BLF1 Affects ATP Hydrolysis Catalyzed by Native and Mutated eIF4A1 and eIF4A2 Proteins
Abstract
1. Introduction
2. Results
2.1. eIF4A1 and eIF4A2 Catalyze ATP Hydrolysis Differently
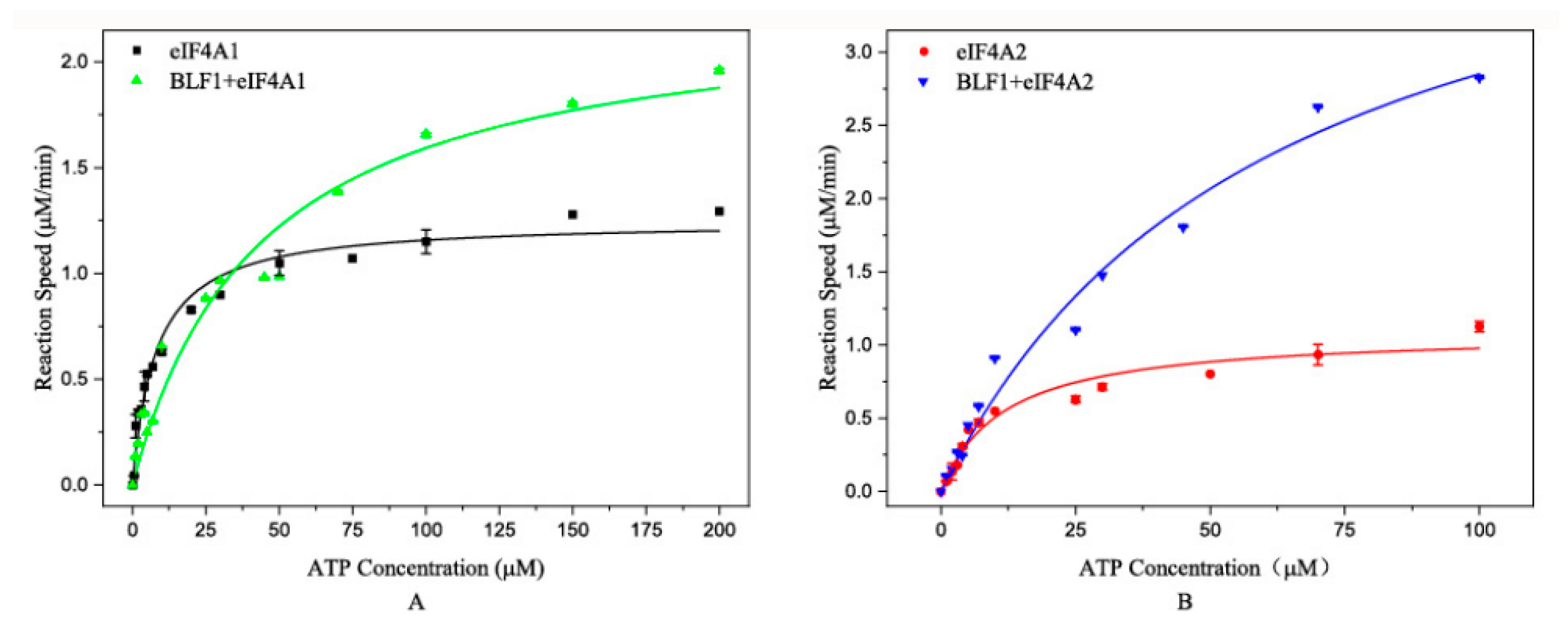
2.2. BLF1 Affects eIF4A1- and eIF4A2-Catalyzed ATP Hydrolysis in Different Ways
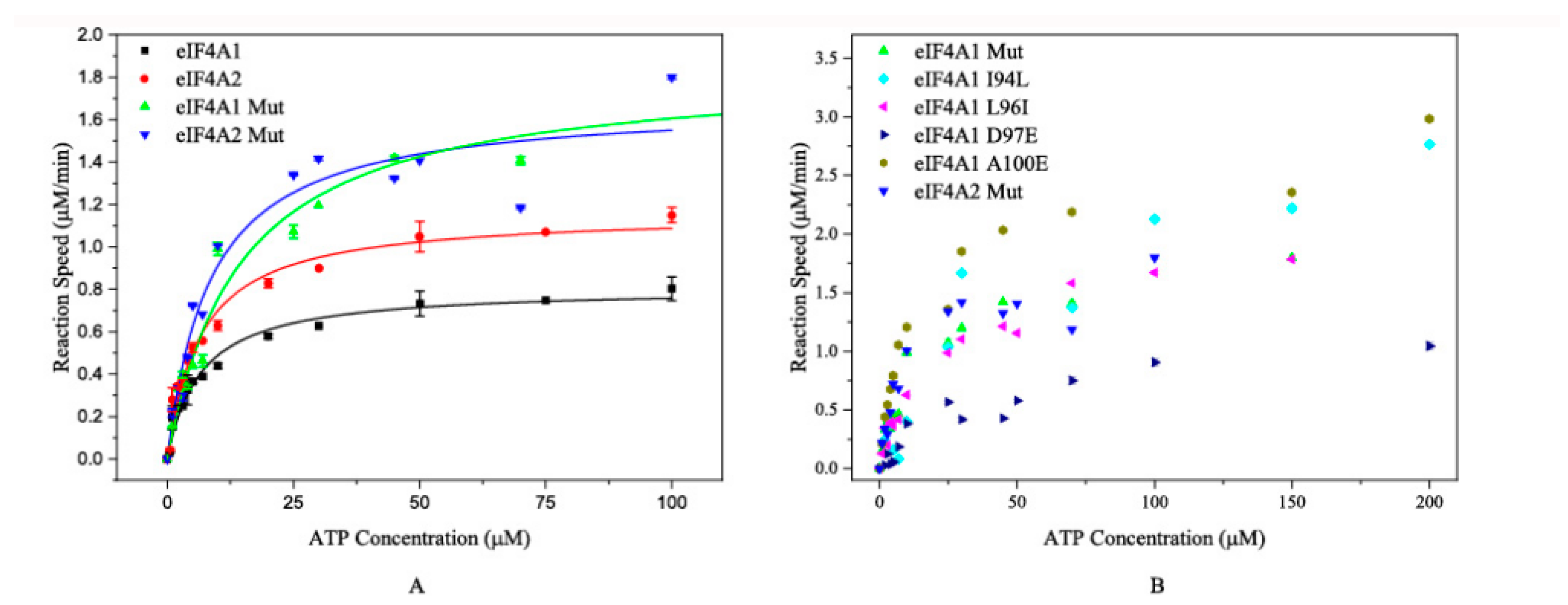
2.3. ATP Hydrolysis Catalyzed by the Mutated Proteins
2.4. BLF1 Also Affects Mutated eIF4A-Catalyzed ATP Hydrolysis
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Evaluating the Environmental Factors (Temperature, Mg2+ Concentration, and pH) on eIF4A1- and eIF4A2-Mediated ATP Hydrolysis
5.2.1. ATP Hydrolysis Measurement
5.2.2. Effects of BLF1 on Wildtype and Mutated eIF4A Proteins
5.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rust, A.; Shah, S.; Hautbergue, G.M.; Davletov, B. Burkholderia Lethal Factor 1, a Novel Anti-Cancer Toxin, Demonstrates Selective Cytotoxicity in MYCN-Amplified Neuroblastoma Cells. Toxins 2018, 10, 261. [Google Scholar] [CrossRef]
- Singh, K.; Singh, A.K.; Uppalapati, S.R.; Kingston, J.J.; Parida, M. Immunogenicity and protective efficacy of Burkholderia pseudomallei BLF1-N and BLF1-C terminal domains against BLF1 toxin. Int. Immunopharmacol. 2019, 77, 105917. [Google Scholar] [CrossRef]
- Babaei Rikan, S.; Sorayaie Azar, A.; Naemi, A.; Bagherzadeh Mohasefi, J.; Pirnejad, H.; Wiil, U.K. Survival prediction of glioblastoma patients using modern deep learning and machine learning techniques. Sci. Rep. 2024, 14, 2371. [Google Scholar] [CrossRef]
- Andreou, A.Z.; Klostermeier, D. The DEAD-box helicase eIF4A: Paradigm or the odd one out? RNA Biol. 2013, 10, 19–32. [Google Scholar] [CrossRef]
- Cruz-Migoni, A.; Hautbergue, G.M.; Artymiuk, P.J.; Baker, P.J.; Bokori-Brown, M.; Chang, C.T.; Dickman, M.J.; Essex-Lopresti, A.; Harding, S.V.; Mahadi, N.M.; et al. A Burkholderia pseudomallei toxin inhibits helicase activity of translation factor eIF4A. Science 2011, 334, 821–824. [Google Scholar] [CrossRef]
- Mobbs, G.W.; Aziz, A.A.; Dix, S.R.; Blackburn, G.M.; Sedelnikova, S.E.; Minshull, T.C.; Dickman, M.J.; Baker, P.J.; Nathan, S.; Raih, M.F.; et al. Molecular basis of specificity and deamidation of eIF4A by Burkholderia Lethal Factor 1. Commun. Biol. 2022, 5, 272. [Google Scholar] [CrossRef]
- Li, C.; Cao, X.W.; Zhao, J.; Wang, F.J. Effective Therapeutic Drug Delivery by GALA3, an Endosomal Escape Peptide with Reduced Hydrophobicity. J. Membr. Biol. 2020, 253, 139–152. [Google Scholar] [CrossRef]
- Walsh, M.J.; Dodd, J.E.; Hautbergue, G.M. Ribosome-inactivating proteins: Potent poisons and molecular tools. Virulence 2013, 4, 774–784. [Google Scholar] [CrossRef]
- Galicia-Vazquez, G.; Cencic, R.; Robert, F.; Agenor, A.Q.; Pelletier, J. A cellular response linking eIF4AI activity to eIF4AII transcription. RNA 2012, 18, 1373–1384. [Google Scholar] [CrossRef]
- Padron, A.; Iwasaki, S.; Ingolia, N.T. Proximity RNA Labeling by APEX-Seq Reveals the Organization of Translation Initiation Complexes and Repressive RNA Granules. Mol. Cell 2019, 75, 875–887 e875. [Google Scholar] [CrossRef]
- Ho, J.J.D.; Lee, S. A Cap for Every Occasion: Alternative eIF4F Complexes. Trends Biochem. Sci. 2016, 41, 821–823. [Google Scholar] [CrossRef]
- Merrick, W.C.; Pavitt, G.D. Protein Synthesis Initiation in Eukaryotic Cells. Cold Spring Harb. Perspect. Biol. 2018, 10, a033092. [Google Scholar] [CrossRef]
- Pestova, T.V.; Hellen, C.U. The structure and function of initiation factors in eukaryotic protein synthesis. Cell. Mol. Life Sci. 2000, 57, 651–674. [Google Scholar] [CrossRef]
- Xue, C.; Gu, X.; Li, G.; Bao, Z.; Li, L. Expression and Functional Roles of Eukaryotic Initiation Factor 4A Family Proteins in Human Cancers. Front. Cell Dev. Biol. 2021, 9, 711965. [Google Scholar] [CrossRef]
- Meijer, H.A.; Kong, Y.W.; Lu, W.T.; Wilczynska, A.; Spriggs, R.V.; Robinson, S.W.; Godfrey, J.D.; Willis, A.E.; Bushell, M. Translational repression and eIF4A2 activity are critical for microRNA-mediated gene regulation. Science 2013, 340, 82–85. [Google Scholar] [CrossRef]
- Shaoyan, X.; Juanjuan, Y.; Yalan, T.; Ping, H.; Jianzhong, L.; Qinian, W. Downregulation of EIF4A2 in non-small-cell lung cancer associates with poor prognosis. Clin. Lung Cancer 2013, 14, 658–665. [Google Scholar] [CrossRef]
- Schütz, P.; Karlberg, T.; van den Berg, S.; Collins, R.; Lehtiö, L.; Högbom, M.; Holmberg-Schiavone, L.; Tempel, W.; Park, H.W.; Hammarström, M.; et al. Comparative structural analysis of human DEAD-box RNA helicases. PLoS ONE 2010, 5, e12791. [Google Scholar] [CrossRef]
- Wu, K.L.; Huang, Y.C.; Wu, Y.Y.; Chang, C.Y.; Chang, Y.Y.; Chiang, H.H.; Liu, L.X.; Tsai, Y.M.; Hung, J.Y. Characterization of the Oncogenic Potential of Eukaryotic Initiation Factor 4A1 in Lung Adenocarcinoma via Cell Cycle Regulation and Immune Microenvironment Reprogramming. Biology 2022, 11, 975. [Google Scholar] [CrossRef]
- Mohr, S.; Bottin, M.C.; Lannes, B.; Neuville, A.; Bellocq, J.P.; Keith, G.; Rihn, B.H. Microdissection, mRNA amplification and microarray: A study of pleural mesothelial and malignant mesothelioma cells. Biochimie 2004, 86, 13–19. [Google Scholar] [CrossRef]
- Yan, L.X.; Wu, Q.N.; Zhang, Y.; Li, Y.Y.; Liao, D.Z.; Hou, J.H.; Fu, J.; Zeng, M.S.; Yun, J.P.; Wu, Q.L.; et al. Knockdown of miR-21 in human breast cancer cell lines inhibits proliferation, in vitro migration and in vivo tumor growth. Breast Cancer Res. 2011, 13, R2. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Zhang, S.; Qiu, L.; Zhang, Y.; Zhou, Y.; Han, J.; Xie, J. Translational Regulation by eIFs and RNA Modifications in Cancer. Genes 2022, 13, 2050. [Google Scholar] [CrossRef]
- Sonenberg, N.; Hinnebusch, A.G. Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell 2009, 136, 731–745. [Google Scholar] [CrossRef]
- Ma, X.M.; Blenis, J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 2009, 10, 307–318. [Google Scholar] [CrossRef]
- Steinberger, J.; Shen, L.; Kiniry, S.J.; Naineni, S.K.; Cencic, R.; Amiri, M.; Aboushawareb, S.A.E.; Chu, J.; Maiga, R.I.; Yachnin, B.J.; et al. Identification and characterization of hippuristanol-resistant mutants reveals eIF4A1 dependencies within mRNA 5′ leader regions. Nucleic Acids Res. 2020, 48, 9521–9537. [Google Scholar] [CrossRef]
- Shen, L.; Pelletier, J. Selective targeting of the DEAD-box RNA helicase eukaryotic initiation factor (eIF) 4A by natural products. Nat. Prod. Rep. 2020, 37, 609–616. [Google Scholar] [CrossRef]
- Zhang, X.; Bi, C.; Lu, T.; Zhang, W.; Yue, T.; Wang, C.; Tian, T.; Zhang, X.; Huang, Y.; Lunning, M.; et al. Targeting translation initiation by synthetic rocaglates for treating MYC-driven lymphomas. Leukemia 2020, 34, 138–150. [Google Scholar] [CrossRef]
- Cencic, R.; Pelletier, J. Hippuristanol—A potent steroid inhibitor of eukaryotic initiation factor 4A. Translation 2016, 4, e1137381. [Google Scholar] [CrossRef]
- Muhamad Ismail, N.A.S.; Yap, S.H.; Mohamad Yussoff, M.A.; Nor Muhammad, N.A.; Firdaus-Raih, M.; Quay, D.H.X. Modeling and computational characterization of a Xanthomonas sp. Hypothetical protein identifies a remote ortholog of Burkholderia lethal factor 1. J. Biomol. Struct. Dyn. 2022, 41, 6027–6039. [Google Scholar] [CrossRef]
- Raza, F.; Waldron, J.A.; Quesne, J.L. Translational dysregulation in cancer: eIF4A isoforms and sequence determinants of eIF4A dependence. Biochem. Soc. Trans. 2015, 43, 1227–1233. [Google Scholar] [CrossRef]
- Tauber, D.; Tauber, G.; Khong, A.; Van Treeck, B.; Pelletier, J.; Parker, R. Modulation of RNA Condensation by the DEAD-Box Protein eIF4A. Cell 2020, 180, 411–426.e416. [Google Scholar] [CrossRef]
- Ripin, N.; Parker, R. Are stress granules the RNA analogs of misfolded protein aggregates? RNA 2022, 28, 67–75. [Google Scholar] [CrossRef]
- Greger, H. Comparative phytochemistry of flavaglines (= rocaglamides), a group of highly bioactive flavolignans from Aglaia species (Meliaceae). Phytochem. Rev. 2022, 21, 725–764. [Google Scholar] [CrossRef]
- Tsumuraya, T.; Ishikawa, C.; Machijima, Y.; Nakachi, S.; Senba, M.; Tanaka, J.; Mori, N. Effects of hippuristanol, an inhibitor of eIF4A, on adult T-cell leukemia. Biochem. Pharmacol. 2011, 81, 713–722. [Google Scholar] [CrossRef]


| Protein | Km (μM) |
|---|---|
| eIF4A1 | 6.55 ± 0.78 |
| eIF4A1 Mut | 15.71 ± 1.51 |
| eIF4A1 I94L | 35.35 ± 2.10 |
| eIF4A1 L96I | 26.65 ± 1.41 |
| eIF4A1 D97E | 36.89 ± 2.05 |
| * eIF4A1 L98F | |
| eIF4A1 A100E | 10.55 ± 0.25 |
| eIF4A2 Mut | 7.85 ± 0.54 |
| eIF4A2 | 11.61 ± 2.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, M.; Cheng, X.; Zhang, Y.; Gu, J.; Mao, X. BLF1 Affects ATP Hydrolysis Catalyzed by Native and Mutated eIF4A1 and eIF4A2 Proteins. Toxins 2025, 17, 232. https://doi.org/10.3390/toxins17050232
An M, Cheng X, Zhang Y, Gu J, Mao X. BLF1 Affects ATP Hydrolysis Catalyzed by Native and Mutated eIF4A1 and eIF4A2 Proteins. Toxins. 2025; 17(5):232. https://doi.org/10.3390/toxins17050232
Chicago/Turabian StyleAn, Min, Xin Cheng, Yu Zhang, Jiang Gu, and Xuhu Mao. 2025. "BLF1 Affects ATP Hydrolysis Catalyzed by Native and Mutated eIF4A1 and eIF4A2 Proteins" Toxins 17, no. 5: 232. https://doi.org/10.3390/toxins17050232
APA StyleAn, M., Cheng, X., Zhang, Y., Gu, J., & Mao, X. (2025). BLF1 Affects ATP Hydrolysis Catalyzed by Native and Mutated eIF4A1 and eIF4A2 Proteins. Toxins, 17(5), 232. https://doi.org/10.3390/toxins17050232




