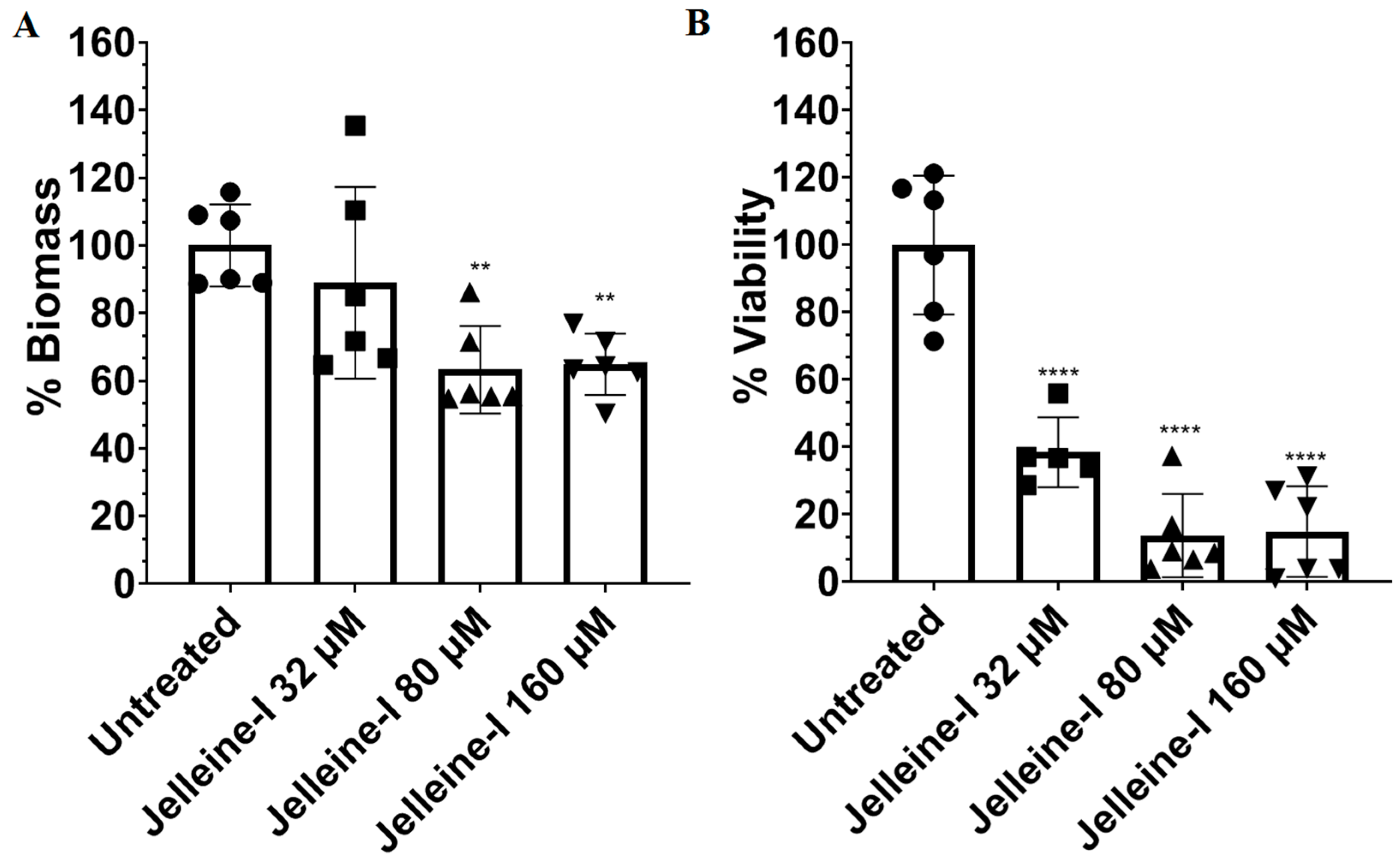
4.9. Anti-Biofilm Assay
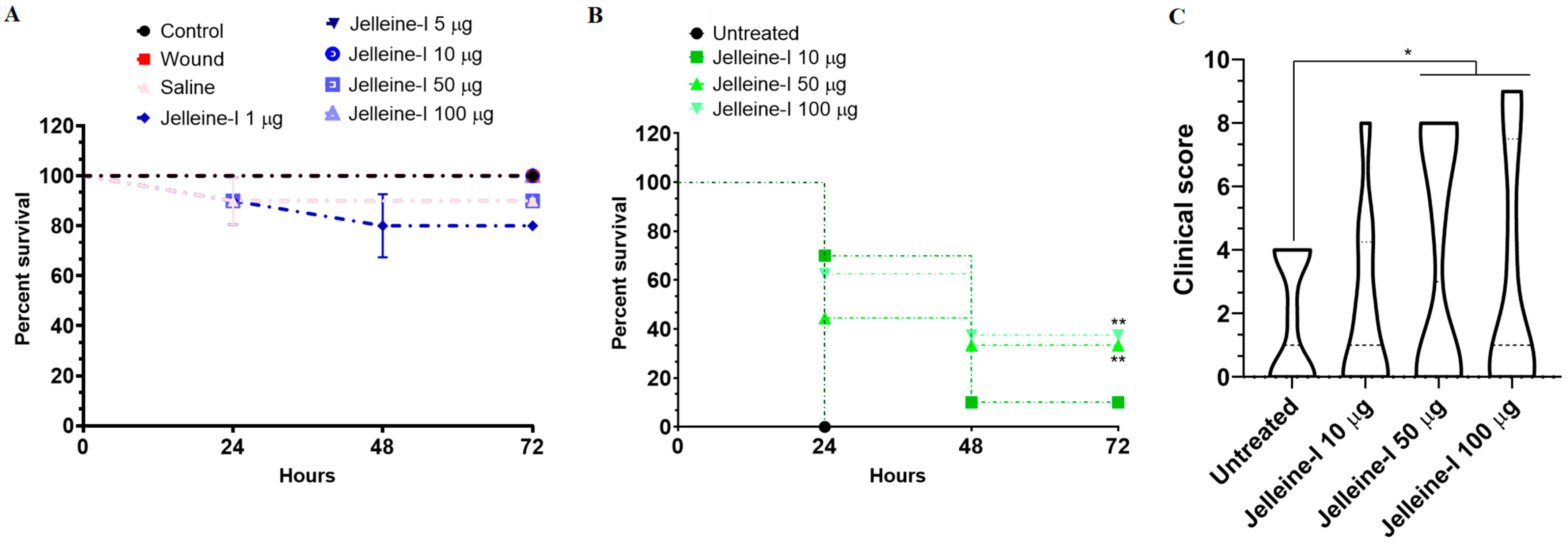
4.10. In Vivo Assay
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Effah, C.Y.; Sun, T.; Liu, S.; Wu, Y. Klebsiella pneumoniae: An Increasing Threat to Public Health. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Wyres, K.L.; Lam, M.M.C.; Holt, K.E. Population Genomics of Klebsiella pneumoniae. Nat. Rev. Microbiol. 2020, 18, 344–359. [Google Scholar] [CrossRef]
- Bengoechea, J.A.; Sa Pessoa, J. Klebsiella pneumoniae Infection Biology: Living to Counteract Host Defences. FEMS Microbiol. Rev. 2019, 43, 123–144. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Sharma, L.; Dela Cruz, C.S.; Zhang, D. Clinical Epidemiology, Risk Factors, and Control Strategies of Klebsiella pneumoniae Infection. Front. Microbiol. 2021, 12, 750662. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.; Aguilar, A.C.; Caicedo, A. Carbapenem-Resistant Klebsiella pneumoniae: Microbiology Key Points for Clinical Practice. Int. J. Gen. Med. 2019, 12, 437–446. [Google Scholar] [CrossRef]
- Caneiras, C.; Lito, L.; Melo-Cristino, J.; Duarte, A. Community- and Hospital-Acquired Klebsiella pneumoniae Urinary Tract Infections in Portugal: Virulence and Antibiotic Resistance. Microorganisms 2019, 7, 138. [Google Scholar] [CrossRef]
- Balkhair, A.; Al-Muharrmi, Z.; Al’Adawi, B.; Al Busaidi, I.; Taher, H.B.; Al-Siyabi, T.; Al Amin, M.; Hassan, K.S. Prevalence and 30-Day All-Cause Mortality of Carbapenem-and Colistin-Resistant Bacteraemia Caused by Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae: Description of a Decade-Long Trend. Int. J. Infect. Dis. 2019, 85, 10–15. [Google Scholar] [CrossRef]
- Karami-Zarandi, M.; Rahdar, H.A.; Esmaeili, H.; Ranjbar, R. Klebsiella pneumoniae: An Update on Antibiotic Resistance Mechanisms. Future Microbiol. 2023, 18, 65–81. [Google Scholar] [CrossRef]
- Lima, W.G.; de Lima, M.E. Therapeutic Prospection of Animal Venoms-Derived Antimicrobial Peptides against Infections by Multidrug-Resistant Acinetobacter baumannii: A Systematic Review of Pre-Clinical Studies. Toxins 2023, 15, 268. [Google Scholar] [CrossRef]
- Bucataru, C.; Ciobanasu, C. Antimicrobial Peptides: Opportunities and Challenges in Overcoming Resistance. Microbiol. Res. 2024, 286, 127822. [Google Scholar] [CrossRef]
- Bechinger, B.; Gorr, S.-U. Antimicrobial Peptides: Mechanisms of Action and Resistance. J. Dent. Res. 2017, 96, 254–260. [Google Scholar] [CrossRef]
- Sahoo, A.; Swain, S.S.; Behera, A.; Sahoo, G.; Mahapatra, P.K.; Panda, S.K. Antimicrobial Peptides Derived From Insects Offer a Novel Therapeutic Option to Combat Biofilm: A Review. Front. Microbiol. 2021, 12, 661195. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, L.; He, Y.; Liu, K.; Zhang, F.; Zhang, H.; Lu, Y.; Yang, C.; Wang, Z.; Fareed, M.S.; et al. An Optimized Analog of Antimicrobial Peptide Jelleine-1 Shows Enhanced Antimicrobial Activity against Multidrug Resistant P. aeruginosa and Negligible Toxicity in Vitro and in Vivo. Eur. J. Med. Chem. 2021, 219, 113433. [Google Scholar] [CrossRef] [PubMed]
- Capparelli, R.; De Chiara, F.; Nocerino, N.; Montella, R.C.; Iannaccone, M.; Fulgione, A.; Romanelli, A.; Avitabile, C.; Blaiotta, G.; Capuano, F. New Perspectives for Natural Antimicrobial Peptides: Application as Antinflammatory Drugs in a Murine Model. BMC Immunol. 2012, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.P.; Brito, J.C.M.; Candido, W.A.B.; Félix, A.S.; Verly, R.M.; Resende, J.M.; Lopes-de-Souza, L.; Chávez-Olórtegui, C.; Fernandes, S.O.A.; Cardoso, V.N. Jelleine-I Membrane Interaction-Related Biological Properties and Antimicrobial Activity against MDR, XDR, and PDR-Acinetobacter baumannii Clinical Isolates. ACS Omega 2025, 10, 10938–10948. [Google Scholar] [CrossRef]
- Nang, S.C.; Han, M.-L.; Yu, H.H.; Wang, J.; Torres, V.V.L.; Dai, C.; Velkov, T.; Harper, M.; Li, J. Polymyxin Resistance in Klebsiella pneumoniae: Multifaceted Mechanisms Utilized in the Presence and Absence of the Plasmid-Encoded Phosphoethanolamine Transferase Gene Mcr-1. J. Antimicrob. Chemother. 2019, 74, 3190–3198. [Google Scholar] [CrossRef]
- Lima, W.G.; Brito, J.C.M.; Verly, R.M.; de Lima, M.E. Jelleine, a Family of Peptides Isolated from the Royal Jelly of the Honey Bees (Apis mellifera), as a Promising Prototype for New Medicines: A Narrative Review. Toxins 2024, 16, 24. [Google Scholar] [CrossRef]
- Jia, F.; Wang, J.; Zhang, L.; Zhou, J.; He, Y.; Lu, Y.; Liu, K.; Yan, W.; Wang, K. Multiple Action Mechanism and in Vivo Antimicrobial Efficacy of Antimicrobial Peptide Jelleine-I. J. Pept. Sci. 2021, 27, e3294. [Google Scholar] [CrossRef]
- Kim, S.R.; Choi, K.-H.; Kim, K.-Y.; Kwon, H.-Y.; Park, S.-W. Development of a Novel Short Synthetic Antibacterial Peptide Derived from the Swallowtail Butterfly Papilio xuthus Larvae. J. Microbiol. Biotechnol. 2020, 30, 1305–1309. [Google Scholar] [CrossRef]
- Fontana, R.; Mendes, M.A.; de Souza, B.M.; Konno, K.; César, L.M.M.; Malaspina, O.; Palma, M.S. Jelleines: A Family of Antimicrobial Peptides from the Royal Jelly of Honeybees (Apis mellifera). Peptides 2004, 25, 919–928. [Google Scholar] [CrossRef]
- Brazilian Committee on Antimicrobial Susceptibility Testing (BrCAST). Tabelas de Pontos de Corte Para Interpretação de CIMs e Diâmetros de Halos. 2025. Available online: https://brcast.org.br/wp-content/uploads/2022/09/Tabela-pontos-de-corte-BrCAST-13-04-2024.pdf (accessed on 12 June 2025).
- Yu, V.L.; Hansen, D.S.; Ko, W.C.; Sagnimeni, A.; Klugman, K.P.; von Gottberg, A.; Goossens, H.; Wagener, M.M.; Benedi, V.J. International Klebseilla Study Group Virulence Characteristics of Klebsiella and Clinical Manifestations of K. pneumoniae Bloodstream Infections. Emerg. Infect. Dis. 2007, 13, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Wang, J.; Peng, J.; Zhao, P.; Kong, Z.; Wang, K.; Yan, W.; Wang, R. The in Vitro, in Vivo Antifungal Activity and the Action Mode of Jelleine-I against Candida Species. Amino Acids 2018, 50, 229–239. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.; Chen, Y.; Wu, J.; Guo, B.; Wu, X.; Zhang, Y.; Wang, M.; Ya, R.; Huang, H. Combined PK/PD Index May Be a More Appropriate PK/PD Index for Cefoperazone/Sulbactam against Acinetobacter baumannii in Patients with Hospital-Acquired Pneumonia. Antibiotics 2022, 11, 703. [Google Scholar] [CrossRef]
- Cabrera, M.P.d.S.; Baldissera, G.; Silva-Gonçalves, L.d.C.; Souza, B.M.d.; Riske, K.A.; Palma, M.S.; Ruggiero, J.R.; Arcisio-Miranda, M. Combining Experimental Evidence and Molecular Dynamic Simulations to Understand the Mechanism of Action of the Antimicrobial Octapeptide Jelleine-I. Biochemistry 2014, 53, 4857–4868. [Google Scholar] [CrossRef]
- Yang, S.; Wang, H.; Zhao, D.; Zhang, S.; Hu, C. Polymyxins: Recent Advances and Challenges. Front. Pharmacol. 2024, 15, 1424765. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.G.; Alves, M.C.; Cruz, W.S.; Paiva, M.C. Chromosomally Encoded and Plasmid-Mediated Polymyxins Resistance in Acinetobacter baumannii: A Huge Public Health Threat. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, S.; Zhu, W.; Bao, Q.; Liang, Y.; Zhao, T.; Li, X.; Zhou, J. Study on the Mechanism of ROS-Induced Oxidative Stress Injury and the Broad-Spectrum Antimicrobial Performance of Nickel Ion-Doped V6O13 Powder. Sci. Rep. 2024, 14, 22374. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Mittal, S.; Sharma, M.; Chaudhary, U. Biofilm and Multidrug Resistance in Uropathogenic Escherichia coli. Pathog. Glob. Health 2015, 109, 26–29. [Google Scholar] [CrossRef]
- Jia, F.; Zhang, Y.; Wang, J.; Peng, J.; Zhao, P.; Zhang, L.; Yao, H.; Ni, J.; Wang, K. The Effect of Halogenation on the Antimicrobial Activity, Antibiofilm Activity, Cytotoxicity and Proteolytic Stability of the Antimicrobial Peptide Jelleine-I. Peptides 2019, 112, 56–66. [Google Scholar] [CrossRef]
- Shen, P.; Ding, K.; Wang, L.; Tian, J.; Huang, X.; Zhang, M.; Dang, X. In Vitro and in Vivo Antimicrobial Activity of Antimicrobial Peptide Jelleine-I against Foodborne Pathogen Listeria monocytogenes. Int. J. Food Microbiol. 2023, 387, 110050. [Google Scholar] [CrossRef]
- Yasir, M.; Willcox, M.D.P.; Dutta, D. Action of Antimicrobial Peptides against Bacterial Biofilms. Materials 2018, 11, 2468. [Google Scholar] [CrossRef] [PubMed]
- CLSI M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
- Lima, W.G.; de Brito, J.C.M.; Cardoso, V.N.; Fernandes, S.O.A. In-Depth Characterization of Antibacterial Activity of Melittin against Staphylococcus aureus and Use in a Model of Non-Surgical MRSA-Infected Skin Wounds. Eur. J. Pharm. Sci. 2021, 156, 105592. [Google Scholar] [CrossRef]
- Kielkopf, C.L.; Bauer, W.; Urbatsch, I.L. Bradford Assay for Determining Protein Concentration. Cold Spring Harb. Protoc. 2020, 2020, 102269. [Google Scholar] [CrossRef] [PubMed]
- Zai, Y.; Ying, Y.; Ye, Z.; Zhou, M.; Ma, C.; Shi, Z.; Chen, X.; Xi, X.; Chen, T.; Wang, L. Broad-Spectrum Antimicrobial Activity and Improved Stability of a D-Amino Acid Enantiomer of DMPC-10A, the Designed Derivative of Dermaseptin Truncates. Antibiotics 2020, 9, 627. [Google Scholar] [CrossRef] [PubMed]
- Araújo-Neto, L.N.; de Lima, M.d.C.A.; de Oliveira, J.F.; de Souza, E.R.; Feitosa Machado, S.E.; de Souza Lima, G.M.; Silva Buonafina, M.D.; Brayner, F.A.; Alves, L.C.; Sandes, J.M.; et al. Thiophene-Thiosemicarbazone Derivative (L10) Exerts Antifungal Activity Mediated by Oxidative Stress and Apoptosis in C. albicans. Chem. Biol. Interact. 2020, 320, 109028. [Google Scholar] [CrossRef]
- Herrera, K.M.S.; Lopes, G.F.M.; Oliveira, M.E.; Sousa, J.F.; Lima, W.G.; Silva, F.K.; Brito, J.C.M.; Gomes, A.J.P.S.; Viana, G.H.R.; Soares, A.C.; et al. A 3-Alkylpyridine-Bearing Alkaloid Exhibits Potent Antimicrobial Activity against Methicillin-Resistant Staphylococcus aureus (MRSA) with No Detectable Resistance. Microbiol. Res. 2022, 261, 127073. [Google Scholar] [CrossRef]
- Trafny, E.A.; Lewandowski, R.; Zawistowska-Marciniak, I.; Stępińska, M. Use of MTT Assay for Determination of the Biofilm Formation Capacity of Microorganisms in Metalworking Fluids. World J. Microbiol. Biotechnol. 2013, 29, 1635–1643. [Google Scholar] [CrossRef]
- Barton, T.E.; Duignan, L.; Kadioglu, A.; Fothergill, J.L.; Neill, D.R. Galleria mellonella as an Antimicrobial Screening Model. J. Vis. Exp. 2024, 212, e67210. [Google Scholar] [CrossRef]
- Andrade-Oliveira, A.L.; Lacerda-Rodrigues, G.; Pereira, M.F.; Bahia, A.C.; Machado, E.d.A.; Rossi, C.C.; Giambiagi-deMarval, M. Tenebrio molitor as a Model System to Study Staphylococcus spp. Virulence and Horizontal Gene Transfer. Microb. Pathog. 2023, 183, 106304. [Google Scholar] [CrossRef]
- Canteri de Souza, P.; Custódio Caloni, C.; Wilson, D.; Sergio Almeida, R. An Invertebrate Host to Study Fungal Infections, Mycotoxins and Antifungal Drugs: Tenebrio molitor. J. Fungi 2018, 4, 125. [Google Scholar] [CrossRef] [PubMed]
- de Souza, P.C.; Morey, A.T.; Castanheira, G.M.; Bocate, K.P.; Panagio, L.A.; Ito, F.A.; Furlaneto, M.C.; Yamada-Ogatta, S.F.; Costa, I.N.; Mora-Montes, H.M.; et al. Tenebrio molitor (Coleoptera: Tenebrionidae) as an Alternative Host to Study Fungal Infections. J. Microbiol. Methods 2015, 118, 182–186. [Google Scholar] [CrossRef] [PubMed]
| Microorganisms | Origin | Carbapenem Resistance a | Hypermucoviscity Phenotype b | Jelleine-I (µM) | Colistin (µg/mL) | ||
|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | Profile c | ||||
| K. pneumoniae ATCC 700603 | Urine | No | No | 32 | 128 | 0.25 | Sensible |
| K. pneumoniae ATCC 43816 | Secretion | No | Yes | 32 | 128 | 0.5 | Sensible |
| K. pneumoniae 17 | Secretion | Yes | Yes | 64 | 64 | 32 | Resistant |
| K. pneumoniae 27 | Secretion | Yes | No | 64 | >128 | 64 | Resistant |
| K. pneumoniae 68 | Secretion | Yes | Yes | 16 | 32 | 8 | Resistant |
| K. pneumoniae 76 | Secretion | Yes | Yes | 64 | 64 | 32 | Resistant |
| K. pneumoniae 104 | Urine | Yes | Yes | 64 | 64 | 64 | Resistant |
| K. pneumoniae 106 | Urine | Yes | No | 128 | 128 | >128 | Resistant |
| K. pneumoniae 110 | Urine | Yes | Yes | 64 | 64 | 32 | Resistant |
| K. pneumoniae 135 | Blood | Yes | Yes | 64 | 128 | 64 | Resistant |
| K. pneumoniae 139 | Blood | Yes | Yes | 64 | 128 | 64 | Resistant |
| K. pneumoniae 169 | Urine | Yes | No | 64 | 64 | 32 | Resistant |
| K. pneumoniae 242 | Secretion | Yes | No | 128 | 128 | 32 | Resistant |
| K. pneumoniae 260 | Secretion | Yes | No | 64 | 64 | 16 | Resistant |
| K. pneumoniae 289 | Blood | Yes | No | 64 | 128 | 32 | Resistant |
| K. pneumoniae 395 | Blood | Yes | No | 64 | 128 | 32 | Resistant |
| K. pneumoniae 627 | Secretion | Yes | No | 64 | >128 | 32 | Resistant |
| K. pneumoniae 642 | Secretion | Yes | No | 64 | 64 | 32 | Resistant |
| K. pneumoniae 689 | Blood | Yes | Yes | 64 | 128 | 32 | Resistant |
| K. pneumoniae 699 | Blood | Yes | Yes | 64 | 128 | 128 | Resistant |
| Control | Ca2+ | Mg2+ | Ascorbic Acid 100 µg/mL | |||||
|---|---|---|---|---|---|---|---|---|
| 20 µM | 50 µM | 100 µM | 20 µM | 50 µM | 100 µM | |||
| Jelleine-I | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
| Colistin | 8 | 8 | 16 | >64 | 8 | 16 | >64 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, W.G.; Laia, R.M.R.; Brito, J.C.M.; Michel, D.A.G.R.; Verly, R.M.; Resende, J.M.; de Lima, M.E. Antibacterial Activity of Jelleine-I, a Peptide Isolated from Royal Jelly of Apis mellifera, Against Colistin-Resistant Klebsiella pneumoniae. Toxins 2025, 17, 325. https://doi.org/10.3390/toxins17070325
Lima WG, Laia RMR, Brito JCM, Michel DAGR, Verly RM, Resende JM, de Lima ME. Antibacterial Activity of Jelleine-I, a Peptide Isolated from Royal Jelly of Apis mellifera, Against Colistin-Resistant Klebsiella pneumoniae. Toxins. 2025; 17(7):325. https://doi.org/10.3390/toxins17070325
Chicago/Turabian StyleLima, William Gustavo, Rayssa Maria Rodrigues Laia, Julio Cesar Moreira Brito, Daniel Augusto Guedes Reis Michel, Rodrigo Moreira Verly, Jarbas Magalhães Resende, and Maria Elena de Lima. 2025. "Antibacterial Activity of Jelleine-I, a Peptide Isolated from Royal Jelly of Apis mellifera, Against Colistin-Resistant Klebsiella pneumoniae" Toxins 17, no. 7: 325. https://doi.org/10.3390/toxins17070325
APA StyleLima, W. G., Laia, R. M. R., Brito, J. C. M., Michel, D. A. G. R., Verly, R. M., Resende, J. M., & de Lima, M. E. (2025). Antibacterial Activity of Jelleine-I, a Peptide Isolated from Royal Jelly of Apis mellifera, Against Colistin-Resistant Klebsiella pneumoniae. Toxins, 17(7), 325. https://doi.org/10.3390/toxins17070325






