Clinical Evidence of Bee Venom Acupuncture for Ankle Pain: A Review of Clinical Research
Abstract
1. Introduction
2. Results
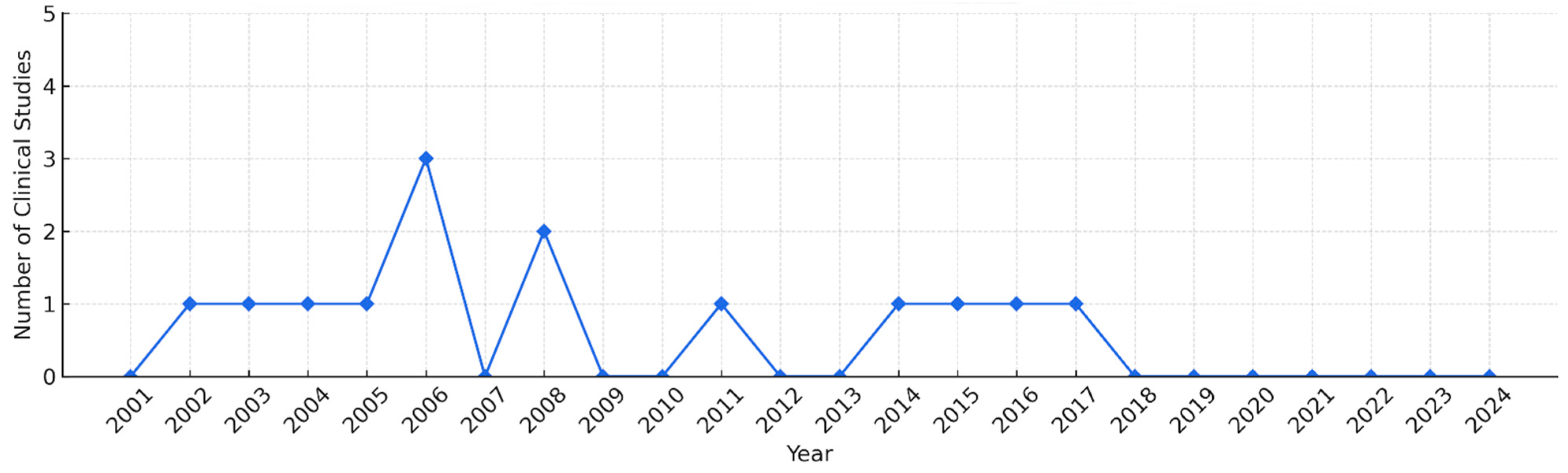
2.1. Study Description
2.2. Medical Conditions and Sample Size
2.3. BVA Treatment
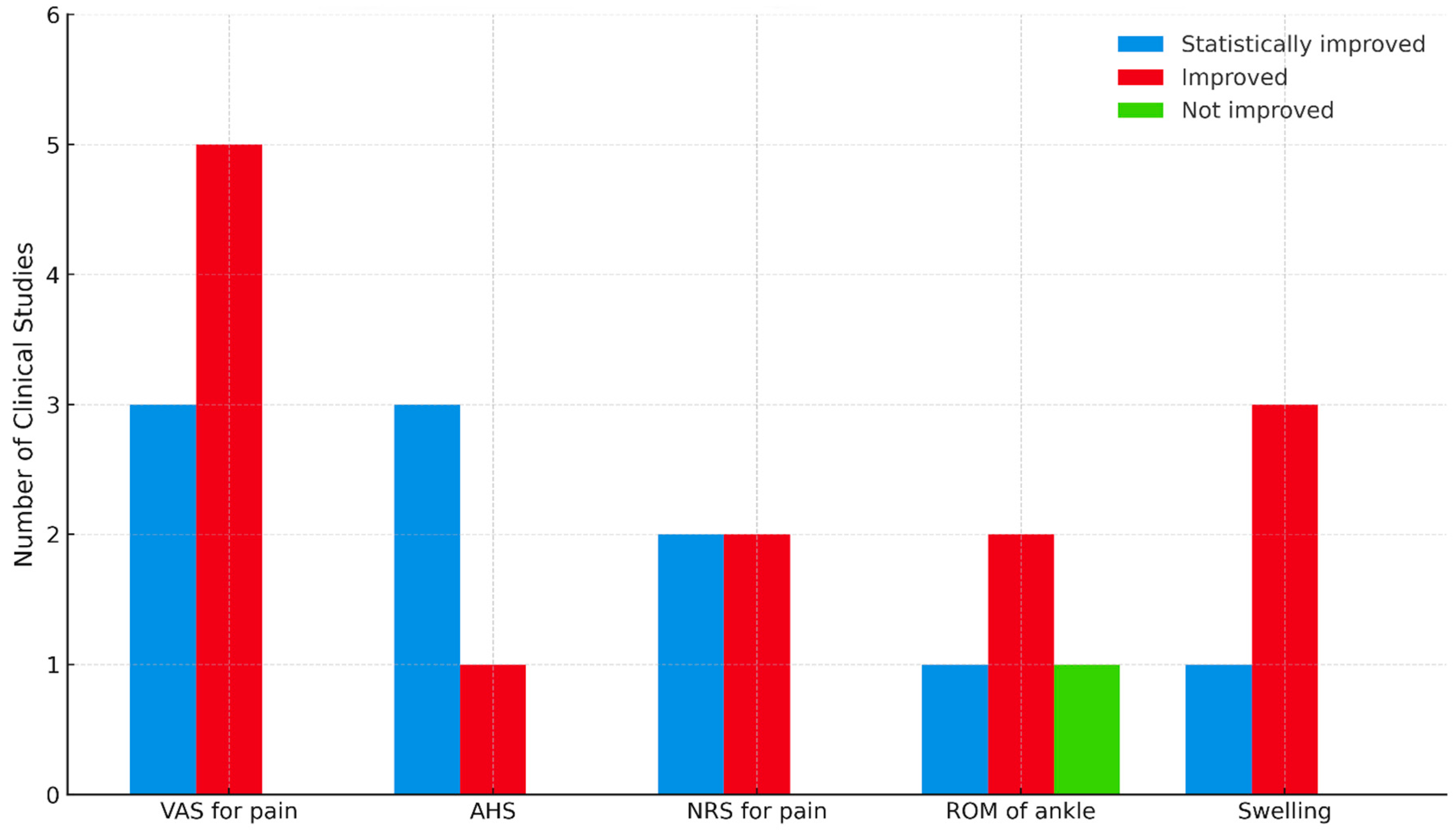
2.4. Outcome Measures
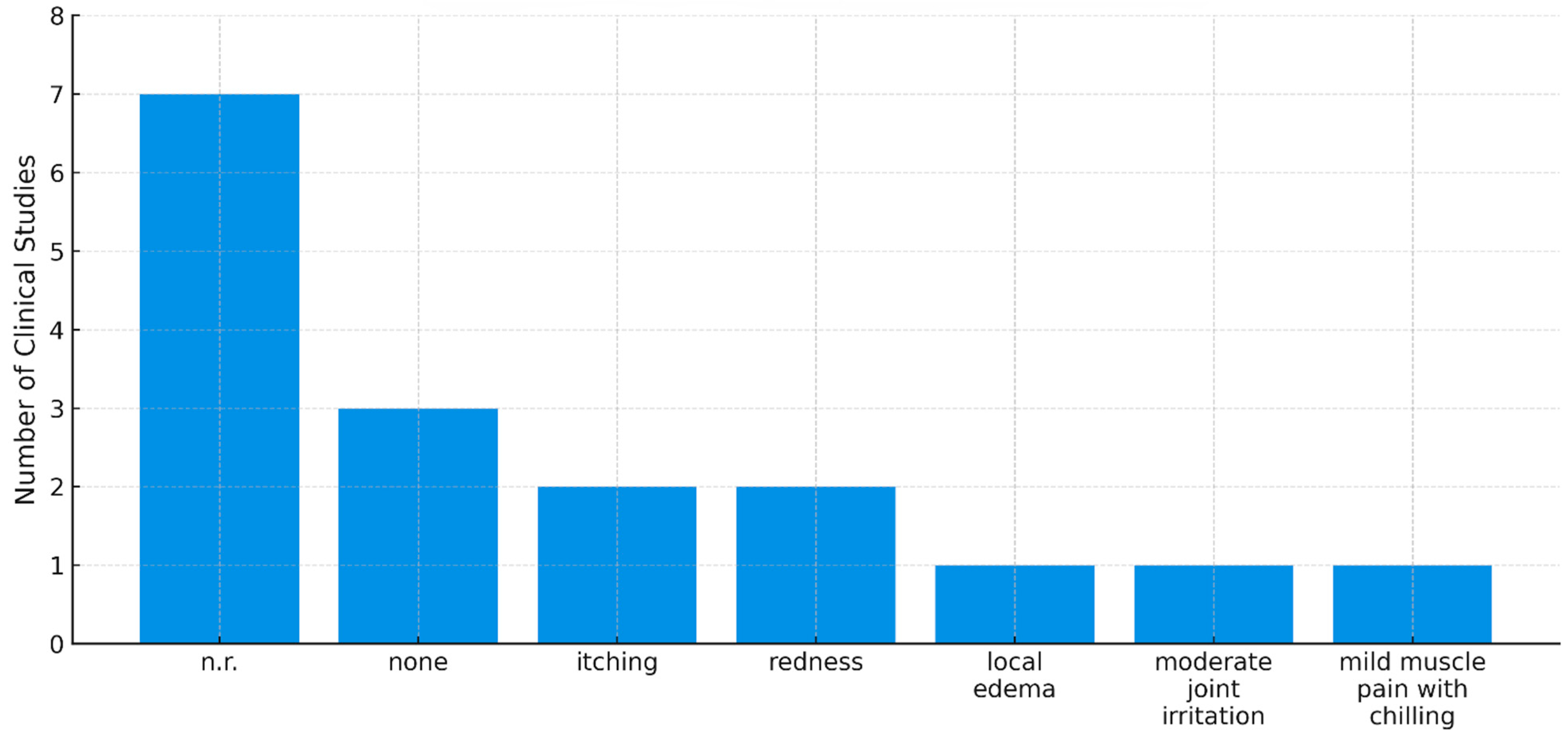
2.5. Adverse Events
2.6. Risk-of-Bias Assessment (ROB)
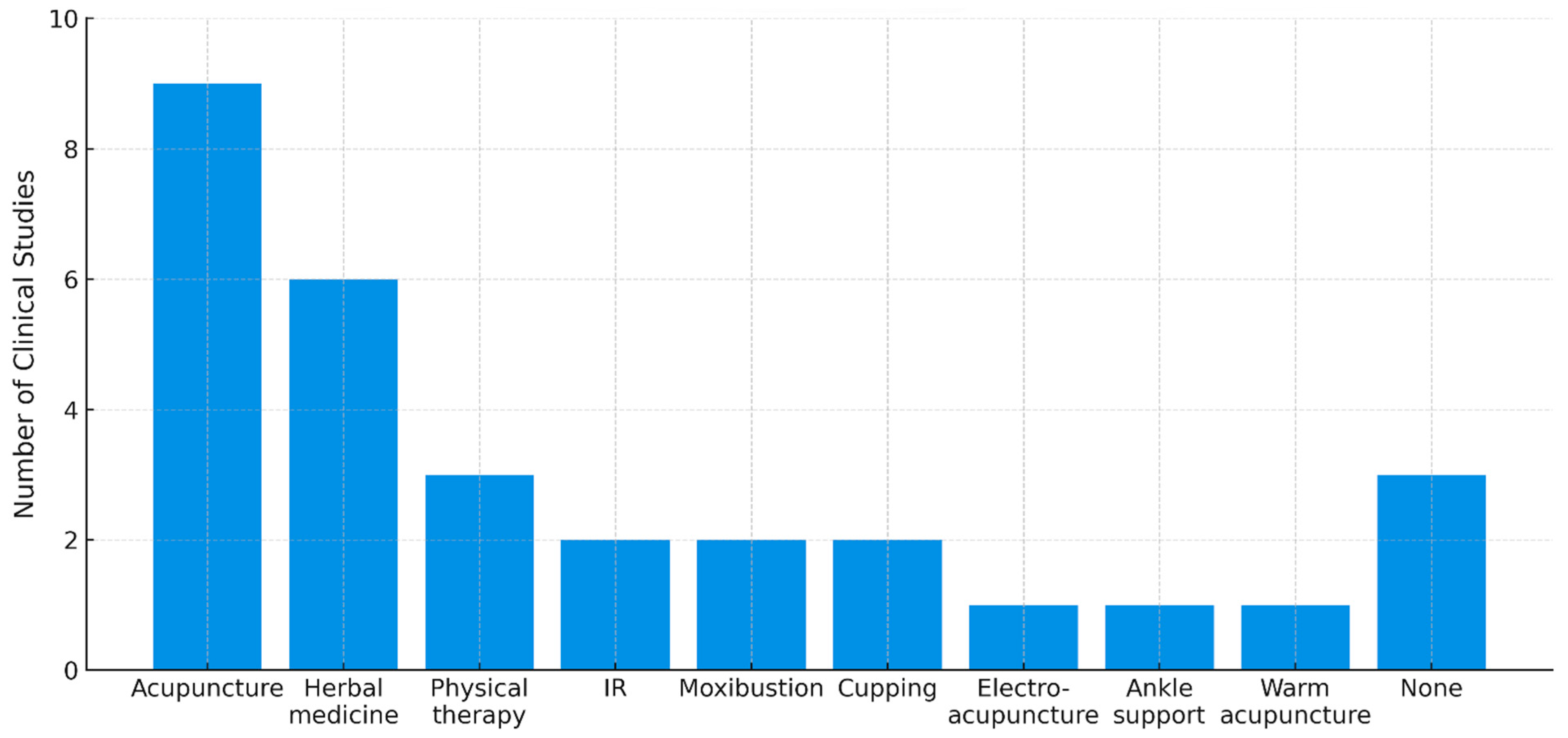
2.7. Co-Interventions
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Data Sources and Searches
5.2. Study Selection
5.3. Data Extraction
5.4. Effectiveness and Safety Assessment
5.5. Quality Assessment of RCTs
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garrick, J.G. The frequency of injury, mechanism of injury, and epidemiology of ankle sprains. Am. J. Sports Med. 1977, 5, 241–242. [Google Scholar] [CrossRef] [PubMed]
- Garrett, W.E., Jr. Strains and sprains in athletes. Postgrad. Med. 1983, 73, 200–209. [Google Scholar] [CrossRef]
- Murray, C.; Marshall, M.; Rathod, T.; Bowen, C.J.; Menz, H.B.; Roddy, E. Population prevalence and distribution of ankle pain and symptomatic radiographic ankle osteoarthritis in community dwelling older adults: A systematic review and cross-sectional study. PLoS ONE 2018, 13, e0193662. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.J.; Roddy, E.; Zhang, W.; Menz, H.B.; Hannan, M.T.; Peat, G.M. The population prevalence of foot and ankle pain in middle and old age: A systematic review. Pain 2011, 152, 2870–2880. [Google Scholar] [CrossRef]
- Vihaan, M. Ankle Pain: A Comprehensive Review of Causes, Evaluation, and Treatment. Clin. Res. Foot Ankle 2023, 11, 430. [Google Scholar] [CrossRef]
- Martin, R.L.; Stewart, G.W.; Conti, S.F. Posttraumatic ankle arthritis: An update on conservative and surgical management. J. Orthop. Sports Phys. Ther. 2007, 37, 253–259. [Google Scholar] [CrossRef]
- Jungmann, P.M.; Lange, T.; Wenning, M.; Baumann, F.A.; Bamberg, F.; Jung, M. Ankle sprains in athletes: Current epidemiological, clinical and imaging trends. Open Access J. Sports Med. 2023, 14, 29–46. [Google Scholar] [CrossRef]
- Safran, M.R.; Zachazewski, J.E.; Benedetti, R.S.; Bartolozzi, A.R., 3rd; Mandelbaum, R. Lateral ankle sprains: A comprehensive review part 2: Treatment and rehabilitation with an emphasis on the athlete. Med. Sci. Sports Exerc. 1999, 31, S438–S447. [Google Scholar] [CrossRef]
- Martin, R.L.; Davenport, T.E.; Paulseth, S.; Wukich, D.K.; Godges, J.J. Ankle stability and movement coordination impairments: Ankle ligament sprains. J. Orthop. Sports Phys. Ther. 2013, 43, A1–A40. [Google Scholar] [CrossRef]
- Vuurberg, G.; Hoorntje, A.; Wink, L.M.; van der Doelen, B.F.W.; van den Bekerom, M.P.; Dekker, R.; van Dijk, C.N.; Krips, R.; Loogman, M.C.M.; Ridderikhof, M.L.; et al. Diagnosis, treatment and prevention of ankle sprains: Update of an evidence-based clinical guideline. Br. J. Sports Med. 2018, 52, 956. [Google Scholar] [CrossRef]
- Liu, A.-F.; Gong, S.-W.; Chen, J.-X.; Zhai, J.-B. Efficacy and Safety of Acupuncture Therapy for Patients with Acute Ankle Sprain: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evid. Based Complement Alternat. Med. 2020, 2020, 9109531. [Google Scholar] [CrossRef] [PubMed]
- Aufschnaiter, A.; Kohler, V.; Khalifa, S.; Abd El-Wahed, A.; Du, M.; El-Seedi, H.; Büttner, S. Apitoxin and Its Components against Cancer, Neurodegeneration and Rheumatoid Arthritis: Limitations and Possibilities. Toxins 2020, 12, 66. [Google Scholar] [CrossRef]
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.M.; Fajloun, Z. Bee venom: Overview of main compounds and bioactivities for therapeutic interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef]
- Lee, S.H.; Choi, S.M.; Yang, E.J. Bee venom acupuncture augments anti-inflammation in the peripheral organs of hSOD1G93A transgenic mice. Toxins 2015, 7, 2835–2844. [Google Scholar] [CrossRef]
- Choi, S.; Chae, H.K.; Heo, H.; Hahm, D.H.; Kim, W.; Kim, S.K. Analgesic effect of melittin on oxaliplatin-induced peripheral neuropathy in rats. Toxins 2019, 11, 396. [Google Scholar] [CrossRef]
- Park, M.; Shin, S. Bee venom acupuncture in traditional Korean medicine: A review of clinical practice guidelines. Toxins 2025, 17, 158. [Google Scholar] [CrossRef]
- Korean Pharmacopuncture Institute. Pharmacopuncturology; Elsevier: Seoul, Republic of Korea, 2011. [Google Scholar]
- Jeong, H.; Kim, K.H.; Ko, S.-g. Effectiveness of Bee Venom Injection for Parkinson’s Disease: A Systematic Review. Toxins 2025, 17, 204. [Google Scholar] [CrossRef]
- Jeong, H.; Jang, S.; Park, J.-K.; Kim, K.H.; Park, J.H.; Lee, G.; Sung, S.-H. Bee Venom Acupuncture for Shoulder Pain: A Literature Review of Clinical Studies. Toxins 2024, 16, 501. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Seo, B.-K. Clinical Effectiveness of Bee Venom Acupuncture for Bone Fractures and Potential Mechanisms: A Narrative Overview. Toxins 2024, 16, 465. [Google Scholar] [CrossRef]
- Sung, S.-H.; Lee, H.-J.; Han, J.-E.; Sung, A.D.-M.; Park, M.; Shin, S.; Jeong, H.I.; Jang, S.; Lee, G. Bee Venom Acupuncture for Neck Pain: A Review of the Korean Literature. Toxins 2023, 15, 129. [Google Scholar] [CrossRef]
- Sung, S.-H.; Han, J.-E.; Lee, H.-J.; Park, M.; Lee, J.-Y.; Jang, S.; Park, J.-K.; Lee, G. Clinical Studies of Bee Venom Acupuncture for Lower Back Pain in the Korean Literature. Toxins 2022, 14, 524. [Google Scholar] [CrossRef] [PubMed]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Simal-Gandara, J. Bee Venom: An Updating Review of Its Bioactive Molecules and Its Health Applications. Nutrients 2020, 12, 3360. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Hsieh, C.-L. Clinical Applications of Bee Venom Acupoint Injection. Toxins 2020, 12, 618. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Ye, Y.; Wang, X.-R.; Lin, L.-T.; Xiao, L.-Y.; Zhou, P.; Shi, G.-X.; Liu, C.-Z. Bee Venom Therapy: Potential Mechanisms and Therapeutic Applications. Toxicon 2018, 148, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Son, M.J.; Choi, J.; Jun, J.H.; Kim, J.I.; Lee, M.S. Bee Venom Acupuncture for Rheumatoid Arthritis: A Systematic Review of Randomised Clinical Trials. BMJ Open 2014, 4, e006140. [Google Scholar] [CrossRef] [PubMed]
- Son, D.J.; Lee, J.W.; Lee, Y.H.; Song, H.S.; Lee, C.K.; Hong, J.T. Therapeutic Application of Anti-Arthritis, Pain-Releasing, and Anti-Cancer Effects of Bee Venom and Its Constituent Compounds. Pharmacol. Ther. 2007, 115, 246–270. [Google Scholar] [CrossRef]
- Ahn, K.H.; Kim, K.H.; Hwang, H.S.; Song, H.S.; Kwon, S.J.; Lee, S.N.; Byun, I.J.; Kang, M.S. The effect of bee-venom acupuncture on heel pain. J. Acupunct. Res. 2002, 19, 149–160. [Google Scholar]
- Ryu, S.M.; Jung, D.Y.; Kim, Y.S.; Lee, S.H. Effect of intra-articular bee venom injection on synovitis of ankle joint with osteonecrosis of talus: A case report. J. Korean Med. Rehab. 2003, 13, 121–127. [Google Scholar]
- Lee, H. The comparative study on the bee-venom therapy and common acupuncture therapy for the acute ankle sprain. Korean J. Acupunct. 2004, 21, 133–143. [Google Scholar]
- Song, H.S. The effect of bee venom acupuncture(BVA) on acute ankle sprain : A randomized controlled trial and double blinding—Pilot study. J. Pharmacopunct. 2005, 8, 11–16. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, K.-U.; Lee, Y.-K.; Lee, K.-M.; Lim, S.-C.; Jung, T.-Y.; Seo, J.-C. A case report of sequela of operation of talus osteonecrosis. J. Pharmacopunct. 2006, 9, 115–120. [Google Scholar] [CrossRef]
- Kim, K.T.; An, B.J.; Kang, M.S.; Song, H.S. A clinical study of bee venom acupuncture therapy on chronic arthritis of ankle. J. Acupunct. Res. 2006, 23, 21–26. [Google Scholar]
- Seo, J.W.; Park, M.J.; Sung, I.H.; Kim, N.O.; Ahn, C.K. A clinical study of bee venom acupuncture therapy on the treatment of acute ankle sprain. J. Acupunct. Res. 2006, 23, 95–103. [Google Scholar]
- Choi, Y.-H.; Kang, J.-H.; Hong, S.-Y.; Heo, D.-S.; Yoon, I.-J. A case study of ankle pain induced rheumatoid arthritis. J. Hyehwa Health Bio Med. 2008, 17, 167–172. [Google Scholar]
- Kang, I.; Moon, J.Y.; Lim, M.J.; Cho, J.H.; Lee, H.E. The comparison Study between different interventions for treating acute ankle sprain. J. Acupunct. Res. 2008, 25, 89–95. [Google Scholar]
- Park, Z.W.; Shin, J.M.; Kim, M.S. A clinical study the case of anterior impingement syndromes of ankle treated by sweet bee venom objectives pharmacopuncture. Korean J. Sports Med. 2011, 11, 69–76. [Google Scholar]
- Won, J.-H.; Ahn, H.-D.; Woo, C.-H. A case report on tarsal tunnel syndrome applied by bee venom and electro-acupuncture therapy. J. East-West Med. 2014, 38, 33–39. [Google Scholar]
- Oh, S.J.; Kim, J.S.; Lee, Y.K.; Lim, S.C.; Lee, H.J. Effects of pharmacopuncture and danggwisu-powder for lateral malleolus avulsion fracture: A case report. J. Acupunct. Res. 2015, 32, 203–210. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, J.Y.; Yeom, S.-R.; Kwon, Y.-D. Case report of peroneal nerve palsy with foot drop treated with complex Korean medical treatment. J. Physiol. Pathol. Korean Med. 2016, 30, 360–365. [Google Scholar] [CrossRef]
- Oh, J.H.; Kim, Y.H.; Kim, J.S.; Lee, H.J.; Lee, Y.K. A case report of post-traumatic acute inflammatory arthritis of ankle joint treated with complex Korean medical treatment including bee-venom therapy. J. Spine Jt. Korean Med. 2017, 14, 89–95. [Google Scholar]
- Sung, S.-H.; Kim, J.-W.; Han, J.-E.; Shin, B.-C.; Park, J.-K.; Lee, G. Animal Venom for Medical Usage in Pharmacopuncture in Korean Medicine: Current Status and Clinical Implication. Toxins 2021, 13, 105. [Google Scholar] [CrossRef]
- Herzog, M.M.; Kerr, Z.Y.; Marshall, S.W.; Wikstrom, E.A. Epidemiology of ankle sprains and chronic ankle instability. J. Athl. Train. 2019, 54, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Hootman, J.M.; Dick, R.; Agel, J. Epidemiology of collegiate injuries for 15 sports: Summary and recommendations for injury prevention initiatives. J. Athl. Train. 2007, 42, 311–319. [Google Scholar] [PubMed] [PubMed Central]
- Wukich, D.K.; Tuason, D.A. Diagnosis and treatment of chronic ankle pain. Instr. Course Lect. 2011, 60, 335–350. [Google Scholar] [PubMed]
- Moreno, M.; Giralt, E. Three valuable peptides from bee and wasp venoms for therapeutic and biotechnological use: Melittin, apamin and mastoparan. Toxins 2015, 7, 1126–1150. [Google Scholar] [CrossRef]
- Bellik, Y. Bee venom: Its potential use in alternative medicine. Anti-Infect. Agents 2015, 13, 3–16. [Google Scholar] [CrossRef]
- Tilinca, M.; Florea, A. Ultrastructural analysis of early toxic effects produced by bee venom phospholipase A2 and melittin in Sertoli cells in rats. Toxicon 2018, 141, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Gajski, G.; Domijan, A.M.; Žegura, B.; Štern, A.; Gerić, M.; Novak Jovanović, I.; Vrhovac, I.; Madunić, J.; Breljak, D.; Filipič, M.; et al. Melittin induced cytogenetic damage, oxidative stress and changes in gene expression in human peripheral blood lymphocytes. Toxicon 2016, 110, 56–67. [Google Scholar] [CrossRef]
- Soltan-Alinejad, P.; Alipour, H.; Meharabani, D.; Azizi, K. Therapeutic potential of bee and scorpion venom Phospholipase A2 (PLA2): A narrative review. Iran. J. Med. Sci. 2022, 47, 300–313. [Google Scholar] [PubMed]
- Liu, N.K.; Xu, X.M. Phospholipase A2 and its molecular mechanism after spinal cord injury. Mol. Neurobiol. 2010, 41, 197–205. [Google Scholar] [CrossRef]
- Gu, H.; Han, S.M.; Park, K.K. Therapeutic effects of apamin as a bee venom component for non-neoplastic disease. Toxins 2020, 12, 195. [Google Scholar] [CrossRef]
- Lallement, G.; Fosbraey, P.; Baille-Le-Crom, V.; Tattersall, J.E.; Blanchet, G.; Wetherell, J.R.; Rice, P.; Passingham, S.L.; Sentenac-Roumanou, H. Compared toxicity of the potassium channel blockers, apamin and dendrotoxin. Toxicology 1995, 104, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Huang, L.; Zhao, S.; Lv, J.; Zhao, H. Acute toxicity of bee venom from Apis mellifera L. in mice: A histopathological study. Indian J. Exp. Biol. 2024, 62, 4. [Google Scholar] [CrossRef]
- Yoo, J.; Lee, G. Adverse events associated with the clinical use of bee venom: A review. Toxins 2022, 14, 562. [Google Scholar] [CrossRef] [PubMed]
- Hariton, E.; Locascio, J.J. Randomised controlled trials—The gold standard for effectiveness research: Study design: Randomised controlled trials. BJOG 2018, 125, 1716. [Google Scholar] [CrossRef]
- Ministry of Health and Welfare; National Development Institute of Korean Medicine; Gallup Korea. 2020 Years National Survey for Traditional Korean Medicine (TKM) Usage; National Development Institute of Korean Medicine: Seoul, Republic of Korea, 2021; Available online: https://nikom.or.kr/koms/board/index.do?menu_idx=19&manage_idx=142 (accessed on 15 March 2025).
- Liu, F.; Panagiotakos, D. Real-world data: A brief review of the methods, applications, challenges and opportunities. BMC Med. Res. Methodol. 2022, 22, 287. [Google Scholar] [CrossRef]
- Minasian, L.M.; O’Mara, A.; Mitchell, S.A. Clinician and patient reporting of symptomatic adverse events in cancer clinical trials: Using CTCAE and PRO-CTCAE® to provide two distinct and complementary perspectives. Patient Relat. Outcome Meas. 2022, 13, 249–258. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]

| First Author (Year) | Study Design | Number of Patients | Diseases | Concentration and Volume | Outcome Measure | Main Result * | Adverse Events | Co-Intervention | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ahn [28] (2002) | Case report | n = 32 | Patients with ankle pain | 1. Concentration: 0.33 mg/mL 2. 1 session: 0.5 mL 3. Total 1 session: n.r. (0.1 cc/point, n.r.) | 1. Symptom Changes | 1. Improved | n.r. | 1. Acupuncture 2. Herbal medicine 3. Cupping |
| 2 | Ryu [29] (2003) | Case report | n = 1 | Synovitis of ankle joint with osteonecrosis of talus patient with ankle pain | 1. Concentration: 0.05 mg/mL 2. 1 session: 0.1–2.5 mL 3. Total 15 sessions: 13.41 mL | 1. Symptom changes (1) Pain (2) Swelling | 1. (1) Improved (2) Improved | Moderate joint irritation, mild muscle pain with chilling in 1 case | None |
| 3 | Lee [30] (2004) | CCT | n = 16 | Acute ankle sprain patients with ankle pain | 1. Concentration: 0.05 mg/mL 2. 1 session: 0.1–2 mL 3. Total 4 sessions: 0.4–8 mL | 1. VAS for pain 2. ROM of ankle 3. Swelling | 1. p < 0.01 2. p < 0.01 3. p < 0.01 | n.r. | None |
| 4 | Song [31] (2005) | RCT | n = 11 | Acute ankle sprain patients with ankle pain | 1. Concentration: 0.33 mg/mL 2. 1 session: 0.04 mL 3. Total 7 sessions: 0.28 mL | 1. VAS for pain 2. AHS | 1. p < 0.01 2. p < 0.001 | Severe itching in 1 case | None |
| 5 | Choi [32] (2006) | Case report | n = 1 | Post-operative patient with ankle pain | 1. Concentration: 0.1 mg/mL, 0.25 mg/mL, or 0.5 mg/mL 2. 1 session: 0.1–0.4 mL (0.1 mg/mL), 0.2–0.4 mL (0.25 mg/mL), or 0. 2 mL (0.5 mg/mL) 3. Total 11 sessions: 1.0 mL (0.1 mg/mL), 1.6 mL (0.25 mg/mL), and 0.2 mL (0.5 mg/mL) | 1. VAS for pain | 1. Improved (10 to 2) | Local edema, redness in 1 case | 1. Acupuncture 2. Herbal medicine |
| 6 | Kim [33] (2006) | CCT | n = 16 | Ankle sprain patients with ankle pain | 1. Concentration: 0.3 mg/mL 2. 1 session: 0.45 mL 3. More than 3 times in total: more than 1.35 mL | VAS for pain | p < 0.05 | None | 1. Acupuncture |
| 7 | Seo [34] (2006) | RCT | n = 11 | Acute ankle sprain patients with ankle pain | 1. Concentration: 0.25 mg/mL or 0.1 mg/mL 2. 1 session: 0.1–0.3 mL 3. Total 3 sessions: 0.3–0.9 mL | 1. NRS for pain 2. AHS | 1. p < 0.01 2. p < 0.01 | n.r. | 1. Acupuncture 2. Physical therapy 3. IR 4. Ankle support |
| 8 | Choi [35] (2008) | Case report | n = 1 | Rheumatoid arthritis patient with ankle pain | 1. Concentration: n.r. 2. 1 session: 1.0 mL 3. Total 16 sessions: 16.0 mL | 1. VAS 2. Walking | 1. Improved (10 to 2) 2. Improved (impossible to possible) | n.r. | 1. Acupuncture 2. Herbal medicine |
| 9 | Kang [36] (2008) | RCT | n = 18 | Acute ankle sprain patients with ankle pain | 1. Concentration: 0.125 mg/mL 2. 1 session: 0.6 mL 3. Total 3 sessions: 1.8 mL | 1. NRS for pain 2. AHS | 1. p < 0.001 2. p < 0.001 | None | IR |
| 10 | Park [37] (2011) | Case report | n = 1 | Anterior impingement syndrome of ankle patient with ankle pain | 1. Concentration: 0.1 mg/mL 2. 1 session: 0.06 mL 3. Total 5 sessions: 0.3 mL | 1. VAS for pain 2. Intensity score (1) Tenderness (2) Swelling | 1. Improved (6 to 1) 2. (1) Improved (2 to 0) (2) Improved (2 to 0) | n.r | 1. Acupuncture 2. Physical therapy |
| 11 | Won [38] (2014) | Case report | n = 1 | Tarsal tunnel syndrome patient with ankle pain | 1. Concentration: 0.1 mg/mL or 0.175 mg/mL 2. 1 session: 0.2–0.6 mL (0.1 mg/mL) or 0.4 mL (0.175 mg/mL) 3. Total 10 sessions: 3.1 mL (0.1 mg/mL) and 1.6 mL (0.175 mg/mL) | 1. VAS for pain | 1. Improved (10 to 3) | n.r. | 1. Electro-acupuncture |
| 12 | Oh [39] (2015) | Case report | n = 1 | Lateral malleolus avulsion fracture patient with ankle pain | 1. Concentration: 0.05 mg/mL or 0.1 mg/mL 2. 1 session: 0.2 mL 3. Total 9 sessions: 0.6 mL(0.05 mg/mL) and 1.2 mL (0.1 mg/mL) | 1. VAS for pain 2. AHS 3. ROM of ankle | 1. Improved (8 to 3) 2. Improved 3. Improved -Flexion: 0° to 20° -Extension: 0° to 10° | Itching, redness in 1 case | 1. Acupuncture 2. Herbal medicine |
| 13 | Kim [40] (2016) | Case report | n = 1 | Peroneal nerve palsy with foot drop patient with ankle pain | 1. Concentration: 0.1 mg/mL 2. 1 session: n.r. 3. Total 15 sessions: n.r. | 1. NRS for pain 2. ROM of ankle (1) Sitting (2) Standing | 1. Improved (10 to 0) 2. (1) Improved (0 to 30°) (2) Improved (0 to 30°) | n.r. | 1. Acupuncture 2. Herbal medicine 3. Moxibustion 4. Cupping 5. Physical therapy |
| 14 | Oh [41] (2017) | Case report | n = 1 | Acute inflammatory arthritis of ankle joint patient with ankle pain | 1. Concentration: 0.05 mg/mL 2. 1 session: 0.3 mL 3. Total 9 sessions: 2.7 mL | 1. NRS for pain 2. Blood test (1) CRP (2) ESR 4. ROM of ankle 5. Swelling | 1. Improved (10 to 2) 2. (1) Improved (Positive to negative) (2) Improved (45 to 23) 4. Not improved 5. Improved (0.7 cm decrease) | None | 1. Acupuncture 2. Warm acupuncture 3. Herbal medicine 4. Moxibustion |
| Medical Conditions of Participants | Number of Studies n (%) | Number of Patients Total (Range) |
|---|---|---|
| Traumatic conditions (ankle sprain, traumatic partial tear, and malleolus avulsion fracture) | 6 (42.9%) | 73 (1–18) |
| Inflammatory conditions (synovitis of ankle joint with osteonecrosis, rheumatoid arthritis, and acute inflammatory arthritis) | 3 (21.4%) | 3 (1) |
| Neuropathic conditions (tarsal tunnel syndrome and peroneal nerve palsy with foot drop) | 2 (14.3%) | 2 (1) |
| Other conditions (post-operative and anterior impingement syndrome) | 2 (14.3%) | 2 (1) |
| Medical Conditions of Participants | Concentration * (mg/mL) | Volume | |
|---|---|---|---|
| Volume Per 1 Session (mL) | Volume for Entire Treatment (mL) | ||
| Traumatic conditions (ankle sprain, traumatic partial tear, and malleolus avulsion fracture) | 0.05–0.33 (3000:1–20,000:1) | 0.04–2.0 | 0.28–8.0 |
| Inflammatory conditions (synovitis of ankle joint with osteonecrosis, rheumatoid arthritis, and acute inflammatory arthritis) | 0.05 (20,000:1) | 0.1–2.5 | 2.7–16.0 |
| Neuropathic conditions (tarsal tunnel syndrome and peroneal nerve palsy with foot drop) | 0.1–0.175 (5700:1–10,000:1) | 0.1–0.6 | 1.6–3.1 |
| Other conditions (post-operative and anterior impingement syndrome) | 0.1–0.5 (2000:1–10,000:1) | 0.06–0.4 | 0.2–1.6 |
| First Author, Year | Selection Bias | Performance Bias | Detection Bias | Attrition Bias | Reporting Bias | |
|---|---|---|---|---|---|---|
| Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting | |
| Song, 2005 [31] | L | L | L | U | L | U |
| Seo, 2006 [34] | L | L | H | U | H | U |
| Kang, 2008 [36] | L | L | H | U | H | U |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, S.-H.; Jeong, H.; Park, J.-H.; Park, M.; Lee, G. Clinical Evidence of Bee Venom Acupuncture for Ankle Pain: A Review of Clinical Research. Toxins 2025, 17, 257. https://doi.org/10.3390/toxins17050257
Sung S-H, Jeong H, Park J-H, Park M, Lee G. Clinical Evidence of Bee Venom Acupuncture for Ankle Pain: A Review of Clinical Research. Toxins. 2025; 17(5):257. https://doi.org/10.3390/toxins17050257
Chicago/Turabian StyleSung, Soo-Hyun, Hyein Jeong, Jong-Hyun Park, Minjung Park, and Gihyun Lee. 2025. "Clinical Evidence of Bee Venom Acupuncture for Ankle Pain: A Review of Clinical Research" Toxins 17, no. 5: 257. https://doi.org/10.3390/toxins17050257
APA StyleSung, S.-H., Jeong, H., Park, J.-H., Park, M., & Lee, G. (2025). Clinical Evidence of Bee Venom Acupuncture for Ankle Pain: A Review of Clinical Research. Toxins, 17(5), 257. https://doi.org/10.3390/toxins17050257






