1. Introduction
Harmful algal blooms (HABs) have been increasingly reported over recent decades, with evidence linking their frequency and intensity to climate change and accelerated eutrophication driven by domestic, industrial, and agricultural runoff [
1,
2,
3,
4,
5,
6]. These blooms encompass a broad diversity of phytoplankton species that vary in toxicity and geographic range. While HABs are often localized events, many causative species have global distributions. Blooms are characterized by excessive biomass accumulation, frequently in combination with the intracellular production of potent toxins [
7]. Given the diversity of HAB-forming species, their responses to environmental change are not always predictable: toxicity can vary within and between species, and climate-driven shifts may favor the rise in novel or invasive taxa.
HABs pose serious ecological and socioeconomic threats, particularly in coastal systems. They can compromise public health via shellfish poisoning and respiratory irritation, disrupt fisheries and aquaculture, and degrade water quality [
8,
9,
10,
11,
12]. In Canadian coastal waters, historical red tide events on the Pacific and Atlantic coasts suggest that rising atmospheric and oceanic temperatures are the key to bloom dynamics [
13]. These temperature increases are extending the growing season [
5,
6], altering bloom phenology [
14,
15,
16], and influencing phytoplankton traits such as growth [
14], motility [
17], and life cycle transitions [
18].
Climate change also affects salinity through evaporation, precipitation, and freshwater runoff changes, particularly in coastal and estuarine systems [
6,
19,
20,
21,
22,
23,
24,
25,
26,
27]. Intensification of the hydrologic cycle [
20,
21] increases the influx of freshwater into marine systems, leading to significant spatial and temporal variability in salinity, a critical stressor for marine phytoplankton [
22,
23,
28].
One of the most profound changes in marine environments is the rise in atmospheric CO
2 concentrations, from pre-industrial levels of 280 ppm to over 426 ppm as of June 2024 [
29]. Approximately half of this CO
2 is absorbed by oceans [
30,
31], leading to the acidification of surface waters [
32,
33]. The resultant decrease in pH can disrupt cellular processes in phytoplankton, including enzyme activity, transmembrane potential, nutrient uptake [
34,
35,
36,
37,
38,
39,
40], motility [
39], and even ichthyotoxicity [
38].
Understanding species-specific impacts is critical, given the complex interplay between climate change and phytoplankton responses.
Heterosigma akashiwo, a euryhaline raphidophyte, has emerged as a dominant HAB species in many coastal regions. Its blooms are often associated with mass fish mortality [
41,
42,
43]. The organism transitions between vegetative cells and benthic resting cysts, activated by temperature and light conditions [
43,
44,
45,
46]. Previous studies have shown that its growth is stimulated by temperatures exceeding 20 °C and CO
2 concentrations above 700 ppm, which are the levels projected for the end of the 21st century [
32,
46,
47,
48,
49,
50,
51,
52,
53].
Moreover,
H. akashiwo exhibits a high tolerance to salinity variability, enabling it to thrive under fluctuating estuarine conditions [
43,
50,
54,
55,
56]. Alteration in membrane permeability in response to salinity may represent a stress mitigation strategy to maintain osmotic balance [
50]. For example, major blooms have occurred in English Bay, Vancouver, Canada, at 15 °C and low salinity (15) following snowmelt-driven runoff [
57]. Despite the recognized influence of temperature, salinity, and CO
2, three major environmental stressors associated with climate change, on
H. akashiwo, few studies have assessed the combined effects of these factors. This study addresses this gap using a Design of Experiment (DOE) approach to systematically examine the interactions between temperature, salinity, and CO
2 on the growth, yield, and membrane permeability of
H. akashiwo [
51,
58]. Compared to the traditional one-factor-at-a-time (OFAT) approach, DOE provides a more robust statistical framework that simultaneously evaluates multiple factors and their interactions while significantly minimizing the number of experimental runs required [
51,
58,
59,
60]. This approach enhances efficiency and improves the reliability and interpretability of the results, particularly when investigating complex, multifactorial environmental stressors such as those associated with climate change. To our knowledge, this is the first study to apply DOE to identify the optimal ecological conditions for
H. akashiwo’s performance. The findings enhance our predictive understanding of HAB dynamics in a rapidly changing ocean.
3. Discussion
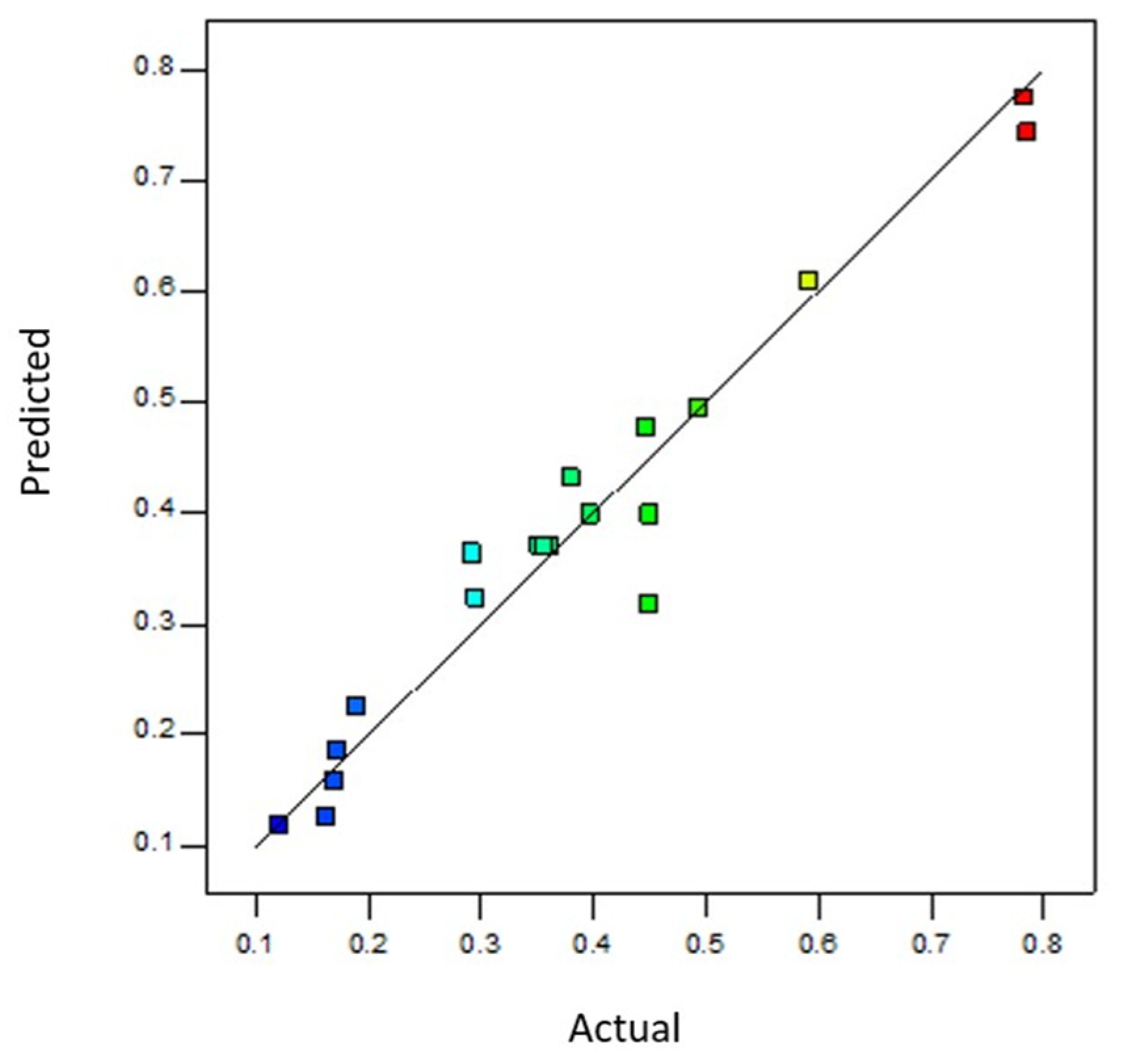
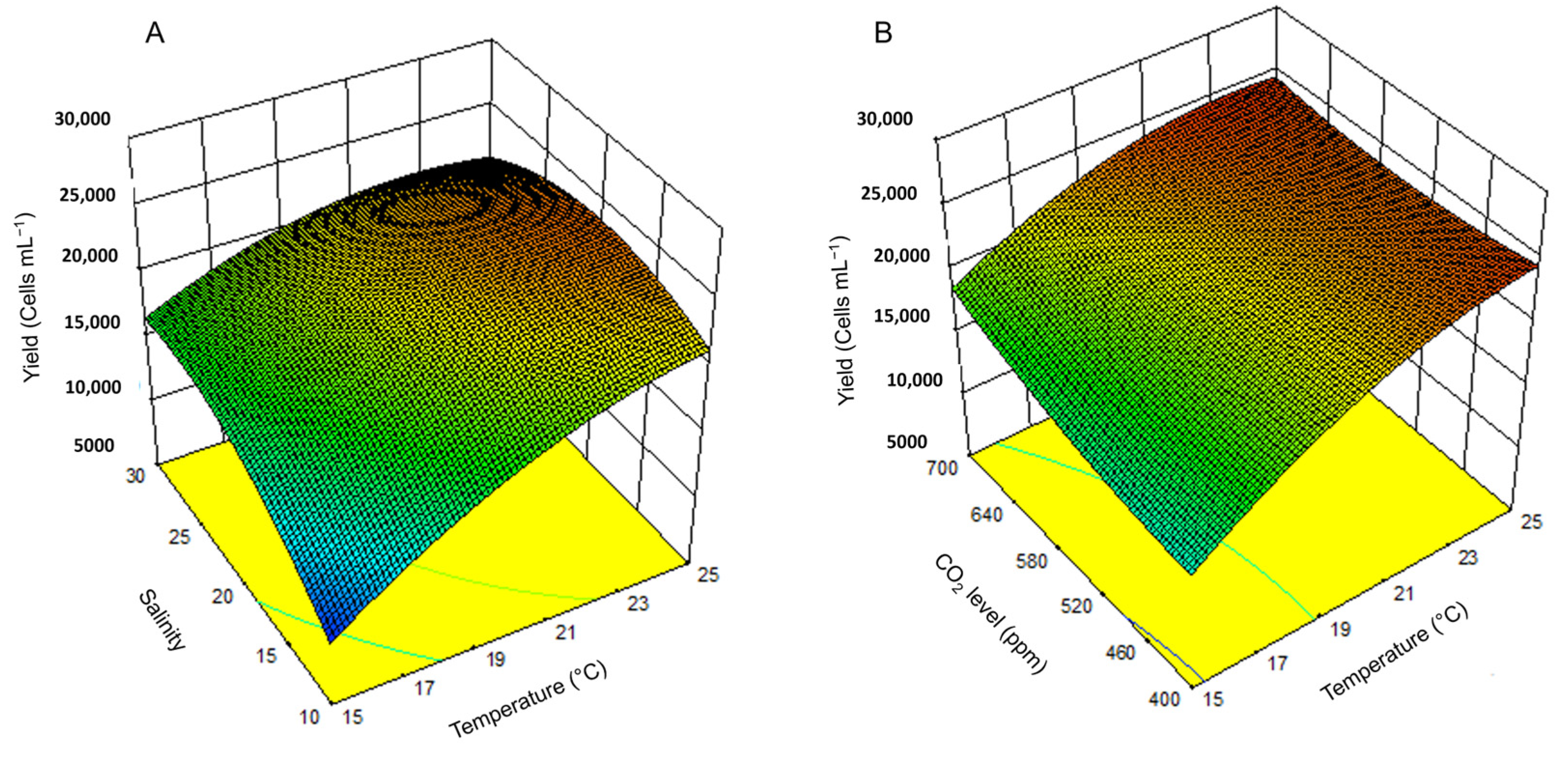
Despite increasing evidence of HABs under changing climate conditions, our understanding of how specific HAB species respond to simultaneous environmental stressors remains limited. Most studies adopt an OFAT approach, which fails to capture the interactive effects of multiple drivers that more accurately reflect natural conditions. In contrast, our study demonstrates the advantages of using a DOE methodology to explore how
H. akashiwo responds to combined changes in temperature, salinity, and CO
2 concentration [
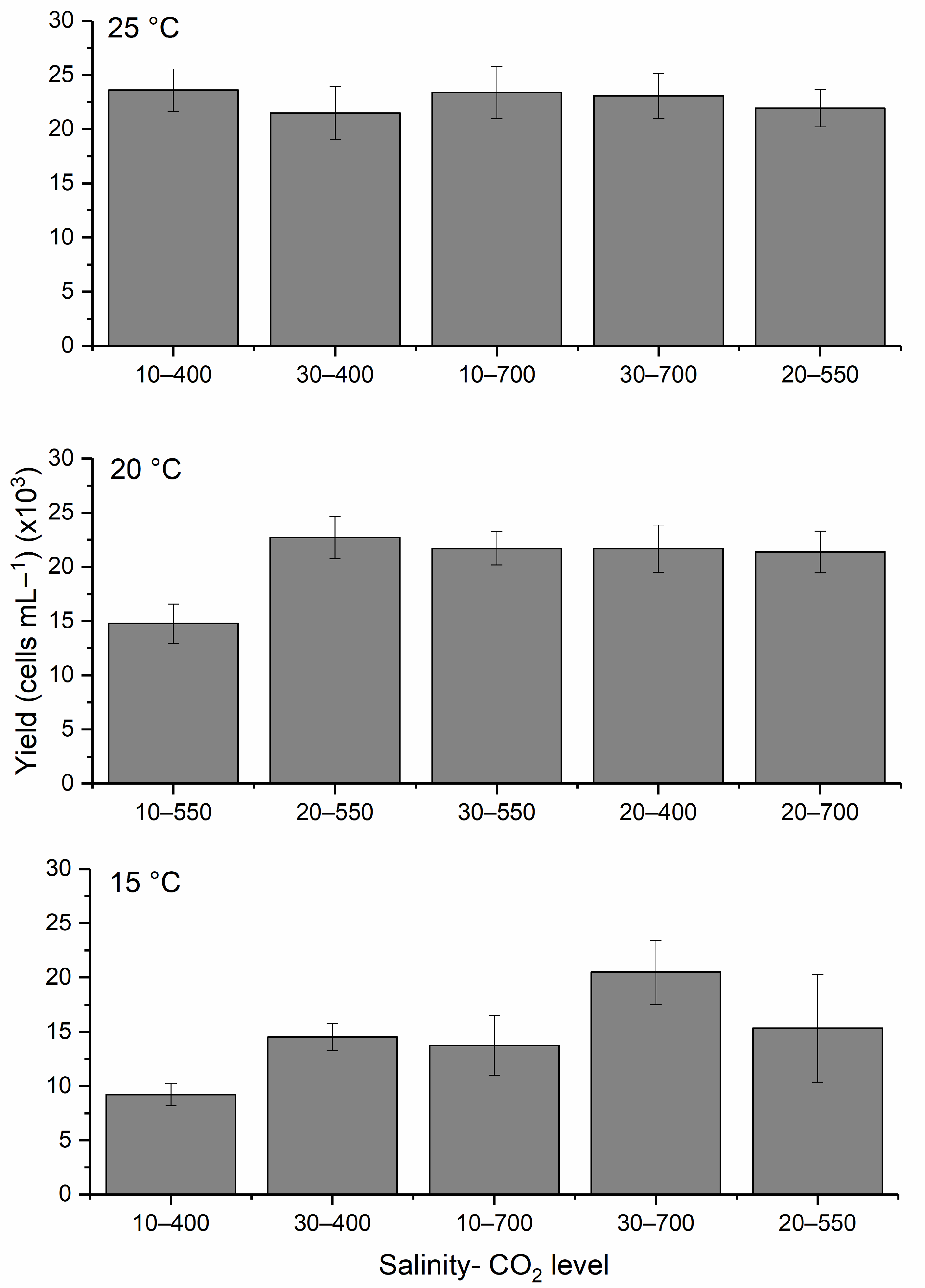
59]. The DOE approach comprehensively evaluated multiple stressors and revealed distinct growth, yield, and cell permeability patterns. The results indicated that warmer temperatures and elevated salinities promote higher growth rates and greater biomass production. This is consistent with earlier findings that
H. akashiwo thrives in warm, high-salinity waters [
47,
48,
49,
50,
51,
63,
64,
65,
66]. Moreover, the study confirmed that elevated CO
2 levels (up to 700 ppm) enhanced growth when paired with optimal temperature conditions, supporting projections that
H. akashiwo may benefit from climate-induced ocean acidification [
48,
52,
53].
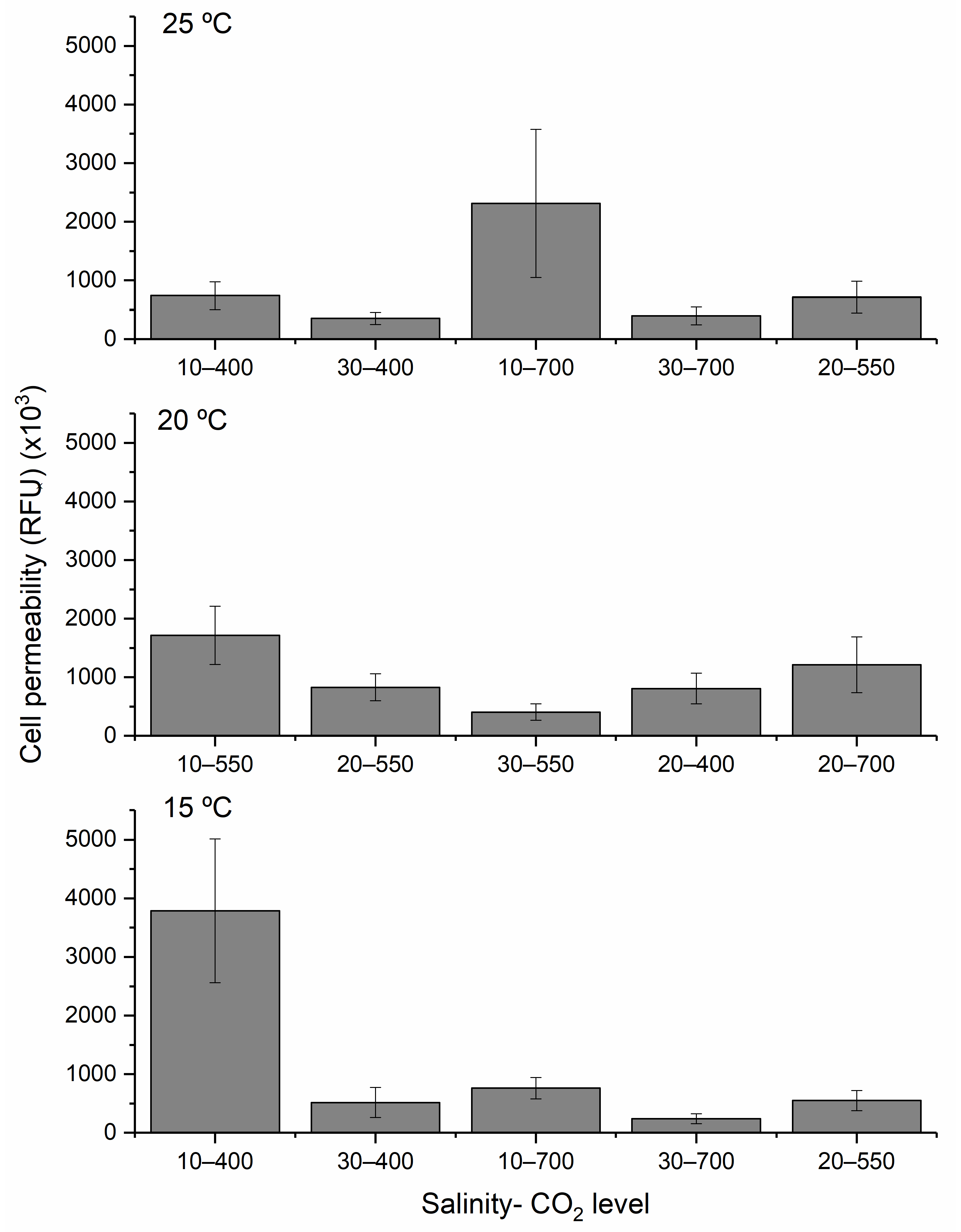
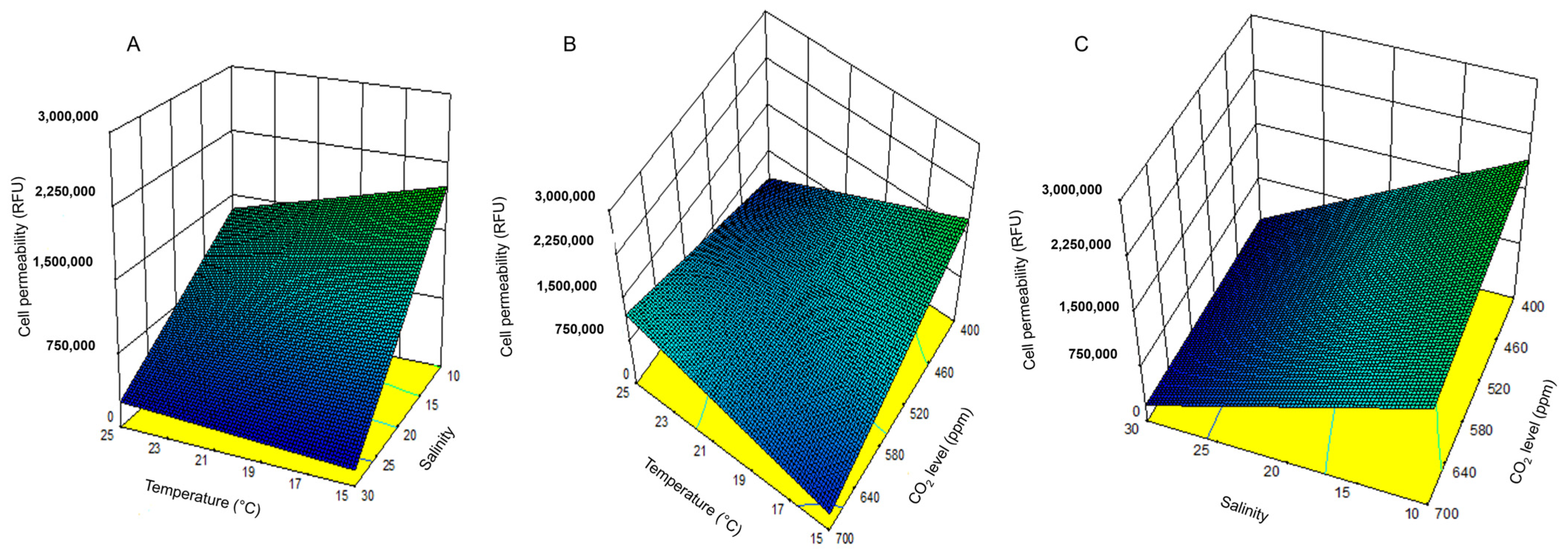
Cell permeability, interpreted here as a proxy for physiological stress, showed an inverse pattern. The highest permeability was recorded under the lowest temperature, salinity, and CO
2 conditions, environments that inhibited growth and yield. This suggests that
H. akashiwo exhibits stress-related membrane responses under suboptimal conditions, potentially contributing to its ichthyotoxicity in colder, fresher, and less buffered waters. Under stress conditions, in particular, some strains of
H. akashiwo are known to release ichthyotoxic metabolites such as reactive oxygen species (ROS), including superoxide and hydrogen peroxide, as well as other bioactive compounds that may disrupt gill function in fish [
43].
Notably, this study found no linear relationship between growth rate and cell permeability, implying that physiological stress and productivity are not necessarily coupled traits. However, a negative correlation between yield and cell permeability suggests a trade-off: conditions that promote biomass accumulation may suppress stress-induced permeability responses. This reinforces the idea that HAB toxicity cannot be predicted solely by cell density and must also consider physiological responses to environmental conditions.
By identifying optimal and suboptimal combinations of temperature, salinity, and CO2, this study provides insight into the ecological niche of H. akashiwo. These findings are particularly relevant for aquaculture risk management, bloom forecasting, and ecosystem health assessments.
4. Conclusions
DOE analysis is a powerful tool for predicting bloom dynamics in future oceans. This study demonstrates that the DOE framework can effectively identify the environmental conditions that optimize growth, yield, and cell membrane permeability in H. akashiwo. Key findings include the following: (1) Maximum growth rates occurred at 25 °C, a salinity of 30, and a CO2 of 400 ppm, with temperature being the most influential factor. (2) The highest yield was recorded at 25 °C, a salinity of 20, and at a CO2 of 700 ppm, indicating a synergistic effect of warming and acidification on biomass accumulation. (3) Peak cell permeability, used as a proxy for ichthyotoxicity in the absence of a known analyte, was observed under the most physiologically stressful conditions (15 °C, lower salinity water, and low CO2), highlighting the concern of this species in developing cooler, fresher waters.
While future ocean conditions may enhance the growth and biomass yield of H. akashiwo, they may concurrently reduce cell permeability and the associated ichthyotoxic potential. However, these conclusions are based on a single strain of H. akashiwo, and the species is known to exhibit strain-specific variability in ichthyotoxicity. Therefore, caution is warranted when generalizing these results. Future research should include additional strains and incorporate other stressors, such as nutrient enrichment and light variability.
Overall, this study contributes to our predictive understanding of how climate change may reshape harmful algal species’ distribution, productivity, and ichthyotoxicity in coastal ecosystems.
5. Materials and Methods
5.1. Culture of Microalgae
A unialgal strain of
H. akashiwo (NWFSC-513), isolated initially from Clam Bay, WA, USA, in 2010, was cultured in f/2 medium (minus silicate) prepared with artificial seawater (ESAW) [
67]. Cultures were maintained in 250 mL Erlenmeyer flasks at 20 ± 1 °C and under continuous illumination (80 ± 5 μmol photons m
−2 s
−1).
5.2. Experimental Conditions
Glassware was acid-washed in 1% HCl overnight and rinsed with ultrapure water. All experimental conditions were tested in triplicate to ensure reproducibility. Salinity was adjusted to 10, 20, or 30 by dissolving NaCl (Sigma-Aldrich, Oakville, ON, Canada) into ESAW. Before each experiment, H. akashiwo was acclimated to the designated salinity and grown to the mid-exponential phase (at Day 3–4 during the exponential growth phase). Cultures were diluted to 10,000 cells mL⁻1 in 50 mL Pyrex tubes (Corning, Corning, NY, USA), each sealed with a silicone stopper equipped with ports for gas, sampling, and pressure release.
Temperature control was achieved using refrigerated/heating circulators (VWR, Mississauga, ON, Canada), with temperature monitored three times daily using a Traceable™ Waterproof Thermometer (Fisher Scientific™, Ottawa, ON, Canada). Light intensity was maintained at 250 ± 10 μmol photons m⁻
2 s⁻
1 using a Quantum Scalar Laboratory sensor (Biospherical Instruments Inc., San Diego, CA, USA). CO
2 (Praxair Canada Inc., London, ON, Canada) was filtered (0.45 μm) and bubbled into cultures for five minutes daily to maintain target concentrations, minimizing shear stress due to the delicate cell-wall-lacking morphology of
H. akashiwo [
43].
5.3. Growth Measurements
Cell density was assessed every 24 ± 1 h using a 0.5 mL aliquot, from which a 30 μL subsample was analyzed via flow cytometry (Turner Designs PhytoCyt flow cytometer (Sunnyvale, CA, USA)) using CFlow
® Plus software, version 1.0.227.5. Cells were gated using forward scatter and chlorophyll-a fluorescence, and density was calculated as:
g is the gated count and
v is the sample volume (μL).
The specific growth rate (
Ke) during the exponential phase was calculated using [
68].
where
N0 and
Nt are the cell concentrations (cells mL
−1) over the (
tt −
t0) period.
The doublings per day (
k) was computed as:
The yield was defined as the mean of the three highest cell densities measured at the end of the exponential or early stationary phases.
5.4. Cell Permeability Assay
Membrane permeability was quantified using SYTOX
® Green (Life Technologies, Carlsbad, CA, USA), which binds nucleic acids in membrane-compromised cells [
69]. A 50 μM stock solution was stored at −20 °C. Background fluorescence was measured using Lugol’s iodine (0.5%
v/
v), and 30 μL samples were analyzed by flow cytometry (Ex: 488 nm, Em: 523 nm). For the test samples, 0.6 μM SYTOX
® Green was added and incubated in the dark for 15 min before measurement. Permeability was expressed as relative fluorescence units (RFU) after background subtraction. This approach offered a reliable proxy for evaluating membrane damage, which is often linked to ichthyotoxin release in
H. akashiwo and other harmful algal species.
5.5. Design of Experiments (DOE)
A two-level complete factorial design (FFD) was used to assess the effects of temperature, salinity, and CO
2 concentration, three key environmental stressors shaped by climate change, on growth, yield, and permeability. High (+1), low (−1), and center (0) coded values were assigned for each factor (
Table 8). The selected ranges reflected values observed in natural aquatic environments as well as projected future conditions, ensuring their ecological relevance and realism.
Following FFD screening, response surface methodology (RSM) was used to explore the optimal conditions using a second-order polynomial model:
where
β0 is the constant parameter;
k is the number of variables;
xi and
xj are the design variables in coded values; and
βi,
βii, and
βij are the coefficients of linear parameters, coefficients of quadratic parameters, and interaction parameters, respectively.
5.6. Statistical Analysis
Design Expert software (v10.0.3.1, Stat-Ease, Inc., Minneapolis, MS, USA) was used to create and analyze the experimental data and to conduct analysis of variance (ANOVA) or experimental design and model fitting. Statistical significance was determined at p < 0.05. All samples and experiments were performed in triplicate. The data were presented as a mean value ± standard deviation.