Procedural Pain Management in Patients with Cerebral Palsy Undergoing Botulinum Toxin Injection: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Results
2.1. Risk of Bias Assessment
2.2. Evidence Synthesis
Side Effects
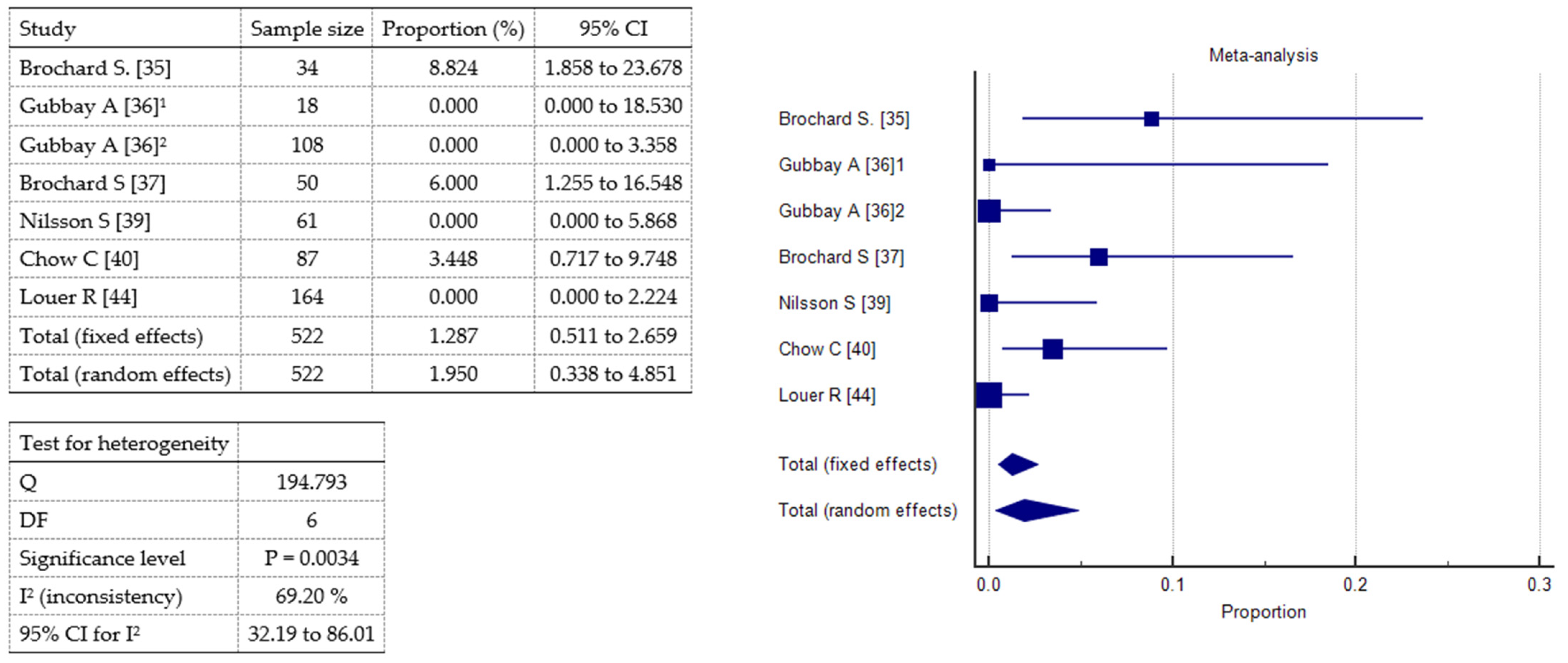
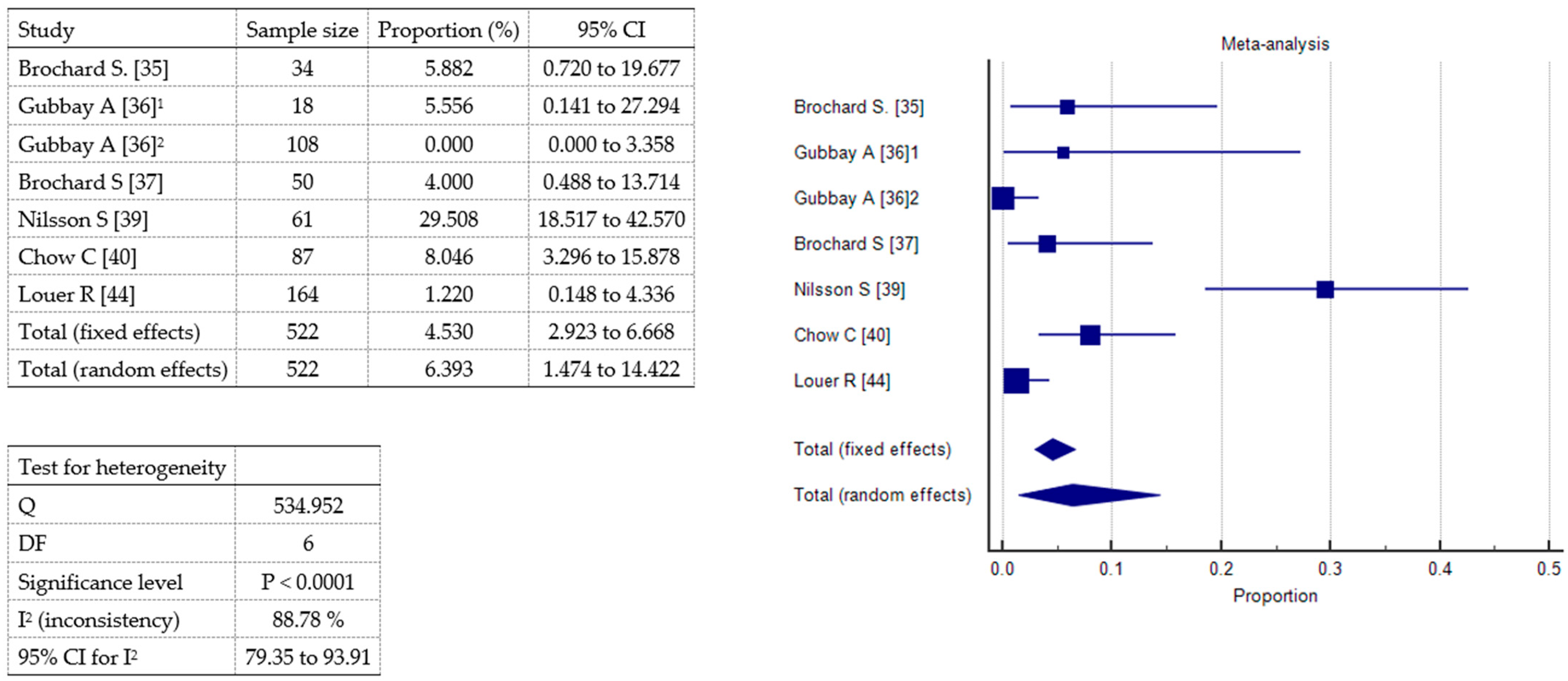
2.3. Meta-Analysis
3. Discussion
Limitations
4. Conclusions
5. Materials and Methods
- PICO1
- –
- P: population with cerebral palsy undergoing botulinum toxin injections.
- –
- I: procedural pain control techniques.
- –
- C: no treatment or any other treatment.
- –
- O: procedural pain.
- PICO2
- –
- P: population with cerebral palsy undergoing botulinum toxin injections.
- –
- I: procedural pain control techniques.
- –
- C: no treatment or any other treatment.
- –
- O: adverse effects.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alexander, J.; Manno, M. Underuse of analgesia in very young pediatric patients with isolated painful injuries. Ann. Emerg. Med. 2003, 41, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Neighbor, M.L.; Honner, S.; Kohn, M.A. Factors affecting emergency department opioid administration to severely injured patients. Acad. Emerg. Med. 2004, 11, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Klein, E.J.; Lewis, C.W.; Johnston, B.D.; Cummings, P. Emergency department analgesia for fracture pain. Ann. Emerg. Med. 2003, 42, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Petrack, E.M.; Christopher, N.C.; Kriwinsky, J. Pain management in the emergency department: Patterns of analgesic utilization. Pediatrics 1997, 99, 711–714. [Google Scholar] [CrossRef]
- Drendel, A.L.; Kelly, B.T.; Ali, S. Pain assessment for children: Overcoming challenges and optimizing care. Pediatr. Emerg. Care. 2011, 27, 773–781. [Google Scholar] [CrossRef]
- Borland, M.; Esson, A.; Babl, F.; Krieser, D. Procedural sedation in children in the emergency department: A PREDICT study. Emerg. Med. Australas. 2009, 21, 71–79. [Google Scholar] [CrossRef]
- Chen, E.; Zeltzer, L.K.; Craske, M.G.; Katz, E.R. Children’s memories for painful cancer treatment procedures: Implications for distress. Child. Dev. 2000, 71, 933–947. [Google Scholar] [CrossRef]
- Weisman, S.J.; Bernstein, B.; Schechter, N.L. Consequences of inadequate analgesia during painful procedures in children. Arch. Pediatr. Adolesc. Med. 1998, 152, 147–149. [Google Scholar] [CrossRef]
- Heinen, F.; Molenaers, G.; Fairhurst, C.; Carr, L.J.; Desloovere, K.; Valayer, E.C.; Morel, E.; Papavassiliou, A.S.; Tedroff, K.; Pascual-Pascual, S.I.; et al. European consensus Table 2006 on botulinum toxin for children with cerebral palsy. Eur. J. Paediatr. Neurol. 2006, 10, 215–225. [Google Scholar] [CrossRef]
- Simpson, D.M.; Blitzer, A.; Brashear, A.; Comella, C.; Dubinsky, R.; Hallett, M.; Jankovic, J.; Karp, B.; Ludlow, C.L.; Miyasaki, J.M.; et al. Assessment: Botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2008, 70, 1699–1706. [Google Scholar] [CrossRef]
- Molenaers, G.; Schörkhuber, V.; Fagard, K.; Van Campenhout, A.; De Cat, J.; Pauwels, P.; Ortibus, E.; De Cock, P.; Desloovere, K. Long-term use of botulinum toxin type A in children with cerebral palsy: Treatment consistency. Eur. J. Paediatr. Neurol. 2009, 13, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, A.; Seyhan, K.; Değer, Ü.; Kutlutürk, S.; Mutlu, A. Should botulinum toxin A injections be repeated in children with cerebral palsy? A systematic review. Dev. Med. Child. Neurol. 2016, 58, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Symons, F.J.; Rivard, P.F.; Nugent, A.C.; Tervo, R.C. Parent evaluation of spasticity treatment in cerebral palsy using botulinum toxin type A. Arch. Phys. Med. Rehabil. 2006, 87, 1658–1660. [Google Scholar] [CrossRef] [PubMed]
- Linder-Lucht, M.; Kirschner, J.; Herrmann, J.; Geth, K.; Korinthenberg, R.; Berweck, S.; Heinen, F.; Mall, V. ‘Why do children with cerebral palsy discontinue therapy with botulinum toxin A? ’ Dev. Med. Child Neurol. 2006, 48, 319–320. [Google Scholar] [CrossRef]
- Kinnett, D. Botulinum toxin A injections in children: Technique and dosing issues. Am. J. Phys. Med. Rehabil. 2004, 83 (Suppl. 10), S59–S64. [Google Scholar] [CrossRef]
- Statement on ASA Physical Status Classification System. Available online: https://www.asahq.org/standards-and-practice-parameters/statement-on-asa-physical-status-classification-system (accessed on 11 May 2025).
- Gorlin, A.W.; Rosenfeld, D.M.; Ramakrishna, H. Intravenous sub-anesthetic ketamine for perioperative analgesia. J. Anaesthesiol. Clin. Pharmacol. 2016, 32, 160–167. [Google Scholar] [CrossRef]
- McCann, M.E.; Kain, Z.N. The management of preoperative anxiety in children: An update. Anesth. Analg. 2001, 93, 98–105. [Google Scholar] [CrossRef]
- Proesmans, M. Respiratory illness in children with disability: A serious problem? Breathe 2016, 12, e97–e103. [Google Scholar] [CrossRef]
- Seddon, P.C.; Khan, Y. Respiratory problems in children with neurological impairment. Arch. Dis. Child. 2003, 88, 75–78. [Google Scholar] [CrossRef]
- Hayakawa, H.; Pincott, E.S.; Ali, U. Anaesthesia and cerebral palsy. BJA Educ. 2022, 22, 26–32. [Google Scholar] [CrossRef]
- St-Laurent, A.; Zysman-Colman, Z.; Zielinski, D. Respiratory prehabilitation in pediatric anesthesia in children with muscular and neurologic disease. Paediatr. Anaesth. 2022, 32, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Heinen, F.; Desloovere, K.; Schroeder, A.S.; Berweck, S.; Borggraefe, I.; van Campenhout, A.; Andersen, G.L.; Aydin, R.; Becher, J.G.; Bernert, G.; et al. The updated European Consensus 2009 on the use of Botulinum toxin for children with cerebral palsy. Eur. J. Paediatr. Neurol. 2010, 14, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Vova, J.A.; Green, M.M.; Brandenburg, J.E.; Davidson, L.; Paulson, A.; Deshpande, S.; Oleszek, J.L.; Inanoglu, D.; McLaughlin, M.J. A consensus statement on the use of botulinum toxin in pediatric patients. PM&R 2022, 14, 1116–1142. [Google Scholar] [CrossRef]
- Gormley, M.; Fehlings, D.; Kim, H.; Bonikowski, M.; Banach, M.; Gul, F.; Meilahn, J.; Racette, B.; Huang, N.; Niu, X.; et al. Efficacy and Safety of OnabotulinumtoxinA for the Treatment of Pediatric Upper and Lower Limb Spasticity: Results From 2 Open-Label, Long-term Extension Trials. J. Child. Neurol. 2024, 40, 168–179. [Google Scholar] [CrossRef]
- Krauss, B.; Green, S.M. Procedural sedation and analgesia in children. Lancet 2006, 367, 766–780. [Google Scholar] [CrossRef]
- Friedrichsdorf, S.J.; Goubert, L. Pediatric pain treatment and prevention for hospitalized children. Pain. Rep. 2019, 5, e804. [Google Scholar] [CrossRef]
- von Baeyer, C.L.; Marche, T.A.; Rocha, E.M.; Salmon, K. Children’s memory for pain: Overview and implications for practice. J. Pain. 2004, 5, 241–249. [Google Scholar] [CrossRef]
- Zier, J.L.; Rivard, P.F.; Krach, L.E.; Wendorf, H.R. Effectiveness of sedation using nitrous oxide compared with enteral midazolam for botulinum toxin A injections in children. Dev. Med. Child. Neurol. 2008, 50, 854–858. [Google Scholar] [CrossRef]
- Kumar, R.; Sneade, C.; Littler, K. Effectiveness of sedation using nitrous oxide compared with enteral midazolam for botulinum toxin A injections in children. Dev. Med. Child Neurol. 2009, 51, 838–839. [Google Scholar] [CrossRef]
- Fung, S.; Phadke, C.P.; Kam, A.; Ismail, F.; Boulias, C. Effect of topical anesthetics on needle insertion pain during botulinum toxin type A injections for limb spasticity. Arch. Phys. Med. Rehabil. 2012, 93, 1643–1647. [Google Scholar] [CrossRef]
- Cantador-Hornero, M.; Jiménez-Espuch, P.; de Torres-Garcia, I.; Contreras-Jiménez, M.; Martínez-Mezo, G.L.; de los Santos, J.M.M.; Fernández-Jurado, M.I.; Tirado-Reyes, M. Protocolo sedoanalgésico para la infiltración de toxina botulínica A en parálisis cerebral [Sedation-analgesia protocol for the injection of botulinum toxin A in cerebral palsy]. An. Pediatr. 2019, 91, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Ben-Pazi, H.; Cohen, A.; Kroyzer, N.; Lotem-Ophir, R.; Shvili, Y.; Winter, G.; Deutsch, L.; Pollak, Y. Clown-care reduces pain in children with cerebral palsy undergoing recurrent botulinum toxin injections- A quasi-randomized controlled crossover study. PLoS ONE 2017, 12, e0175028. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, K.; Paget, S.P.; Webb, A.; Khut, G.P.; Morrow, A.M. Biofeedback assisted relaxation training and distraction therapy for pain in children undergoing botulinum neurotoxin A injections: A crossover randomized controlled trial. Dev. Med. Child. Neurol. 2022, 64, 1507–1516. [Google Scholar] [CrossRef]
- Brochard, S.; Blajan, V.; Lempereur, M.; Le Moine, P.; Peudenier, S.; Lefranc, J.; Rémy-Néris, O. Effectiveness of nitrous oxide and analgesic cream (lidocaine and prilocaine) for prevention of pain during intramuscular botulinum toxin injections in children. Ann. Phys. Rehabil. Med. 2009, 52, 704–716. [Google Scholar] [CrossRef]
- Gubbay, A.; Langdon, K. Effectiveness of sedation using nitrous oxide compared with enteral midazolam for botulinum toxin A injections in children. Dev. Med. Child Neurol. 2009, 51, 491–492. [Google Scholar] [CrossRef]
- Brochard, S.; Blajan, V.; Lempereur, M.; Garlantezec, R.; Houx, L.; Le Moine, P.; Peudenier, S.; Lefranc, J.; Rémy-Néris, O. Determining the technical and clinical factors associated with pain for children undergoing botulinum toxin injections under nitrous oxide and anesthetic cream. Eur. J. Paediatr. Neurol. 2011, 15, 310–315. [Google Scholar] [CrossRef]
- Forrester, M.; Srinivasan, J.; Mihrshahi, S.; Waugh, M.; O’Flaherty, S.; Rice, J.; Graham, K.; Scheinberg, A. Conscious sedation or general anaesthetic for intramuscular botulinum toxin injections in children—A two centre cross-sectional prospective audit. Eur. J. Paediatr. Neurol. 2012, 16, 215–217. [Google Scholar] [CrossRef]
- Nilsson, S.; Brunsson, I.; Askljung, B.; Påhlman, M.; Himmelmann, K. A rectally administered combination of midazolam and ketamine was easy, effective and feasible for procedural pain in children with cerebral palsy. Acta Paediatr. 2017, 106, 458–462. [Google Scholar] [CrossRef]
- Chow, C.; Choong, C.T. Ketamine-based procedural sedation and analgesia for botulinum toxin A injections in children with cerebral palsy. Eur. J. Paediatr. Neurol. 2016, 20, 319–322. [Google Scholar] [CrossRef]
- Mondon-Willaume, A. Pain management during botulinum toxin A injections in cerebral palsy children Pediatric PMR. Ann. Phys. Rehabil. Med. 2017, 60, e61–e63. [Google Scholar] [CrossRef]
- Fisher, M.T.; Zigler, C.K.; Houtrow, A.J. Factors affecting procedural pain in children during and immediately after intramuscular botulinum toxin injections for spasticity. J. Pediatr. Rehabil. Med. 2018, 11, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Chau, B.; Chi, B.; Wilson, T. Decreasing pediatric pain and agitation during botulinum toxin injections for spasticity with virtual reality: Lessons learned from clinical use. J. Pediatr. Rehabil. Med. 2018, 11, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Louer, R.; McKinney, R.C.; Abu-Sultaneh, S.; Lutfi, R.; Abulebda, K. Safety and Efficacy of a Propofol and Ketamine Based Procedural Sedation Protocol in Children with Cerebral Palsy Undergoing Botulinum Toxin A Injections. PM&R 2019, 11, 1320–1325. [Google Scholar] [CrossRef]
- Houx, L.; Dubois, A.; Brochard, S.; Pons, C. Do clowns attenuate pain and anxiety undergoing botulinum toxin injections in children? Ann. Phys. Rehabil. Med. 2020, 63, 393–399. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomized studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- Ramsay, M.A.; Savege, T.M.; Simpson, B.R.; Goodwin, E.R. Controlled sedation with alphaxalone-alphadolone. Br. Med. J. 1974, 22, 656–659. [Google Scholar] [CrossRef]
- Standard Clinico-Organizzativi SIAARTI-SARNePI per l’anestesia in età pediatrica. Available online: https://www.siaarti.it/news/371363 (accessed on 11 May 2025).
- Analgesia e sedazione procedurale in radiologia diagnostica ed operativa pediatrica. Available online: https://www.siaarti.it/news/1788257. SIAARTI. (accessed on 11 May 2025).
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- MedCalc Statistical Software version 14.8.1. MedCalc Software bvba: Ostend, Belgium. Available online: http://www.medcalc.org (accessed on 18 May 2025).




| Reference | Study Type | Population * | Diagnosis | Intervention | Outcome | Results *# | Side Effects |
|---|---|---|---|---|---|---|---|
Zier JL [29]  | RCT double-blind | 50 (29 M, 21 F); mean age 8 y 2 mo ± 4 y 5 mo | CP: DP 11, TrP 3, QP 16, HP 16, other 3; GMFCS I 4, II 24, III 4, IV 13, V 5 | Rectal midazolam vs. N₂O (operator titrated 70% maximum nitrous oxide⁄oxygen mixture) | Pain by means of FLACC assessed by parents and nurse. Parents comparation with prior sedation for similar procedures. Sedation levels at discharge. | Median FLACC: N₂O 4 (0–10); midazolam 6 (0–10). N₂O better in parental comparison to prior sedation experience. Sedation levels at discharge higher in midazolam: 0 in 3 children, 1 in 10 children, 2 in 9 children, 3 in 3 children, 4 none; in N₂O: 0 in 17 children, 1 in 7 children, 2 in 1 child, 3 and 4 none. | Midazolam: 1 desaturation < 92%. N₂O: 1 nausea, 1 headache and pallor, 4 vomit, 2 desaturation < 92%. |
Kumar R [30]  | Non-randomized controlled trial | 33 (21 M, 12 F); mean age 8 y 1 mo ± 4 y 11 mo | CP: GMFCS I 11, II 6, III 6, IV 5, V 5; HP 8, DP 11, QP 7, other 7 | Entonox® (N₂O: 50% nitrous oxide⁄oxygen) vs. enteral midazolam with Emla® | Pain measured by means of FLACC, FPS, or VAS (range 0–10). Parents satisfaction. | Median pain scores: midazolam 3, Entonox® 2. Children with pain scores > 5: midazolam 4, Entonox® 3. Parent satisfaction: midazolam 13 satisfied, 0 not satisfied, 3 unknown; Entonox® 18 satisfied, 2 not satisfied, 4 unknown. | None |
Fung S [31]  | RCT single-blind | 30; mean age 55 y ± 16 y | Ankle spasticity | 3 anesthetics (Emla®, Gebauer Pain Ease vapocoolant spray, ice) compared with no anesthetic (controls) | Mean pain: NRS (range 0–7) and the FPS (range 0–6). | Emla®: NRS 2.20 ± 2.10, FPS 2.10 ± 2.00; Spray: NRS 3.70 ± 2.40, FPS 3.60 ± 2.50; Ice: NRS 2.20 ± 2.20, FPS 1.80 ± 2.20; Controls: NRS 3.70 ± 1.95, FPS 3.07 ± 1.89. | / |
Cantador Hornero M [32]  | Cross-sectional study | 124; mean age 6.75 y | CP: QP 17, DP 66, HP 41 GMFCS I 41, II 35, III 28, IV 8, V 12 | Group I, without sedation or topical anesthetic cream; group II, N₂O; group III, deep iv sedation; group IV, light sedation oral or rectal benzodiazepines | Pain and percentage of patients who experienced pain ≤ 2 assessed by means of FLACC < 3 y, WBS 3–7 y, VNS > 8 y (range 0–10). Secondary outcome measures: associations between the level of pain and different variables (GMFCS score, dose of BoNTA, number of injections, MAS score, functional classification of CP, and age); level of satisfaction of parents and physicians (5-point Likert scales). | Median pain scores: group I 4 (3–5), group II 2 (0–5), group III 0 (0–0), group IV 6 (4.6–9) Pain level ≤ 2: group III 100%, group II 57.6%, group I 25%, group IV 0% (p < 0.001). Parent satisfaction: group III higher compared to all other groups. Mean number of injections: group III 7.8 ± 2.1, groups I 5.2 ± 2.5, group II 5.7 ± 2.3, group IV 5.6 ± 3.3. Pain was inversely correlated with age. | / |
Ben-Pazi H [33]  | Quasi-randomized crossover trial | 45 (31 M,14 F); mean age 7.04 y ± 4.68 y | CP: 16 DP, 13 HP, 15 QP, 1 TrP; GMFCS 2.86 (1.07) | Clown care vs. standard care | Expected pain and post-procedural pain measured by means of VAS: FPS (range 1–5) for children or VAS for parent. | Mean VAS-after 1st: clown care 2.89 ± 1.36, controls 3.85 ± 1.39. Mean VAS-after 2nd (carryover effect): clown care 2.84 ± 1.38, controls 4.11 ± 0.93. Mean difference between experienced pain and expected pain: clown care −0.34 ± 1.58, controls +0.74 ± 1.78. Younger children reported higher pain. | / |
Ostojic K [34]  | Crossover RCT | 38 (20 M,18 F), mean age 13 y 5 mo ± 3 y 4 mo; 10 dropouts | 34 CP; 4 HSP or ABI | Biofeedback-assisted relaxation training (BART) vs. distraction therapy | Mean and worst pain by means of FPS (range 0–10). Fear by means of Children’s Fear Scale (range 0–4). Anxiety by means of state–trait Anxiety inventory (range 0–60). | No significant difference in mean and worst pain, fear, and anxiety. | / |
| Reference | Study Type | Population * | Diagnosis | Procedures | Intervention | Outcome | Results *# | Side Effects |
|---|---|---|---|---|---|---|---|---|
Brochard S [35]  | Prospective study | 34 (18 M, 16 F); mean age 5,94 y ±4.21 y | 33 CP: 10 HP, 12 DP, 11 QP; 1 adductor hypertonus relating to an orthopedic problem | 209 | N₂O and Emla® | Maximal pain over the whole procedure by means of CHEOPS (range 4–13). Pain during localize-by-electrostimulation, puncture, and injection phases. Pain assessed 30’ after the procedure by means of VAS for parents and children ≥ 6 y or FPS for children < 6 y (range 0–10). | Mean maximal CHEOPS 8.50 ± 3.56. Mean CHEOPS during injection 7.40 ± 3.56 > localize 6.24 ± 3.07 > puncture 5.3 ± 2.44. Mean children VAS/FPS 2.41 ±2.51 in 24/51 sessions. Mean parents VAS 3.59 ±2.58 in 38/51 sessions. No correlation between max CHEOPS and age. | 3 children vomited after the session with no other complications; 2 children had particularly vivid dreams. |
Gubbay A [36]  | Comparative study (1st audit) | 105 (72 M, 33 F); mean age 7 y 1 mo ± 3 y 11 mo | CP | 105 | Oral midazolam (0.5 mg/kg, max dose 12 mg) vs. MIKE (midazolam 0.5 mg/kg ketamine 0.3 mg/kg orally) in 18 patients | Parents satisfaction. | Oral midazolam: 89.5% tolerated the procedure well; 85% satisfied. MIKE: 67% tolerated the procedure adequately; of 11 children who had previously undergone BoNT-A with midazolam alone, 8 parents reported that the procedure was better tolerated when using MIKE versus midazolam alone. | 1 transient distress after MIKE procedure. |
 | Comparative study (2nd audit) | 108 children (62 M, 46 F); mean age 8 y 11 mo ± 3 y 11 mo | CP: GMFCS I 63, II 30, III 15; majority spastic DP | 108 | Intranasal fentanyl (1.5 µg/kg, max dose 75 µg) and topical anesthetic cream vs. 1st audit sedation methods | Effectiveness reported by parents and safety. | 75% parents reported that intranasal fentanyl provided superior analgesia compared to previous methods (more effective in children weighing over 20 kg). | No serious adverse events. |
Brochard S [37]  | Monocentric prospective study | 50 (31 M, 19 F); mean age 6.6 y ± 4.32 y § | CP: 18 DP, 20 HP, 12 QP; GMFCS: 10 I, 20 II, 6 III, 10 IV, 4 V | 199 | N₂O (50% nitrous oxide/oxygen) and Emla® | Maximal pain over the whole procedure (CHEOPS). Pain during localize-by-electrostimulation, puncture, and injection phases. Pain in injected muscle. | Mean maximal CHEOPS 8.16 ± 3.5 (38% CHEOPS > 9). Mean CHEOPS during injection (6.77 ± 3.30) > localization (5.46 ± 2.03) > puncture (4.88 ± 2.03). CHEOPS in adductors < gastrocnemius. | § 3 children vomited after the session with no other complications; 2 children had particularly vivid dreams. |
Forrester M [38] | Prospective observational cross-sectional audit | 171 CP; mean age 7 y 1 mo | CP | 171 | N₂O and topical anesthetic (conscious sedation) vs. GA | Carer perception of their child’s pain (no or slight, moderate, severe to extreme) and satisfaction. Median number of staff required. | Perception under conscious sedation: 78% no or slight, 14% moderate, 8% severe to extreme > under GA: 98% no or slight, 2% severe to extreme. Overall satisfaction: no sign. diff. Median number of staff required: conscious sedation 3 < GA 4. | Minimal adverse events. |
Nilsson S [39] | Retrospective study | 61 (36 M, 25 F); mean age 5.7 y (1.4–13.8 y) | 16 USCP, 38 BSCP, 7 DCP. GMFCS I 25, II 5, III 8, IV 11, V 12. | 128 | Rectal midazolam (mean dose 0.26 mg/kg, range 0.15–0.37 mg/kg) and racemic ketamine (mean dose 3.9 mg/kg, range 2.50–4.90 mg/kg) + Emla® and oral or rectal paracetamol (30 mg/kg) 1 h before injection | Pain by means of FLACC for nurse and CAS (0–10) for parents. Feasibility (0–10 scale). Total time spent in the clinic. Frequency of side effects in 24 h (questionnaire for parents). | Median FLACC 2.0 (0–8): 85% 1–3; 10.8% 4–5; 4.2% > 5. Median CAS 2.0 (0–10). Median feasibility 9 (6–10). Median time 3.25 h (1.5–6.0 h). | Nausea 21.7%; pain 8.9%; sleep disturbance 7.7%. |
Chow C [40] | Retrospective study | 87 (46 M, 41 F); median age 5 y 5 mo (range: 1 y 5 mo −13 y 2 mo) | CP: GMFCS II 52, III 21, IV 8, V 6. | 152 | Topical anesthetic cream, iv ketamine (median dose 1.0 mg/kg, range 0.7−2.2 mg/kg), and/or iv midazolam (median dose 0.1 mg/kg, range 0.06−0.45 mg/kg); In 92.1% procedures, iv atropine to reduce airway secretions | Efficacy: frequency of successfully completed procedures. Safety: frequency of adverse events at 2–3 weeks post-procedure follow-up review. | 100% (152) successfully completed procedures. 6.6% were associated with mild, self-limiting adverse events. See box on the right. | 4 rashes, 3 nausea and vomiting, 1 limb tremors, 1 mild headache, 1 nightmare in the post-procedure night. No serious adverse events. |
Mondon-Willaume A [41] | Prospective study | 29; mean age 6 y 8 mo | CP and spastic children | 30 | N₂O, topical anesthetic, systematic analgesia with more or less sedative drugs | Maximal pain over the whole procedure (puncture, stimulation for localizing, injection) by means of FLACC (range 0–10). Percentage of patients with FLACC max < 3/10 (controlled pain group). | Mean max FLACC 2.10 ± 2.45. Controlled pain group: 65.5% (FLACC max < 3/10). Painful group (4 y 9 mo) significantly younger than controlled pain group (7 y 8 mo). | / |
Fisher MT [42] | Retrospective study | 249; mean age 9.2 y ± 5.6 y | 189 CP, 60 other neurologic and musculoskeletal conditions | 563 | Vapocoolant spray vs. no vapocoolant, and in a 11 procedures topical anesthetic, and in 6 procedures, oral sedative | Pain by means of FPS (0–10) for the child or the parent during and after the procedure. Regression analysis to determine predictors of pain during and post procedure. | Mean FPS: overall 3.8 ± 3.0; vapocoolant spray: 3.9 ± 3.0; no vapocoolant 3.1 ± 2.7. Predictors of procedural pain: topical anesthetic; leg, hand, thigh injections; younger age. Predictors of post-procedural pain: pain during the procedure; older age. | / |
Chau B [43] | Retrospective study | 14 (5 M, 9 F); mean age 7.79 y ± 2.39 y | 12 CP median GMFCS * 2.25 (1.38–4), 1 spinal cord injury, 1 pontine hemorrhage | 14 | Individually set VR experience by means of VR headset and VR-capable smartphone, from publicly-available 360° videos via YouTube | Pain (FLACC). Caregiver feedback: positive, neutral, or negative experience with VR. | 9 positive: median FLACC 2.5 (1 patient NR); 2 neutral: median FLACC 5.5; 2 negative: median FLACC 9.5; 1 unable to complete. | No adverse events. |
Louer, R. [44] | Retrospective study | 164 (102 M,62 F); median age 9 y (4–11) | CP, GMFCS I 1, II 21, III 50, IV 55, V 33 | 345 | Propofol and ketamine | Frequency of adverse events. Median procedure time, recovery time, total sedation time. | Adverse events: overall, 10.1% of procedures; all episodes were transient and resolved with supplemental oxygen. See box on the right. Median procedure time 10’, recovery time 11’, total sedation time 33’. | Hypoxemia 9.6%; transient apnea 1.4%; 0.9% hypoxemia and transient apnea. No serious adverse events. |
Houx, L [45] | Prospective observational study | 59 (35 M, 24 F); median age clowns 8 y (5–10) vs. controls 7.5 y (5–11) | 52 CP, 12 cognitive disorders. | 88 | N₂O and Emla® + medical clowns vs. usual distractions (music, television, video games as controls) | Pain: FLACC and VAS for the child and parent. Anxiety: VAS for the child and parent(s). Success of the sessions: 4-point Likert scale for physician and parents. Benefits of the distraction: 4-point Likert scale for parents. | Median maximal FLACC: clowns 2.5 (1–4) vs. controls 3 (1–4.3). Median VAS self-reported: clowns 2.5 (0–5) vs. controls 3 (1–6.3). Median VAS proxy: clowns 2.5 (0.3–3.4) vs. controls 3 (1–4.5). Median Likert success for parents: clowns 4 (4–4) vs. controls 3 (4–4) Median Likert benefits: clowns 4 (4–4) vs. controls 4 (3–4). | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faccioli, S.; Ehsani, A.; Kaleci, S.; Tonini, G.; Tagliani, I.; Vetrano, M.; Sassi, S. Procedural Pain Management in Patients with Cerebral Palsy Undergoing Botulinum Toxin Injection: A Systematic Review and Meta-Analysis. Toxins 2025, 17, 317. https://doi.org/10.3390/toxins17070317
Faccioli S, Ehsani A, Kaleci S, Tonini G, Tagliani I, Vetrano M, Sassi S. Procedural Pain Management in Patients with Cerebral Palsy Undergoing Botulinum Toxin Injection: A Systematic Review and Meta-Analysis. Toxins. 2025; 17(7):317. https://doi.org/10.3390/toxins17070317
Chicago/Turabian StyleFaccioli, Silvia, Alessandro Ehsani, Shaniko Kaleci, Giulia Tonini, Ilaria Tagliani, Mario Vetrano, and Silvia Sassi. 2025. "Procedural Pain Management in Patients with Cerebral Palsy Undergoing Botulinum Toxin Injection: A Systematic Review and Meta-Analysis" Toxins 17, no. 7: 317. https://doi.org/10.3390/toxins17070317
APA StyleFaccioli, S., Ehsani, A., Kaleci, S., Tonini, G., Tagliani, I., Vetrano, M., & Sassi, S. (2025). Procedural Pain Management in Patients with Cerebral Palsy Undergoing Botulinum Toxin Injection: A Systematic Review and Meta-Analysis. Toxins, 17(7), 317. https://doi.org/10.3390/toxins17070317





