A Comprehensive Review of Hypotheses About the Biological Function of Zearalenone, and a New Hypothesis for the Function of Resorcylic and Dihydroxyphenylacetic Macrolactones in Fungi
Abstract
1. Objective and Scope
2. Discovery and Practical Use of ZEN
2.1. Zearalenone Derivatives as Commercial Anabolics
2.2. High Yields of ZEN from Fermentation
2.3. Laboratory Synthesis of ZEN
2.4. Zeranol as a Trigger of Trade War Between the European Union and the United States
2.5. Zeranol as an Illicit Doping Agent
2.6. Note About Nomenclature
3. Biosynthesis of ZEN and Its Control
4. Which Fusarium Species Produce ZEN?
4.1. Disentangling the Claim That F. moniliforme or F. verticillioides Produced ZEN
| Year | ZEN Prod. | No. of Strains | Chemistry | Taxonomy 1 | Ref. | Remark |
|---|---|---|---|---|---|---|
| 1969 | yes | 2 | TLC, UV, and IR spectra, GC | ? | [88] | |
| 1970 | no | 8 | Mouse bioassay, TLC | ? | [89] | |
| 1970 | no | 5 | Bioassay, TLC, UV spectrum | ? | [90] | |
| 1974 | no | 3 | TLC | ? | [91] | |
| 1975 | yes | 1 | TLC | ? | [92] | |
| 1975 | yes | ? | TLC, GC-ID, IR | ? | [84] | |
| 1976/77 | yes/no | 1/31 | TLC, UV spectrum | ? | [93,94] | |
| 1976 | yes/no | 2/5 | TLC, UV maxima | Booth 1971 | [95] | |
| 1978 | yes | ? | TLC, UV spectrum, bioassay | ? | [83] | |
| 1978 | yes | 1 | TLC | ? | [96] | |
| 1981 | yes | review | - | - | [97] | P. 893, 902; refers to [95] |
| 1985 | yes | ? | TLC, GC-MS | ? | [86] | |
| 1985 | no | 4 | TLC, GC-MS | Booth 1971 | [98] | |
| 1986 | yes | 1 | HPLC-UV, MS, NMR | ? | [82] | |
| 1986 | yes | review | - | - | [99] | Refers to [88] 5 |
| 1989 | no | 52 2 | TLC | Nelson 1983 | [100] | |
| 1990 | yes | ? | TLC (after agar with mycelia pressed on TLC plates) | Booth 1971, Nelson 1983 | [101] | |
| 1991 | yes | 1 | TLC 4 | Burgess 1983 | [102] | |
| 1991 | yes/no | 1/1 | TLC | Booth 1971 | [103] | |
| 1991 | yes/no | 9/42 | TLC | ? | [104] | |
| 1994 | yes | 1 | TLC | ? | [105] | |
| 1994 | yes | 1 | TLC | ? | [106] | |
| 1996 | review | yes | - | - | [107] | Refers to [82,101,102] |
| 1997 | yes/no | 7/1 | TLC | ? | [108] | |
| 1997 | no | 28 2 | HPLC-UV, UV spectra | Booth 1971, Nelson 1983 | [109] | |
| 1999 | yes/no | 3/695 | TLC | ? | [110] | |
| 2002 | yes | review | - | - | [111] | No reference for the claim 6 |
| 2003 | yes | review | - | - | [112] | Refers to [113] 7 and [114] 8 |
| 2004 | yes | review | - | - | [115] | Refers to [116] 9 |
| 2005 | yes/no | 2 or 3/18 3 | TLC | Hoog 2000, FusKey | [117] | |
| 2007 | yes | review | - | - | [118] | No reference for the claim |
| 2009 | no | 5 | HPLC-MS/MS | Nelson 1983 | [119] | |
| 2010 | yes | review | - | - | [120] | Refers to [121] 9 |
| 2012 | yes | review | - | - | [122] | Refers to [117] |
| 2013 | yes | review | - | - | [123] | No reference for the claim |
| 2013 | yes | 16 | ELISA 10 (R-Biopharm) | Sequencing αTEF | [124] | |
| 2014 | yes | 16 | ELISA (R-Biopharm) | Sequencing αTEF | [125,126] | |
| 2014 | yes | review | - | - | [127] | P. 105, no reference |
| 2016 | yes | review | - | - | [128] | No support for the claim 11 |
| 2021 | yes | review | - | - | [129] | Refers to [118] |
| 2024 | yes/no | 2/14 | ELISA (R-Biopharm) | Seq. αTEF, ITS | [130] | |
| 2025 | yes | review | - | - | [131] | Refers to [129] |
4.2. Misleading Results of ELISA Without Proper Negative Controls
4.3. Perpetuation of Disputed Claims in Reviews
4.4. Search for Homologues of PKS4 and PKS13 in Fusarium Genomes
4.5. Can We Be Certain That No Strain of the Disputed Species Produces ZEN?
4.6. Unexpected Discovery of Genes for ZEN Biosynthesis in the F. verticillioides Genome
4.7. Remembering Wally Marasas
5. HYPOTHESIS 1: ZEN Is a Fungal Hormone Controlling Sexual Development
5.1. Foundation and Experimental Support
5.2. Criticism
5.3. Can the Results Be Explained Without Invoking a Hormone Hypothesis?
6. HYPOTHESIS 2: ZEN Is a Plant Hormone
6.1. Stimulatory Effects of ZEN on Plants
| Year | Plant | Effect | Ref. |
|---|---|---|---|
| 1968 | Tobacco | Growth of callus, formation of shoots and roots | [189] |
| 1978 | Maize | Growth of embryo | [190] |
| 1991 | Lemna perpusilla | Enhancement of flowering | [193] |
| 1993 | Lemna gibba | Enhancement of flowering | [194] |
| 1996 | Maize | Growth of embryo, primary root, and shoot 2 | [191] |
| 1998 | Wheat | Generative growth | [195] |
| 1998 | Wheat | Growth of haploid embryos | [196] |
| 1999 | Wheat | Embryogenesis of wheat callus | [197] |
| 2003 | Wheat | Growth of haploid embryos after pollination with maize | [198] |
| 2006 | Wheat | Wheat production | [199] |
| 2006 | Soybean | Soybean production | [200] |
| 2009 | Soybean, wheat | Photosynthesis rate, seed number, and weight | [201] |
| 2010 | Soybean, wheat | Regeneration of plants from callus | [202] |
| 2010 | Winter wheat | Acceleration of vernalization | [203] |
| 2011 | Soybean and wheat | Efficiency of photosynthesis 3 | [204] |
| 2017 | Maize, wheat, sorghum | Tolerance to osmotic stress | [205] |
| 2019 | Wheat | Microspore embryogenesis | [175] |
| 2021 | Legumes | Yield, protein content, sugar content | [206] |
| 2022 | Tetrastigma hemsleyanum | Growth of shoots and roots | [207] |
| 2023 | Tetrastigma hemsleyanum | Root growth and miRNA accumulation | [208] |
| 2024 | Soybean | ZEN lactonase may stabilize the plant hormone levels | [192] |
6.2. ZEN as an Endogenous Plant Regulator
6.3. Criticism
7. HYPOTHESIS 3: ZEN Is a Virulence Factor of Fusarium spp.
7.1. Origin and Support
7.2. Criticism
7.2.1. Inhibition of Plant Heat Shock Protein by ZEN and Detoxification of ZEN by Plants [231]
7.2.2. Infection of Maize Stalks with ZEN-Nonproducing Mutant [230]
7.2.3. Production of RALs by Virulent Strains of Ilyonectria spp. [232]
7.3. How to Recognize That a Fungal Metabolite Is a Virulence Factor?
7.4. Disclaimer
8. HYPOTHESIS 4: ZEN Is a Defense Metabolite Protecting Fusarium from Mycoparasites and Suppressing Competitors
8.1. Supporting Observations
8.1.1. Inhibition of Fungal Growth by ZEN
8.1.2. Stimulation of ZEN Production by Competing Fungi
8.1.3. Stimulation of ZEN Production by Competing Fungi Overlooked Due to Lack of Normalization
8.1.4. Degradation or Detoxification of ZEN by Fungi That Parasitize ZEN Producers
8.2. Proof by Gene Disruption Experiments
8.3. Cautionary Note About the Use of ZEN and ZEN Mutants as Research Tools
9. Biological Function of Fungal Resorcylic Acid (RALs) and Dihydroxyphenylacetic Acid (DHPLs) Macrolactones
9.1. The Hypothesis
9.2. Fungal Homologues of Polyketide Synthases Responsible for ZEN Synthesis
9.3. Antifungal Activity of RALs and DHPLs
9.4. Conservation of Molecular Targets Hinders Adaptation of Antagonists
| Name | Chemical Structure (Example) 1 | Fungal Producers (Incomplete List) | Ref. to Isolation | Ref. to Anti- Fungal Activity |
|---|---|---|---|---|
| Cryptosporiopsin A |  | Cryptosporiopsis sp. | [345] | [345] |
| Curvularin dehydrocurvularin | 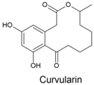 | Curvularia aeria, Alternaria, Penicillium sp., Cochliobolus spicifer, Alternaria longipes | [346,347,348,349] | [350] |
| Hypothemycin | 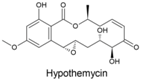 | Hypomyces trichothecoides, Hypomyces subiculosus, Podospora sp. | [351,352,353] | [354] |
| Lasiodiplodin |  | Lasiodiplodia theobromae, L. pseudotheobromae | [355] | [355] |
| Monocillin VI and 4′-hydroxymonocillin IV | 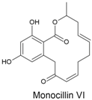 | Paecilomyces | [356,357] | [358] |
| Queenslandon | 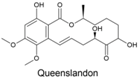 | Chrysosporium queenslandicum | [359] | [359] |
| Radicicol (monorden) |  | Many 2 | Many 2 | [321,322,338,360] |
9.5. Self-Protection of Macrolactone Producers Is Inefficient
9.6. Fitness Costs and Control of Macrolactone Synthesis
9.7. Regulatory Networks and Life History Scenarios
10. Research Challenges
10.1. Asking the Right Questions
10.2. Example: Fusaristatin A in F. pseudograminearum
10.3. Avoiding Pitfalls
10.4. Access to Gene Disruption Mutants
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Proportion of α-ZEL and β-ZEL in Infected Grains in the Field
| Year | Origin | Occurrence Maize | Concentration Maize | Occurrence Wheat | Concentration Wheat | Ref. |
|---|---|---|---|---|---|---|
| 2007 | Nigeria | α-ZEL >> β-ZEL | α-ZEL >> β-ZEL | - | - | [396] |
| 2012 | Belgium | α-ZEL ≈ β-ZEL | α-ZEL > β-ZEL | Low | - 2 | [397] |
| 2014 | Nigeria | Low | - 3 | - | - | [398] |
| 2014 | Belgium | - | - | α-ZEL >> β-ZEL | α-ZEL >> β-ZEL 4 | [399] |
| 2015 | Finland | - | - | α-ZEL ≈ β-ZEL | α-ZEL << β-ZEL | [400] |
| 2016 | Poland | - | - | α-ZEL << β-ZEL | α-ZEL << β-ZEL | [401] |
| 2016 | Nigeria | α-ZEL ≈ β-ZEL | α-ZEL ≈ β-ZEL | - | - | [402] |
| 2021 | Nigeria | α-ZEL ≈ β-ZEL | α-ZEL > β-ZEL | - | - | [403] |
| 2025 | Poland | - | - | α-ZEL << β-ZEL | α-ZEL << β-ZEL 5 | [404] |
References
- European Commission. Commission Recommendation (EU) 2016/1319 of 29 July 2016 amending Recommendation 2006/576/EC as regards deoxynivalenol, zearalenone and ochratoxin A in pet food. Off. J. Eur. Union 2016, L208, 58–60. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on the risks for public health related to the presence of zearalenone in food. EFSA J. 2011, 9, 2197. [Google Scholar] [CrossRef]
- IARC. Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization: Geneva, Switzerland, 2002; Volume 82, ISBN 978-92-832-1282-9. [Google Scholar]
- McNutt, S.; Purwin, P.; Murray, C. Vulvovaginitis in swine. J. Am. Vet. Med. Assoc. 1928, 73, 484–492. [Google Scholar]
- Pullar, E.M.; Lerew, W.M. Vulvovaginitis of swine. Aust. Vet. J. 1937, 13, 28–31. [Google Scholar] [CrossRef]
- McErlean, B.A. Vulvovaginitis of swine. Vet. Rec. 1952, 64, 539–540. [Google Scholar]
- Hennig-Pauka, I.; Koch, F.-J.; Schaumberger, S.; Woechtl, B.; Novak, J.; Sulyok, M.; Nagl, V. Current challenges in the diagnosis of zearalenone toxicosis as illustrated by a field case of hyperestrogenism in suckling piglets. Porc. Health Manag. 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Andrews, F.N.; Stob, M. Anabolic and Estrogenic Compound and Process of Making. U.S. Patent No. 3,196,019, 20 July 1965. [Google Scholar]
- Urry, W.H.; Wehrmeister, H.L.; Hodge, E.B.; Hidy, P.H. The structure of zearalenone. Tetrahedron Lett. 1966, 7, 3109–3114. [Google Scholar] [CrossRef]
- Stob, M.; Baldwin, R.S.; Tuite, J.; Andrews, F.N.; Gillette, K.G. Isolation of an anabolic, uterotrophic compound from corn infected with Gibberella zeae. Nature 1962, 196, 1318. [Google Scholar] [CrossRef]
- Christensen, C.M.; Nelson, G.H.; Mirocha, C.J. Effect on the white rat uterus of a toxic substance isolated from Fusarium. Appl. Microbiol. 1965, 13, 653–659. [Google Scholar] [CrossRef]
- Mirocha, C.J.; Christensen, C.M.; Nelson, G.H. Estrogenic metabolite produced by Fusarium graminearum in stored corn. Appl. Microbiol. 1967, 15, 497–503. [Google Scholar] [CrossRef]
- Shipchandler, M.T. Chemistry of zearalenone and some of its derivatives. Heterocycles 1975, 3, 471–520. [Google Scholar] [CrossRef]
- Strauch, T.; Carroll, J.; Berg, E.; Salfen, B. Zeranol administration to gestating sows alters sow blood serum IGF-I concentrations and improves piglet performance. J. Anim. Vet. Adv. 2004, 3, 270–277. [Google Scholar]
- Hidy, P. Synthetic Fermentation Medium and Process Using Same for Cultivating Gibberella zeae. U.S. Patent No. 3,580,811, 25 May 1971. [Google Scholar]
- Woodings, E.T. Process for Producing Zearalenone. U.S. Patent No. 3,661,714, 9 May 1972. [Google Scholar]
- Keith, C.L. Process for Producing Zearalenone. U.S. Patent No. 3,661,712, 9 May 1972. [Google Scholar]
- McMullen, J.R. Process for Producing Zearalenone. U.S. Patent No. 3,661,713, 9 May 1972. [Google Scholar]
- Young, V.V. Separation of Mixed Diastereoisomers of Zearalanol. U.S. Patent No. 3,687,982, 29 August 1972. [Google Scholar]
- Young, V.V. Preparation of Zearalanone or of Racemic Mixtures of Zearalanol Dimers. U.S. Patent No. 3,818,044, 18 June 1974. [Google Scholar]
- Young, V.V.; Kosewicz, J.S.; Schmitz, F.W. Method for the Recovery of Zearalenone. U.S. Patent No. 4,010,167, 1 March 1977. [Google Scholar]
- Bethell, J.R.; Reid, G.R.; Affleck, K.M.; Breining, T. Process for Manufacturing Zeranol. U.S. Patent No. 8,674,120, 18 March 2014. [Google Scholar]
- Bethell, J.R.; Reid, G.R.; Affleck, K.M.; Breining, T. A Process for Manufacturing Zeranol. European Patent No. 2417118, 27 January 2016. [Google Scholar]
- Czaja, R.; Grenda, V.; Chamberlin, E. Synthesis of the Biologically Active Diastereoisomer of Zearalanol. U.S. Patent No. 3,704,249, 28 November 1972. [Google Scholar]
- Moimas, F.; Clauti, G. A Process for the Separation of Macrocyclic Diastereomers. European Patent Application No. 89103624.6, 13 September 1989. [Google Scholar]
- Gelo, M.; Šunjić, V. Enzymatic kinetic separation of stereoisomeric macrocyclic lactone derivatives, 7α,β-O-acyl trans-zearalenols and 7α,β-O-acyl zearanols. Tetrahedron 1992, 48, 6511–6520. [Google Scholar] [CrossRef]
- Taub, D.; Kuo, C.-H. Zearalenone Ethers. British Patent No. 1,198,953, 15 July 1970. [Google Scholar]
- Robertson, D.E. Zearaline Glycoside Compounds. U.S. Patent No. 3,960,835, 1 June 1976. [Google Scholar]
- Nelson, R. Hormonal Involvement in Sexual Reproduction in the Fungi with Special Reference to F-2, a Fungal Estrogen. In Morphological and Biochemical Events in the Plant-Parasite Interaction; Mochizuki Publishing Co. for the Phytopathological Society of Japan: Tokyo, Japan, 1971; pp. 181–205. [Google Scholar]
- Windels, C.E.; Mirocha, C.J.; Abbas, H.K.; Xie, W. Perithecium production in Fusarium graminearum populations and lack of correlation with zearalenone production. Mycologia 1989, 81, 272–277. [Google Scholar] [CrossRef]
- Marasas, W.F.O.; Nelson, P.E.; Toussoun, T.A. Toxigenic Fusarium Species, Identity and Mycotoxicology; Pennsylvania State University Press: University Park, TX, USA, 1984; ISBN 978-0-271-00348-1. [Google Scholar]
- Speers, G.M.; Meronuck, R.A.; Barnes, D.M.; Mirocha, C.J. Effect of feeding Fusarium roseum F. sp. graminearum contaminated corn and the mycotoxin F-2 on the growing chick and laying hen. Poult. Sci. 1971, 50, 627–633. [Google Scholar] [CrossRef]
- Taub, D.; Girotra, N.N. Zearalenone Intermediates and Their Preparation. U.S. Patent No. 3,624,144, 30 November 1971. [Google Scholar]
- Taub, D.; Girotra, N.N.; Hoffsommer, R.D.; Kuo, C.H.; Slates, H.L.; Weber, S.; Wendler, N.L. Total synthesis of the macrolide zearalenone. Chem. Commun. 1967, 225–226. [Google Scholar] [CrossRef]
- Taub, D.; Girotra, N.N.; Hoffsommer, R.D.; Kuo, C.H.; Slates, H.L.; Weber, S.; Wendler, N.L. Total synthesis of the macrolide, zearalenone. Tetrahedron 1968, 24, 2443–2461. [Google Scholar] [CrossRef] [PubMed]
- Umehara, A.; Kawakita, K.; Sasaki, M. Total synthesis of (−)-zearalenone and (−)-zearalanone: A macrocyclization strategy by Ni/Zr/Cr-mediated reductive ketone coupling. J. Org. Chem. 2024, 89, 13800–13805. [Google Scholar] [CrossRef]
- Nichols, W. History of the TBA Implant Database; Texas Tech University: Lubbock, TX, USA, 2022. [Google Scholar]
- European Council. Council directive of 31 July 1981 concerning the prohibition of certain substances having a hormonal action and of any substances having a thyrostatic action. Off. J. Eur. Communities 1981, 222, 32–33. [Google Scholar]
- European Council. Council Directive 88/299/EEC of 17 May 1988 on trade in animals treated with certain substances having a hormonal action and their meat. Off. J. Eur. Communities 1988, 128, 36–38. [Google Scholar]
- Anonymous. Request by the European Communities for Arbitration Under Article 22.6 of the DSU—WTO Report; WTO: Geneva, Switzerland, 2009. [Google Scholar]
- Johnson, R.; Hanrahan, C. The U.S.-EU Beef Hormone Dispute—Report for United State Congress; R40449; Congressional Research Service: Washington, DC, USA, 2010. [Google Scholar]
- Directorate-General for Communication European Commission. EU Complies with WTO Ruling on Hormone Beef and Calls on USA and Canada to Lift Trade Sanctions—Press Release; Communication European Commission: Brussels, Belgium, 2003. [Google Scholar]
- Congressional Research Service US Congress. U.S.—European Union Disputes in the World Trade Organization; RL31860; Congressional Research Service US Congress: Washington, DC, USA, 2003. [Google Scholar]
- Anonymous. European Communities—Measures Concerning Meat and Meat Products (Hormones)—WTO Report; WTO: Geneve, Switzerland, 2009. [Google Scholar]
- Anonymous. Joint Communication from the European Communities and the United States—WTO Report; WTO: Geneva, Switzerland, 2009. [Google Scholar]
- Laurenza, E. Latest Developments in the Implementation of EC-Hormones II. Eur. J. Risk Regul. 2012, 3, 408–409. [Google Scholar] [CrossRef]
- Anonymous. The U.S.-EU Beef Hormone Dispute—Congress Report; Congressional Research Service: Washington, DC, USA, 2017. [Google Scholar]
- Johnson, R. U.S.-EU Beef Hormone Dispute. In Major Agricultural Trade Issues in 2020—Congretional Research Service Report; Congretional Research Service Reports; United States Congress: Washington, DC, USA, 2020; pp. 35–36. [Google Scholar]
- The Prohibited List. Available online: https://www.wada-ama.org/en/prohibited-list (accessed on 19 December 2024).
- Ayotte, C.; Goudreault, D.; Charlebois, A. Testing for natural and synthetic anabolic agents in human urine. J. Chromatogr. B 1996, 687, 3–25. [Google Scholar] [CrossRef]
- Thevis, M.; Fußhöller, G.; Schänzer, W. Zeranol: Doping offence or mycotoxin? A case-related study. Drug Test. Anal. 2011, 3, 777–783. [Google Scholar] [CrossRef]
- Kintz, P.; Ameline, A.; Raul, J.-S. Discrimination between zeranol and zearalenone exposure using hair analysis. Drug Test. Anal. 2018, 10, 906–909. [Google Scholar] [CrossRef] [PubMed]
- Pompa, G.; Montesissa, C.; Di Lauro, F.M.; Fadini, L. The metabolism of zearalenone in subcellular fractions from rabbit and hen hepatocytes and its estrogenic activity in rabbits. Toxicology 1986, 42, 69–75. [Google Scholar] [CrossRef]
- Pompa, G.; Montesissa, C.; Di Lauro, F.M.; Fadini, L.; Capua, C. Zearanol metabolism by subcellular fractions from lamb liver. J. Vet. Pharmacol. Ther. 1988, 11, 197–203. [Google Scholar] [CrossRef]
- Metzler, M. Proposal for a uniform designation of zearalenone and its metabolites. Mycotoxin Res. 2011, 27, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Mirocha, C.J.; Pathre, S.V. Mycotoxins—Their biosynthesis in fungi: Zearalenone biosynthesis. J. Food Prot. 1979, 42, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Gaffoor, I.; Trail, F. Characterization of two polyketide synthase genes involved in zearalenone biosynthesis in Gibberella zeae. Appl. Environ. Microbiol. 2006, 72, 1793–1799. [Google Scholar] [CrossRef]
- Lysøe, E.; Klemsdal, S.S.; Bone, K.R.; Frandsen, R.J.N.; Johansen, T.; Thrane, U.; Giese, H. The PKS4 gene of Fusarium graminearum is essential for zearalenone production. Appl. Environ. Microbiol. 2006, 72, 3924–3932. [Google Scholar] [CrossRef]
- Kim, Y.-T.; Lee, Y.-R.; Jin, J.; Han, K.-H.; Kim, H.; Kim, J.-C.; Lee, T.; Yun, S.-H.; Lee, Y.-W. Two different polyketide synthase genes are required for synthesis of zearalenone in Gibberella zeae. Mol. Microbiol. 2005, 58, 1102–1113. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-E.; Son, H.; Lee, Y.-W. Biosynthetic mechanism and regulation of zearalenone in Fusarium graminearum. JSM Mycotoxins 2018, 68, 1–6. [Google Scholar] [CrossRef]
- Park, A.R.; Son, H.; Min, K.; Park, J.; Goo, J.H.; Rhee, S.; Chae, S.-K.; Lee, Y.-W. Autoregulation of ZEB2 expression for zearalenone production in Fusarium graminearum. Mol. Microbiol. 2015, 97, 942–956. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhou, X.; Gu, X.; Cao, S.; Wang, C.; Xu, J.-R. The cAMP-PKA pathway regulates growth, sexual and asexual differentiation, and pathogenesis in Fusarium graminearum. Mol. Plant Microbe Interact. 2014, 27, 557–566. [Google Scholar] [CrossRef]
- Park, A.R.; Fu, M.; Shin, J.Y.; Son, H.; Lee, Y.-W. The protein kinase a pathway regulates zearalenone production by modulating alternative ZEB2 transcription. J. Microbiol. Biotechnol. 2016, 26, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Bayram, Ö.S.; Bayram, Ö.; Valerius, O.; Park, H.S.; Irniger, S.; Gerke, J.; Ni, M.; Han, K.-H.; Yu, J.-H.; Braus, G.H. LaeA control of velvet family regulatory proteins for light-dependent development and fungal cell-type specificity. PLoS Genet. 2010, 6, e1001226. [Google Scholar] [CrossRef]
- Lee, J.; Myong, K.; Kim, J.-E.; Kim, H.-K.; Yun, S.-H.; Lee, Y.-W. FgVelB globally regulates sexual reproduction, mycotoxin production and pathogenicity in the cereal pathogen Fusarium graminearum. Microbiology 2012, 158, 1723–1733. [Google Scholar] [CrossRef]
- Kim, H.-K.; Lee, S.; Jo, S.-M.; McCormick, S.P.; Butchko, R.A.E.; Proctor, R.H.; Yun, S.-H. Functional roles of FgLaeA in controlling secondary metabolism, sexual development, and virulence in Fusarium graminearum. PLoS ONE 2013, 8, e68441. [Google Scholar] [CrossRef]
- Bok, J.W.; Noordermeer, D.; Kale, S.P.; Keller, N.P. Secondary metabolic gene cluster silencing in Aspergillus nidulans. Mol. Microbiol. 2006, 61, 1636–1645. [Google Scholar] [CrossRef]
- Yang, K.; Tian, J.; Keller, N.P. Post-translational modifications drive secondary metabolite biosynthesis in: A review. Environ. Microbiol. 2022, 24, 2857–2881. [Google Scholar] [CrossRef]
- Palmer, J.M.; Theisen, J.M.; Duran, R.M.; Grayburn, W.S.; Calvo, A.M.; Keller, N.P. Secondary metabolism and development is mediated by LlmF control of VeA subcellular localization in Aspergillus nidulans. PLoS Genet. 2013, 9, e1003193. [Google Scholar] [CrossRef]
- Bachleitner, S.; Sulyok, M.; Sørensen, J.L.; Strauss, J.; Studt, L. The H4K20 methyltransferase Kmt5 is involved in secondary metabolism and stress response in phytopathogenic Fusarium species. Fungal Genet. Biol. 2021, 155, 103602. [Google Scholar] [CrossRef]
- Lysøe, E.; Bone, K.R.; Klemsdal, S.S. Identification of up-regulated genes during zearalenone biosynthesis in Fusarium. Eur. J. Plant Pathol. 2008, 122, 505–516. [Google Scholar] [CrossRef]
- Lee, S.; Son, H.; Lee, J.; Lee, Y.-R.; Lee, Y.-W. A putative ABC transporter gene, ZRA1, is required for zearalenone production in Gibberella zeae. Curr. Genet. 2011, 57, 343–351. [Google Scholar] [CrossRef]
- Abou Ammar, G.; Tryono, R.; Doell, K.; Karlovsky, P.; Deising, H.B.; Wirsel, S.G.R. Identification of ABC transporter genes of Fusarium graminearum with roles in azole tolerance and/or virulence. PLoS ONE 2013, 8, e79042. [Google Scholar] [CrossRef]
- Aoki, T.; Ward, T.J.; Kistler, H.C.; O’Donnell, K. Systematics, phylogeny and trichothecene mycotoxin potential of Fusarium head blight cereal pathogens. Mycotoxins 2012, 62, 91–102. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Costa, M.M.; Broders, K.; Becker, Y.; Maier, W.; Yurkov, A.; Kermode, A.; Buddie, A.G.; Ryan, M.J.; Schumacher, R.K.; et al. An integrative re-evaluation of the Fusarium sambucinum species complex. Stud. Mycol. 2025, 110, 1–110. [Google Scholar] [CrossRef]
- Xia, J.W.; Sandoval-Denis, M.; Crous, P.W.; Zhang, X.G.; Lombard, L. Numbers to names—Restyling the Fusarium incarnatum-equiseti species complex. Persoonia Mol. Phylogeny Evol. Fungi 2019, 43, 186–221. [Google Scholar] [CrossRef] [PubMed]
- Munkvold, G.P.; Proctor, R.H.; Moretti, A. Mycotoxin production in Fusarium according to contemporary species concepts. Annu. Rev. Phytopathol. 2021, 59, 373–402. [Google Scholar] [CrossRef]
- Nirenberg, H. Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-Sektion Liseola. Mitt. Biol. Bundesanst. Land-u. Forstwirtsch. Berlin-Dahlem 1976, 169, 1–117. [Google Scholar]
- O’Donnell, K.; Cigelnik, E.; Nirenberg, H.I. Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 1998, 90, 465–493. [Google Scholar] [CrossRef]
- Leslie, J.F.; Zeller, K.A.; Lamprecht, S.C.; Rheeder, J.P.; Marasas, W.F.O. Toxicity, pathogenicity, and genetic differentiation of five species of Fusarium from sorghum and millet. Phytopathology 2005, 95, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Fotso, J.; Leslie, J.F.; Wu, X.; Vandervelde, D.; Thakur, R.A. Characterization of Bostrycoidin: An Analytical Analog of Zearalenone. J. Food Sci. 2004, 69, 227–232. [Google Scholar] [CrossRef]
- Chakrabarti, D.; Ghosal, S. Occurrence of free and conjugated 12,13-epoxytrichothecenes and zearalenone in banana fruits infected with Fusarium moniliforme. Appl. Environ. Microbiol. 1986, 51, 217–219. [Google Scholar] [CrossRef]
- Ghosal, S.; Biswas, K.; Srivastava, R.S.; Chakrabarti, D.K.; Chaudhary, K.C.B. Toxic substances produced by Fusarium V: Occurrence of zearalenone, diacetoxyscirpenol, and T-2 toxin in moldy corn infected with Fusarium moniliforme Sheld. J. Pharm. Sci. 1978, 67, 1768–1769. [Google Scholar] [CrossRef] [PubMed]
- Lásztity, R.; Wöller, L. Toxinerzeugung von Fusariumarten und ihr Vorkommen in Landwirtschaftlichen Produkten. Period. Polytech. Chem. Eng. 1975, 19, 249–262. [Google Scholar]
- El-Kady, I.A.; El-Maraghy, S.S. Screening of zearalenone-producing Fusarium species in Egypt and chemically defined medium for production of the toxin. Mycopathologia 1982, 78, 25–29. [Google Scholar] [CrossRef]
- Richardson, K.E.; Hagler, W.M.; Campbell, C.L.; Hamilton, P.B. Production of zearalenone, T-2 toxin, and deoxynivalenol by Fusarium spp. isolated from plant materials grown in North Carolina. Mycopathologia 1985, 90, 155–160. [Google Scholar] [CrossRef]
- Chakrabarti, D.K.; Ghosal, S. Mycotoxins produced by Fusarium oxysporum in the seeds of Brassica campestris during storage. Mycopathologia 1987, 97, 69–75. [Google Scholar] [CrossRef]
- Mirocha, C.J.; Christensen, C.M.; Nelson, G.H. Biosynthesis of the fungal estrogen F-2 and a naturally occuring derivative (F-3) by Fusarium moniliforme. Appl. Microbiol. 1969, 17, 482–483. [Google Scholar] [CrossRef]
- Caldwell, R.W.; Tuite, J.; Stob, M.; Baldwin, R. Zearalenone production by Fusarium species. Appl. Microbiol. 1970, 20, 31–34. [Google Scholar] [CrossRef]
- Eugenio, C.P.; Christensen, C.M.; Mirocha, C.J. Factors affecting production of the mycotoxin F-2 by Fusarium roseum. Phytopathology 1970, 60, 1055–1057. [Google Scholar] [CrossRef]
- Ishii, K.; Sawano, M.; Ueno, Y.; Tsunoda, H. Distribution of zearalenone-producing Fusarium species in Japan. Appl. Microbiol. 1974, 27, 625–628. [Google Scholar] [CrossRef]
- Kotsonis, F.N.; Smalley, E.B.; Ellison, R.A.; Gale, C.M. Feed refusal factors in pure cultures of Fusarium roseum graminearum. Appl. Microbiol. 1975, 30, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Bottalico, A. La presenza di Fusarium moniliforme Sheld. nelle cariossidi del Granturco (Zea mays L.) quale problema fitopatologico e micotossicologico in Italia. II. Aspetti micotossicolo. Phytopathol. Mediterr. 1976, 15, 54–58. [Google Scholar]
- Bottalico, A. Production of zearalenone by Fusarium spp. from cereals, in Italy. Phytopathol. Mediterr. 1977, 16, 75–78. [Google Scholar]
- Hacking, A.; Rosser, W.R.; Dervish, M.T. Zearalenone-producing species of Fusarium on barley seed. Ann. Appl. Biol. 1976, 84, 7–11. [Google Scholar] [CrossRef]
- Martin, P.M.D.; Keen, P. The occurrence of zearalanone in raw and fermented products from Swaziland and Lesotho. Sabouraudia J. Med. Vet. Mycol. 1978, 16, 15–22. [Google Scholar] [CrossRef]
- Cole, R.J.; Cox, R.H. Fusarium Toxins. In Handbook of Toxic Fungal Metabolites; Academic Press: New York, NY, USA; London, UK; Toronto, ON, Canada, 1981; pp. 893–910. [Google Scholar]
- Bottalico, A.; Visconti, A.; Logrieco, A.; Solfrizzo, M.; Mirocha, C.J. Occurrence of zearalenols (diastereomeric mixture) in corn stalk rot and their production by associated Fusarium species. Appl. Environ. Microbiol. 1985, 49, 547–551. [Google Scholar] [CrossRef]
- Haschek, W.M.; Haliburton, J. Fusarium Moniliforme and Zearalenone Toxicoses in Domestic Animals: A Review. In Diagnosis of Mycotoxicosis; Martinus Nijhoff Publishers: Dordrecht, The Netherlands, 1986; pp. 213–235. [Google Scholar]
- Abbas, H.K.; Mirocha, C.J.; Kommedahl, T.; Vesonder, R.F.; Golinski, P. Production of trichothecene and non-trichothecene mycotoxins by Fusarium species isolated from maize in Minnesota. Mycopathologia 1989, 108, 55–58. [Google Scholar] [CrossRef]
- Hashmi, M.H.; Thrane, U. Mycotoxins and other secondary metabolites in species of Fusarium isolated from seeds of capsicum, coriander and fenugreek. Pak. J. Bot. 1990, 22, 106–116. [Google Scholar]
- Hussein, H.M.; Baxter, M.; Andrew, I.G.; Franich, R.A. Mycotoxin production by Fusarium species isolated from New Zealand maize fields. Mycopathologia 1991, 113, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Bresler, G.; Vaamonde, G.; Brizzio, S. Natural occurrence of zearalenone and toxicogenic fungi in amaranth grain. Int. J. Food Microbiol. 1991, 13, 75–80. [Google Scholar] [CrossRef]
- Ranjan, K.S.; Sinha, A.K. Occurrence of mycotoxigenic fungi and mycotoxins in animal feed from Bihar, india. J. Sci. Food Agric. 1991, 56, 39–47. [Google Scholar] [CrossRef]
- Gupta, R. Effect of food preservatives on zearalenone production by Fusarium moniliforme. J. Phytol. Res. 1994, 7, 21–24. [Google Scholar]
- El-Kady, I.A.; Abdel-Mallek, A.Y.; El Maraghy, S.S.M.; Hassan, H.A.H. Toxigenic moulds in pesticide treated liquid medium. Cryptogam. Mycol. 1994, 15, 75–81. [Google Scholar] [CrossRef]
- De Nijs, M.; Rombouts, F.; Notermans, S. Fusarium molds and their mycotoxins. J. Food Saf. 1996, 16, 15–58. [Google Scholar] [CrossRef]
- Chandra, R.; Sarbhoy, A. Production of aflatoxins and zearalenone by the toxigenic fungal isolates obtained from stored food grains of commercial crops. Indian Phytopathol. 1997, 50, 458–468. [Google Scholar]
- Jimenez, M.; Huerta, T.; Mateo, R. Mycotoxin production by Fusarium species isolated from bananas. Appl. Environ. Microbiol. 1997, 63, 364–369. [Google Scholar] [CrossRef]
- Mubatanhema, W.; Moss, M.O.; Frank, M.J.; Wilson, D.M. Prevalence of Fusarium species of the Liseola section on Zimbabwean corn and their ability to produce the mycotoxins zearalenone, moniliformin and fumonisin B1. Mycopathologia 1999, 148, 157–163. [Google Scholar] [CrossRef]
- Gajecki, M. Zearalenone—Undesirable substances in feed. Pol. J. Vet. Sci. 2002, 5, 117–122. [Google Scholar]
- Conková, E.; Laciaková, A.; Kovác, G.; Seidel, H. Fusarial toxins and their role in animal diseases. Vet. J. 2003, 165, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Gedek, B. Kompendium Der Medizinischen Mykologie; Pareys Studientexte; Verlag Paul Parey: Berlin, Germany, 1980. [Google Scholar]
- Pittet, A. Natural occurence of mycotoxins in foods and feeds—An updated review. Rev. Med. Veterinaire 1998, 149, 479–492. [Google Scholar]
- Alldrick, A.; Hajselova, M. Zearalenone. In Mycotoxins in Food—Detection and Control; Woodhead Publishing Limited: Abington, UK, 2004; pp. 353–366. [Google Scholar]
- Chelkowski, J. Fusarium and Mycotoxins. In Mycotoxins in Agriculture and Food Safety; Marcel Dekker: New York, NY, USA, 1998; pp. 45–64. [Google Scholar]
- Cvetnic, Z.; Pepeljnjak, S.; Segvic, M. Toxigenic potential of Fusarium species isolated from non-harvested maize. Arh. Hig. Rada Toksikol. 2005, 56, 275–280. [Google Scholar]
- Mostrom, M. Zearalenone. In Veterinary Toxicology—Basic and Clinical Principles; Academic Press: Cambridge, MA, USA, 2007; pp. 977–982. [Google Scholar]
- Aoyama, K.; Ishikuro, E.; Nishiwaki, M.; Ichinoe, M. Zearalenone contamination and the causative fungi in sorghum. Food Hyg. Saf. Sci. 2009, 50, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Gajecki, M.; Gajecka, M.; Jakimiuk, E.; Zielonka, L.; Obremski, K. Zearalenone: Undesirable Substance. In Mycotoxins in Food, Feed and Bioweapons; Springer: Heidelberg, Germany; Dordrecht, The Netherlands; London, UK; New York, NY, USA, 2010; pp. 131–144. ISBN 978-3-642-00724-8. [Google Scholar]
- Golinski, P.; Kaczmarek, Z.; Kiecana, I.; Wisniewska, H.; Kaptur, P.; Kostecki, M.; Chelkowski, J. Fusarium head blight of common polish winter wheat cultivars—Comparison of effects of Fusarium avenaceum and Fusarium culmorum on yield components. J. Phytopathol. 2002, 150, 135–141. [Google Scholar] [CrossRef]
- Marín, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Reduction of mycotoxins and toxigenic fungi in the Mediterranean basin maize chain. Phytopathol. Mediterr. 2012, 51, 93–118. [Google Scholar]
- Afsah-Hejri, L.; Jinap, S.; Hajeb, P.; Radu, S.; Shakibazadeh, S. A review on mycotoxins in food and feed: Malaysia case study. Compr. Rev. Food Sci. Food Saf. 2013, 12, 629–651. [Google Scholar] [CrossRef]
- Matny, O. Mycotoxin production by Fusarium spp. isolates on wheat straw in laboratory condition. Res. J. Biotechnol. 2013, 8, 35–41. [Google Scholar] [CrossRef]
- Matny, O.N. Screening of mycotoxin produced by Fusarium verticillioides and F. proliferatum in culture media. Asian J. Agric. Rural Dev. 2014, 4, 36–41. Available online: https://ageconsearch.umn.edu/record/198380/files/5-358-AJARD-4_1_2014-36-41.pdf (accessed on 13 June 2017).
- Matny, O.N. Screening of mycotoxin produced by Fusarium verticillioides and F. proliferatum in culture media. Asian J. Agric. Rural Dev. 2013, 12, 1001–1006. Available online: https://www.researchgate.net/publication/259996854_Screening_of_Mycotoxin_Produced_by_Fusarium_Verticillioides_and_F_Proliferatum_in_Culture_Media (accessed on 13 June 2017).
- Nesic, K.; Ivanovic, S.; Nesic, V. Fusarial toxins: Secondary metabolites of Fusarium fungi. Rev. Environ. Contam. Toxicol. 2014, 228, 101–120. [Google Scholar] [CrossRef]
- Smith, M.-C.; Madec, S.; Coton, E.; Hymery, N. Natural co-occurrence of mycotoxins in foods and feeds and their in vitro combined toxicological effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Ropejko, K.; Twarużek, M. Zearalenone and its metabolites—General overview, occurrence, and toxicity. Toxins 2021, 13, 35. [Google Scholar] [CrossRef]
- Al-Rashdi, F.K.H.; Al-Sadi, A.M.; Waly, M.I.; Hussain, S.; Velazhahan, R. Assessment of fumonisin, deoxynivalenol, and zearalenone levels and the occurrence of mycotoxigenic Fusarium species in cereal grains from Muscat, sultanate of Oman. Agriculture 2024, 14, 2225. [Google Scholar] [CrossRef]
- Anonymous. Wikipedia: Zearalenone. Available online: https://en.wikipedia.org/wiki/Zearalenone (accessed on 2 January 2025).
- Booth, C. The Genus Fusarium; Commonwealth Agricultural Bureaux [for the] Commonwealth Mycological Institute: Wallingford, UK, 1971; ISBN 978-0-85198-046-1. [Google Scholar]
- Nelson, P.E.; Toussoun, T.A.; Marasas, W.F.O. Fusarium Species: An Illustrated Manual for Identification; Pennsylvania State University Press: University Park, TX, USA, 1983; ISBN 978-0-271-00349-8. [Google Scholar]
- Burgess, L.; Liddell, C. Laboratory Manual for Fusarium Research; University of Sydney: Sydney, Australia, 1983. [Google Scholar]
- de Hoog, G.; Guarro, J.; Gené, J.; Figueras, M. Atlas of Clinical Fungi, 2nd ed.; CBS: Utrecht, The Netherlands, 2000. [Google Scholar]
- Fusarium—Interactive Key. Available online: http://res.agr.ca/bdr/Fusarium/Fuskey-Fusarium (accessed on 31 August 1999).
- Glenn, A.E. Mycotoxigenic Fusarium species in animal feed. Anim. Feed Sci. Technol. 2007, 137, 213–240. [Google Scholar] [CrossRef]
- O’Donnell, K.; McCormick, S.P.; Busman, M.; Proctor, R.H.; Ward, T.J.; Doehring, G.; Geiser, D.M.; Alberts, J.F.; Rheeder, J.P. Marasas et al. 1984 “Toxigenic Fusarium species: Identity and mycotoxicology” revisited. Mycologia 2018, 110, 1058–1080. [Google Scholar] [CrossRef]
- Lee, H.J.; Ok, H.E.; Sim, J.H.; Min, S.-G.; Chun, H.S. Estimate of zearalenone exposure through the intake of white and brown rice in the Korean population. Toxicol. Environ. Chem. 2015, 97, 1071–1085. [Google Scholar] [CrossRef]
- Lee, Y.-W.; Kim, J.-G.; Chung, D.-H.; Roh, P.-U.; Pestka, J.J. Natural occurrence of zearalenone in rice and soybean produced in Korea. Mycotoxin Res. 1991, 7, 69–72. [Google Scholar] [CrossRef]
- Lee, K.Y.; Poole, C.F.; Zlatkis, A. Simultaneous multi-mycotoxin determination by high performance thin-layer chromatography. Anal. Chem. 1980, 52, 837–842. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, J.; Sui, K.; Sung, W. Simultaneous thin-layer chromatographic determination of zearalenone and patulin in maize. J. Planar Chromatogr. 1993, 6, 274–277. [Google Scholar]
- Thrane, U. Fusarium Species and Their Specific Profiles of Secondary Metabolites. In Fusarium: Mycotoxins, Taxonomy and Pathogenicity; Topics in Secondary Metabolism; Elsevier Science Publishers: Amsterdam, The Netherlands; New York, NY, USA, 1989; Volume 2, pp. 199–225. [Google Scholar]
- Desjardins, A.E. Overview of Zearalenone-Producing Fusarium Species. In Fusarium Mycotoxins: Chemistry, Genetics, and Biology; American Phytopathological Society: St. Paul, MN, USA, 2006; p. 62. ISBN 978-0-89054-335-1. [Google Scholar]
- Munkvold, G.P. Fusarium Species and Their Associated Mycotoxins. In Mycotoxigenic Fungi: Methods and Protocols; Moretti, A., Susca, A., Eds.; Springer: New York, NY, USA, 2017; pp. 51–106. ISBN 978-1-4939-6707-0. [Google Scholar]
- Mehrabi, R.; Bahkali, A.H.; Abd-Elsalam, K.A.; Moslem, M.; Ben M’Barek, S.; Gohari, A.M.; Jashni, M.K.; Stergiopoulos, I.; Kema, G.H.J.; de Wit, P.J.G.M. Horizontal gene and chromosome transfer in plant pathogenic fungi affecting host range. FEMS Microbiol. Rev. 2011, 35, 542–554. [Google Scholar] [CrossRef]
- Villani, A.; Proctor, R.H.; Kim, H.-S.; Brown, D.W.; Logrieco, A.F.; Amatulli, M.T.; Moretti, A.; Susca, A. Variation in secondary metabolite production potential in the Fusarium incarnatum-equiseti species complex revealed by comparative analysis of 13 genomes. BMC Genom. 2019, 20, 314. [Google Scholar] [CrossRef] [PubMed]
- Navale, V.D.; Sawant, A.M.; Gowda, V.U.; Vamkudoth, K.R. Assembly, annotation, and comparative whole genome sequence of Fusarium verticillioides isolated from stored maize grains. Pathogens 2022, 11, 810. [Google Scholar] [CrossRef] [PubMed]
- Linnemannstöns, P.; Schulte, J.; del Mar Prado, M.; Proctor, R.H.; Avalos, J.; Tudzynski, B. The polyketide synthase gene pks4 from Gibberella fujikuroi encodes a key enzyme in the biosynthesis of the red pigment bikaverin. Fungal Genet. Biol. 2002, 37, 134–148. [Google Scholar] [CrossRef]
- Brown, D.W.; Butchko, R.A.E.; Busman, M.; Proctor, R.H. Identification of gene clusters associated with fusaric acid, fusarin, and perithecial pigment production in Fusarium verticillioides. Fungal Genet. Biol. 2012, 49, 521–532. [Google Scholar] [CrossRef]
- Ma, S.M.; Zhan, J.; Watanabe, K.; Xie, X.; Zhang, W.; Wang, C.C.; Tang, Y. Enzymatic Synthesis of Aromatic Polyketides Using PKS4 from Gibberella fujikuroi. J. Am. Chem. Soc. 2007, 129, 10642–10643. [Google Scholar] [CrossRef]
- Wagner, D.; Schmeinck, A.; Mos, M.; Morozov, I.Y.; Caddick, M.X.; Tudzynski, B. The bZIP transcription factor MeaB mediates nitrogen metabolite repression at specific loci. Eukaryot. Cell 2010, 9, 1588–1601. [Google Scholar] [CrossRef]
- Wiemann, P.; Willmann, A.; Straeten, M.; Kleigrewe, K.; Beyer, M.; Humpf, H.-U.; Tudzynski, B. Biosynthesis of the red pigment bikaverin in Fusarium fujikuroi: Genes, their function and regulation. Mol. Microbiol. 2009, 72, 931–946. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Wingfield, M.J.; Crous, P.W. Awards and Personalia. IMA Fungus 2012, 3, A24–A28. [Google Scholar] [CrossRef]
- Deed—Attribution-NonCommercial 4.0 International—Creative Commons. Available online: https://creativecommons.org/licenses/by-nc/4.0/ (accessed on 22 March 2025).
- Eugenio, C.P. Factors Influencing the Biosynthesis of the Fungal Estrogen (F-2) and the Effects of F-2 on Perithecia Formation by Fusarium Species. Ph.D. Thesis, University of Minnesota, St. Paul, MN, USA, 1968. [Google Scholar]
- Wolf, J.C. Regulation of the Sexual Stage in Fusarium roseum ‘Graminearum’. Master’s Thesis, University of Minnesota, St. Paul, MN, USA, 1971. [Google Scholar]
- Nelson, R.; Mirocha, C.; Huisingh, D.; Tijerina-Menchaca, A. Eflects of F-2, an estrogenic metabolite from Fusasarium, on sexual reproduction of certain ascomycetes. Abstracts of annual APS conference. Phytopathology 1968, 58, 1061–1062. [Google Scholar]
- Barksdale, A.W. Sexual hormones of achlya and other fungi. Science 1969, 166, 831–837. [Google Scholar] [CrossRef]
- Wolf, J.C.; Lieberman, J.; Mirocha, C. Inhibition of F-2 (zearalenone) biosynthesis and perithecium production in Fusarium roseum graminearum. Phytopathology 1972, 62, 937–939. [Google Scholar] [CrossRef]
- Wolf, J.C.; Mirocha, C.J. Regulation of sexual reproduction in Gibberella zeae (Fusarium roxeum “graminearum”) by F-2 (zearalenone). Can. J. Microbiol. 1973, 19, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.C.; Mirocha, C.J. Control of sexual reproduction in Gibberella zeae (Fusarium roseum “graminearum”). Appl. Environ. Microbiol. 1977, 33, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Inaba, T.; Mirocha, C.J. Preferential binding of radiolabeled zearalenone to a protein fraction of Fusarium roseum graminearum. Appl. Environ. Microbiol. 1979, 37, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Mirocha, C.J.; Swanson, S.P. Regulation of perithecia production in Fusarium roseum by zearalenone. J. Food Saf. 1983, 5, 41–53. [Google Scholar] [CrossRef]
- Bacon, C.W.; Robbins, J.D.; Porter, J.K. Media for identification of Gibberella zeae and production of F-2-(Zearalenone). Appl. Environ. Microbiol. 1977, 33, 445–449. [Google Scholar] [CrossRef]
- Betina, V. Differentiation and secondary metabolism in some prokaryotes and fungi. Folia Microbiol. 1995, 40, 51–67. [Google Scholar] [CrossRef]
- O’Neill, K.; Damoglou, A.P.; Patterson, M.F. Toxin production by Fusarium culmorum IMI 309344 and F. graminearum NRRL 5883 on grain substrates. J. Appl. Bacteriol. 1993, 74, 625–628. [Google Scholar] [CrossRef]
- Bosch, U.; Mirocha, C.J. Toxin production by Fusarium species from sugar beets and natural occurrence of zearalenone in beets and beet fibers. Appl. Environ. Microbiol. 1992, 58, 3233–3239. [Google Scholar] [CrossRef] [PubMed]
- Blaney, B.J.; Dodman, R.L. Production of the mycotoxins zearalenone, 4-deoxynivalenol and nivalenol by isolates of Fusarium graminearum Groups 1 and 2 from cereals in Queensland. Aust. J. Agric. Res. 1988, 39, 21–29. [Google Scholar] [CrossRef]
- Aoki, T.; O’Donnell, K. Morphological and molecular characterization of Fusarium pseudograminearum sp. nov., formerly recognized as the Group 1 population of F. graminearum. Mycologia 1999, 91, 597–609. [Google Scholar] [CrossRef]
- Dyer, P.S.; Ingram, D.S.; Johnstone, K. The control of sexual morphogenesis in the Ascomycotina. Biol. Rev. 1992, 67, 421–458. [Google Scholar] [CrossRef]
- Gooday, G.W. Hormones in Mycelial Fungi. In Growth, Differentiation and Sexuality; Wessels, J.G.H., Meinhardt, F., Eds.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 401–411. ISBN 978-3-662-11908-2. [Google Scholar]
- Gaffoor, I.; Brown, D.W.; Plattner, R.; Proctor, R.H.; Qi, W.; Trail, F. Functional analysis of the polyketide synthase genes in the filamentous fungus Gibberella zeae (anamorph Fusarium graminearum). Eukaryot. Cell 2005, 4, 1926–1933. [Google Scholar] [CrossRef]
- Braunsdorf, C.; Mailänder-Sánchez, D.; Schaller, M. Fungal sensing of host environment. Cell. Microbiol. 2016, 18, 1188–1200. [Google Scholar] [CrossRef]
- Weigt, D.; Niemann, J.; Siatkowski, I.; Zyprych-Walczak, J.; Olejnik, P.; Kurasiak-Popowska, D. Effect of zearalenone and hormone regulators on microspore embryogenesis in anther culture of wheat. Plants 2019, 8, 487. [Google Scholar] [CrossRef]
- Biesaga-Koscielniak, J.; Filek, M. Occurrence and Physiology of Zearalenone as a New Plant Hormone. In Sociology, Organic Farming, Climate Change and Soil Science; Lichtfouse, E., Ed.; Sustainable Agriculture Reviews; Springer Netherlands: Dordrecht, The Netherlands, 2010; Volume 3, pp. 419–435. ISBN 978-90-481-3333-8. [Google Scholar]
- Chaurasia, S.; Chand, R.; Joshi, A.K. A simple technique for the induction of sporulation in Alternaria triticina incitant of leaf blight of wheat. J. Plant Dis. Prot. 1998, 105, 17–21. [Google Scholar]
- McRae, C.F.; Heritage, A.D.; Brown, J.F. A simple technique for inducing sporulation by Alternarla carthami on artificial media. Australas. Plant Pathol. 1983, 12, 53–55. [Google Scholar] [CrossRef]
- Charlton, K.M. The sporulation of Alternaria solani in culture. Trans. Br. Mycol. Soc. 1953, 36, 349-IN5. [Google Scholar] [CrossRef]
- Ellers, K.; Baxters, R. Induced conidial formation in Alternaria zinniae on media amended with Morestan. Proc. Am. Phytopathol. Soc. 1974, 1, 159. [Google Scholar]
- Jacobs, S.E.; Marsden, A.W. The role of antibiotics in the decomposition of sawdust. Ann. Appl. Biol. 1948, 35, 18–24. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Kim, D.-W.; Yun, S.-H. Functional analysis of genes specifically expressed during aerial hyphae collapse as a potential signal for perithecium formation induction in Fusarium graminearum. Plant Pathol. J. 2024, 40, 83–97. [Google Scholar] [CrossRef]
- Cohen, D. Maximizing final yield when growth is limited by time or by limiting resources. J. Theor. Biol. 1971, 33, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Utermark, J.; Karlovsky, P. Role of zearalenone lactonase in protection of Gliocladium roseum from fungitoxic effects of the mycotoxin zearalenone. Appl. Environ. Microbiol. 2007, 73, 637–642. [Google Scholar] [CrossRef]
- Amir, S.; Cohen, D. Optimal reproductive efforts and the timing of reproduction of annual plants in randomly varying environments. J. Theor. Biol. 1990, 147, 17–42. [Google Scholar] [CrossRef]
- Sørensen, J.L.; Benfield, A.H.; Wollenberg, R.D.; Westphal, K.; Wimmer, R.; Nielsen, M.R.; Nielsen, K.F.; Carere, J.; Covarelli, L.; Beccari, G.; et al. The cereal pathogen Fusarium pseudograminearum produces a new class of active cytokinins during infection. Mol. Plant Pathol. 2018, 19, 1140–1154. [Google Scholar] [CrossRef] [PubMed]
- Vrabka, J.; Niehaus, E.-M.; Münsterkötter, M.; Proctor, R.H.; Brown, D.W.; Novák, O.; Pěnčik, A.; Tarkowská, D.; Hromadová, K.; Hradilová, M.; et al. Production and role of hormones during interaction of Fusarium species with maize (Zea mays L.) seedlings. Front. Plant Sci. 2019, 9, 1936. [Google Scholar] [CrossRef]
- Swart, A.; Kamerbeek, G.A. Ethylene production and mycelium growth of the tulip strain of Fusarium oxysporum as influenced by shaking of and oxygen supply to the culture medium. Physiol. Plant. 1977, 39, 38–44. [Google Scholar] [CrossRef]
- Mirocha, C.J.; Christensen, C.M.; Nelson, G.H. Physiologic activity of some fungal estrogens produced by Fusarium. Cancer Res. 1968, 28, 2319–2322. [Google Scholar]
- Brodnik, T.; Klemenc, N.; Vospernik, P.; Zust, J. Influence of toxins from maize infected by Aspergillus flavus, Penicillium rubrum and Fusarium graminearum and of aflatoxin B1, rubratoxin A and toxin F-2 on maize embryo growth. Seed Sci. Technol. 1978, 6, 965–970. [Google Scholar]
- McLean, M. The phytotoxicity of selected mycotoxins on mature, germinating Zea mays embryos. Mycopathologia 1996, 132, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, X.; Xu, X.; Guo, L.; Wang, X.; Xiang, W.; Zhao, J. Identification and pathogenicity of Clonostachys spp. and its co-inoculation with Fusarium species on soybean root. Plant Pathol. 2024, 73, 1801–1811. [Google Scholar] [CrossRef]
- Han, Y.-Z.; Meng, F.-J. Studies on zearalenone influencing the growth and development of Lemna perpusilla. Chin. Sci. Bull. 1991, 36, 1037–1040. [Google Scholar]
- Fu, Y.-F.; Meng, F.-J. The effects of zearalenone the growth and development of Lemma Gibba G3. Acta Phytophysiol. Sin. 1993, 19, 395–398. [Google Scholar]
- Biesaga-Koscielniak, J. Investigation of the possibility of stimulating generative development of plants by exogenous zearalenone. Acta Physiol. Plant. 1998, 20, S5. [Google Scholar]
- Biesaga-Koscielniak, J. Effect of replacement of 2,4-D by the zearalenone on the haploid embryos growth in wheat (Triticum aestivum L.) after its pollination by maize (Zea mays L.). Acta Physiol. Plant. 1998, 20, S16. [Google Scholar]
- Biesaga-Koscielniak, J.; Marcinska, I. The effect of various substances on embryogenesis of wheat callus. Acta Biol. Cracoviensia Ser. Bot. Suppl. 1999, 41, S32. [Google Scholar]
- Biesaga-Kościelniak, J.; Marcińska, I.; Wędzony, M.; Kościelniak, J. Effect of zearalenone treatment on the production of wheat haploids via the maize pollination system. Plant Cell Rep. 2003, 21, 1035–1039. [Google Scholar] [CrossRef]
- Biesaga-Koscielniak, J.; Janeczko, A.; Filek, M.; Dziurka, M.; Koscielniak, J. Effect of zearalenone on the growth and productivity of crop plants. I. Effectiveness of application of zearalenone on wheat production. Bibl. Fragm. Agron. 2006, 11, 53–54. [Google Scholar]
- Biesaga-Koscielniak, J.; Janeczko, A.; Filek, M.; Dziurka, M.; Koscielniak, J. Effect of zearalenone on the growth and productivity of crop plants. II. Effectiveness of application of zearalenone on soybean production. Bibl. Fragm. Agron. 2006, 11, 55–56. [Google Scholar]
- Kościelniak, J.; Biesaga-Kościelniak, J.; Janeczko, A.; Filek, W.; Kalaji, H.M. Can the Giberella zeae toxin zearalenone affect the photosynthetic productivity and increase yield formation in spring wheat and soybean plants? Photosynthetica 2009, 47, 586–594. [Google Scholar] [CrossRef]
- Biesaga-Kościelniak, J.; Kościelniak, J.; Janeczko, A. The impact of zearalenone and thidiazuron on indirect plant regeneration of oilseed rape and wheat. Acta Physiol. Plant. 2010, 32, 1047–1053. [Google Scholar] [CrossRef]
- Filek, M.; Biesaga-Kościelniak, J.; Marcińska, I.; Cvikrová, M.; Macháčková, I.; Krekule, J. Contents of polyamines during vernalization in wheat and the effect of zearalenone. Biol. Plant. 2010, 54, 483–487. [Google Scholar] [CrossRef]
- Kościelniak, J.; Ostrowska, A.; Biesaga-Kościelniak, J.; Filek, W.; Janeczko, A.; Kalaji, H.M.; Stalmach, K. The effect of zearalenone on PSII photochemical activity and growth in wheat and soybean under salt (NaCl) stress. Acta Physiol. Plant. 2011, 33, 2329–2338. [Google Scholar] [CrossRef]
- Płażek, A.; Tatrzańska, M.; Maciejewski, M.; Dziurka, M.; Waligórski, P.; Dubert, F. Effects of zearalenone and 24-epibrassinolide on the salt tolerance of select monocotyledonous crop plants. J. Appl. Bot. Food Qual. 2017, 90, 280–287. [Google Scholar] [CrossRef]
- Dziurka, M.; Maksymowicz, A.; Ostrowska, A.; Biesaga-Kościelniak, J. The interaction effect of drought and exogenous application of zearalenone on the physiological, biochemical parameters and yield of legumes. J. Plant Growth Regul. 2021, 40, 1824–1835. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Li, J.; Huang, J.; Bao, S.; He, C.; Zhang, M.; Xiang, T. Metabolites of zearalenone and phytohormones secreted by endophytic fungus strain TH15 regulating the root development in Tetrastigma hemsleyanum. Plant Cell Tissue Organ Cult. PCTOC 2022, 150, 683–694. [Google Scholar] [CrossRef]
- Li, J.; Huang, X.; Zeng, Z.; Chen, Z.; Huang, J.; He, C.; Xiang, T. Zearalenone regulates microRNA156 to affect the root development of Tetrastigma hemsleyanum. Tree Physiol. 2023, 43, 643–657. [Google Scholar] [CrossRef]
- Bonner, J.; Axtman, G. The growth of plant embryos in vitro. Preliminary experiments on the role of accessory substances. Proc. Natl. Acad. Sci. USA 1937, 23, 453–457. [Google Scholar] [CrossRef]
- Slama, K. Animal hormones and antihormones in plants. Biochem. Physiol. Pflanz. 1980, 175, 177–193. [Google Scholar] [CrossRef]
- Jordan, V.C.; Mittal, S.; Gosden, B.; Koch, R.; Lieberman, M.E. Structure-activity relationships of estrogens. Environ. Health Perspect. 1985, 61, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.-F.; Han, Y.-Z.; Zhao, D.-G.; Meng, F.-J. Zearalenone and flower bud formation in thin-cell layers of Nicotiana tabacum L. Plant Growth Regul. 2000, 30, 271–274. [Google Scholar] [CrossRef]
- Meng, F.-J.; Que, Y.-M.; Zhang, S.-Q. Zearalenone-like substance in winter wheat plants and its relation to vernalization. Acta Bot. Sin. 1986, 28, 622–627. [Google Scholar]
- Han, Y.-Z.; Meng, F.-J. Studies on zeralenone-like substance in rape plant and its relation to vernalization. Acta Agric. Univ. Pekin. 1986, 12, 386–388. [Google Scholar]
- Meng, F.-J.; Que, Y.-M.; Han, Y.-Z.; Li, H.-X.; Wang, Z.-C. Isolation of zearalenone from shoot apices of overwintering winter wheat. Sci. China Ser. B 1989, 32, 1099–1105. [Google Scholar]
- Li, H.-L.; Meng, F.-J. Isolation and identification of zearalenone from Apium graveoleus L. Acta Phytophysiol. Sin. 1989, 15, 211–215. [Google Scholar]
- Que, Y.-M.; Liang, Z.-X.; Han, Y.-Z.; Meng, F.-J. Analysis of zearalenone in winter wheat and cotton plants during flowering and fruiting. Acta Agric. Univ. Pekin. 1990, 16, 153–155. [Google Scholar]
- Wang, H.; Meng, F.-J. Formation of endogenous zearalenone and its inhibition by malathion in winter wheat during vernalization. Acta Phytophysiol. Sin. 1990, 16, 197–200. [Google Scholar]
- Meng, F.-J.; Han, Y.-Z.; Que, Y.-M.; Wang, H. Zearalenone, a Key Substance Controlling Plant Development. In Progress in Plant Growth Regulation; Current Plant Science and Biotechnology in Agriculture; Springer: Dordrecht, The Netherlands, 1992; Volume 13, pp. 291–297. ISBN 978-94-011-2458-4. [Google Scholar]
- Fu, Y.-F.; Meng, F.-J. Zearalenone in growth and development of winter wheat. Acta Agron. Sin. 1994, 20, 271–276. [Google Scholar]
- Fu, Y.; Li, H.; Meng, F.-J. The possible role of zearalenone in the floral gradient in Nicotiana tabacum L. J. Plant Physiol. 1995, 147, 197–202. [Google Scholar] [CrossRef]
- Meng, F.-J.; Han, Y.-Z.; Fu, Y.-F.; Guo, F.-L. Zearalenone in higher plants. Flower. Newsl. 1996, 22, 54–57. [Google Scholar]
- Chen, X.-J.; Meng, F.-J. Isolation and identification of conjugated zearalenone in maize and wheat seeds. Acta Phytophysiol. Sin. 1997, 23, 309–312. [Google Scholar]
- Yu, J.-J.; Han, Y.-Z.; Zhao, D.-G.; Fu, Y.-F.; Meng, F.-J. Zearalenone and sex expression in hemp. J. China Agric. Univ. 1998, 3, 24–28. [Google Scholar]
- Zhao, D.-G.; Han, Y.-Z.; Fu, Y.-F.; Guo, F.-L.; Meng, F.-J. Changes of plant hormones in inflorescence during sex determination in maize. Acta Phytophysiol. Sin. 1999, 25, 57–65. [Google Scholar]
- Biesaga-Kościelniak, J. Zearalenone as a New Hypothetical Regulator of Plant Growth and Development; Monograph of Institute of Plant Physiology; Polish Academy of Sciences: Kraków, Poland, 2001. [Google Scholar]
- Filek, M.; Zembala, M.; Dudek, A.; Laggner, P.; Kriechbaum, M. Electric and structural studies of hormone interaction with chloroplast envelope membranes isolated from vegetative and generative rape. J. Plant Physiol. 2007, 164, 861–867. [Google Scholar] [CrossRef]
- Rolli, E.; Righetti, L.; Galaverna, G.; Suman, M.; Dall’Asta, C.; Bruni, R. Zearalenone uptake and biotransformation in micropropagated Triticum durum Desf. plants: A xenobolomic approach. J. Agric. Food Chem. 2018, 66, 1523–1532. [Google Scholar] [CrossRef]
- Chen, X.-J.; Liu, H.-C.; Meng, F.-J. Direct enzyme-linked immunosorbent assay for zearalenone. Plant Physiol. Commun. 1989, 5e, 61–63. [Google Scholar]
- Quesada-Ocampo, L.M.; Al-Haddad, J.; Scruggs, A.C.; Buell, C.R.; Trail, F. Susceptibility of maize to stalk rot caused by Fusarium graminearum deoxynivalenol and zearalenone mutants. Phytopathology 2016, 106, 920–927. [Google Scholar] [CrossRef]
- Torres Acosta, J.A.; Michlmayr, H.; Shams, M.; Schweiger, W.; Wiesenberger, G.; Mitterbauer, R.; Werner, U.; Merz, D.; Hauser, M.-T.; Hametner, C.; et al. Zearalenone and ß-zearalenol but not their glucosides inhibit heat shock protein 90 ATPase activity. Front. Pharmacol. 2019, 10, 1160. [Google Scholar] [CrossRef]
- DesRochers, N.; Walsh, J.P.; Renaud, J.B.; Seifert, K.A.; Yeung, K.K.-C.; Sumarah, M.W. Metabolomic profiling of fungal pathogens responsible for root rot in American ginseng. Metabolites 2020, 10, 35. [Google Scholar] [CrossRef]
- Hagler, W.M.; Mirocha, C.J.; Pathre, S.V.; Behrens, J.C. Identification of the naturally occurring isomer of zearalenol produced by Fusarium roseum “Gibbosum” in rice culture. Appl. Environ. Microbiol. 1979, 37, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Fruhauf, S.; Pühringer, D.; Thamhesl, M.; Fajtl, P.; Kunz-Vekiru, E.; Höbartner-Gussl, A.; Schatzmayr, G.; Adam, G.; Damborsky, J.; Djinovic-Carugo, K.; et al. Bacterial lactonases ZenA with noncanonical structural features hydrolyze the mycotoxin zearalenone. ACS Catal. 2024, 14, 3392–3410. [Google Scholar] [CrossRef] [PubMed]
- Winssinger, N.; Barluenga, S. Chemistry and biology of resorcylic acid lactones. Chem. Commun. 2007, 2, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.V.; Agatsuma, T.; Nakano, H. Targeting of the protein chaperone, HSP90, by the transformation suppressing agent, radicicol. Oncogene 1998, 16, 2639–2645. [Google Scholar] [CrossRef] [PubMed]
- Winssinger, N.; Fontaine, J.-G.; Barluenga, S. Hsp90 inhibition with resorcylic acid lactones (RALs). Curr. Top. Med. Chem. 2009, 9, 1419–1435. [Google Scholar] [CrossRef]
- Turbyville, T.J.; Wijeratne, E.M.K.; Liu, M.X.; Burns, A.M.; Seliga, C.J.; Luevano, L.A.; David, C.L.; Faeth, S.H.; Whitesell, L.; Gunatilaka, A.A.L. Search for Hsp90 inhibitors with potential anticancer activity: Isolation and SAR studies of radicicol and monocillin I from two plant-associated fungi of the Sonoran desert. J. Nat. Prod. 2006, 69, 178–184. [Google Scholar] [CrossRef]
- Zimmermann, T.J.; Niesen, F.H.; Pilka, E.S.; Knapp, S.; Oppermann, U.; Maier, M.E. Discovery of a potent and selective inhibitor for human carbonyl reductase 1 from propionate scanning applied to the macrolide zearalenone. Bioorg. Med. Chem. 2009, 17, 530–536. [Google Scholar] [CrossRef]
- Ugele, M.; Sasse, F.; Knapp, S.; Fedorov, O.; Zubriene, A.; Matulis, D.; Maier, M.E. Propionate analogues of zearalenone bind to Hsp90. ChemBioChem 2009, 10, 2203–2212. [Google Scholar] [CrossRef]
- Chen, B.; Zhong, D.; Monteiro, A. Comparative genomics and evolution of the HSP90 family of genes across all kingdoms of organisms. BMC Genom. 2006, 7, 156. [Google Scholar] [CrossRef]
- Zhang, F.-L.; Zhu, K.-X.; Wang, J.-Y.; Zhang, M.; Yan, J.-M.; Liu, Q.-C.; Zhang, X.-Y.; Guo, J.-C.; Liu, X.; Sun, Q.-C.; et al. Cross-species analysis of transcriptome emphasizes a critical role of TNF-α in mediating MAP2K7/AKT2 signaling in zearalenone-induced apoptosis. J. Hazard. Mater. 2023, 459, 132226. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-Y.; Zheng, Z.-H.; Son, Y.-O.; Shi, X.; Jang, Y.-O.; Lee, J.-C. Mycotoxin zearalenone induces AIF- and ROS-mediated cell death through p53- and MAPK-dependent signaling pathways in RAW264.7 macrophages. Toxicol. Vitr. 2011, 25, 1654–1663. [Google Scholar] [CrossRef]
- Kempken, F.; Rohlfs, M. Fungal secondary metabolite biosynthesis—A chemical defence strategy against antagonistic animals? Fungal Ecol. 2010, 3, 107–114. [Google Scholar] [CrossRef]
- Rohlfs, M. Fungal Secondary Metabolism in the Light of Animal–Fungus Interactions: From Mechanism to Ecological Function. In Biosynthesis and Molecular Genetics of Fungal Secondary Metabolites, Volume 2; Zeilinger, S., Martín, J.-F., García-Estrada, C., Eds.; Springer: New York, NY, USA, 2015; pp. 177–198. ISBN 978-1-4939-2531-5. [Google Scholar]
- Pfeiffer, E.; Hildebrand, A.; Mikula, H.; Metzler, M. Glucuronidation of zearalenone, zeranol and four metabolites in vitro: Formation of glucuronides by various microsomes and human UDP-glucuronosyltransferase isoforms. Mol. Nutr. Food Res. 2010, 54, 1468–1476. [Google Scholar] [CrossRef]
- Frizzell, C.; Uhlig, S.; Miles, C.O.; Verhaegen, S.; Elliott, C.T.; Eriksen, G.S.; Sørlie, M.; Ropstad, E.; Connolly, L. Biotransformation of zearalenone and zearalenols to their major glucuronide metabolites reduces estrogenic activity. Toxicol. In Vitro 2015, 29, 575–581. [Google Scholar] [CrossRef]
- Smedsgaard, J. Micro-scale extraction procedure for standardized screening of fungal metabolite production in cultures. J. Chromatogr. A 1997, 760, 264–270. [Google Scholar] [CrossRef]
- Lepom, P.; Baath, H.; Knabe, O. Vorkommen von Fusarium-Arten und ihren Mykotoxinen auf Silomais. Arch. Tierernaehrung 1988, 38, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, E.; Ellner, F. Distribution of disease symptoms and mycotoxins in maize ears infected by Fusarium culmorum and Fusarium graminearum. Mycotoxin Res. 2015, 31, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Lysøe, E.; Bone, K.R.; Klemsdal, S.S. Real-time quantitative expression studies of the zearalenone biosynthetic gene cluster in Fusarium graminearum. Phytopathology 2009, 99, 176–184. [Google Scholar] [CrossRef]
- Hallen-Adams, H.E.; Wenner, N.; Kuldau, G.A.; Trail, F. Deoxynivalenol biosynthesis-related gene expression during wheat kernel colonization by Fusarium graminearum. Phytopathology 2011, 101, 1091–1096. [Google Scholar] [CrossRef]
- Stephens, A.E.; Gardiner, D.M.; White, R.G.; Munn, A.L.; Manners, J.M. Phases of infection and gene expression of Fusarium graminearum during crown rot disease of wheat. Mol. Plant Microbe Interact. 2008, 21, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, D.M.; Kazan, K.; Manners, J.M. Nutrient profiling reveals potent inducers of trichothecene biosynthesis in Fusarium graminearum. Fungal Genet. Biol. 2009, 46, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.A.; Evans, J.; Mead, A.; Hammond-Kosack, K.E. A spatial temporal analysis of the Fusarium graminearum transcriptome during symptomless and symptomatic wheat infection. Mol. Plant Pathol. 2017, 18, 1295–1312. [Google Scholar] [CrossRef]
- Lemmens, M.; Scholz, U.; Berthiller, F.; Dall’Asta, C.; Koutnik, A.; Schuhmacher, R.; Adam, G.; Buerstmayr, H.; Mesterházy, A.; Krska, R.; et al. The ability to detoxify the mycotoxin deoxynivalenol colocalizes with a major quantitative trait locus for Fusarium head blight resistance in wheat. Mol. Plant Microbe Interact. 2005, 18, 1318–1324. [Google Scholar] [CrossRef]
- Cullen, D.; Caldwell, R.; Smalley, E. Cultural characteristics, pathogenicity, and zearalenone production by strains of Gibberella zeae isolated from corn. Phytopathology 1982, 72, 1415–1418. [Google Scholar] [CrossRef]
- Bagi, F.; Balaž, F.; Marija, Š. Pathogenicity and zearalenone production by different Fusarium graminearum isolates in artificially infected wheat grain. Cereal Res. Commun. 2000, 28, 477–484. [Google Scholar] [CrossRef]
- Cullen, D.; Caldwell, R.W.; Smalley, E. Susceptibility of maize to Gibberella zeae ear rot: Relationship to host genotype pathogen virulence, and zearalenone contamination. Plant Dis. 1983, 67, 89–91. [Google Scholar] [CrossRef]
- Manka, M.; Visconti, A.; Chelkowski, J.; Bottalico, A. Pathogenicity of Fusarium isolates from wheat, rye and triticale towards seedlings and their ability to produce trichothecenes and zearalenone. J. Phytopathol. 1985, 113, 24–29. [Google Scholar] [CrossRef]
- Gang, G.; Miedaner, T.; Schuhmacher, U.; Schollenberger, M.; Geiger, H.H. Deoxynivalenol and nivalenol production by Fusarium culmorum isolates differing in aggressiveness toward winter rye. Phytopathology 1998, 88, 879–884. [Google Scholar] [CrossRef]
- Gardiner, D.M.; Kazan, K.; Manners, J.M. Novel genes of Fusarium graminearum that negatively regulate deoxynivalenol production and virulence. Mol. Plant Microbe Interact. 2009, 22, 1588–1600. [Google Scholar] [CrossRef]
- Hao, G.; McCormick, S.; Tiley, H.; Gutiérrez, S.; Yulfo-Soto, G.; Vaughan, M.M.; Ward, T.J. NX trichothecenes are required for Fusarium graminearum infection of wheat. Mol. Plant-Microbe Interact. 2023, 36, 294–304. [Google Scholar] [CrossRef]
- Desjardins, A.; Spencer, G.; Plattner, R.; Beremand, M. Furanocoumarin phytoalexins, trichothecene toxins, and infection of Pastinaca sativa by Fusarium sporotrichioides. Phytopathology 1989, 79, 170–175. [Google Scholar] [CrossRef]
- Desjardins, A.E.; Hohn, T.; McCormick, S.P. Effect of gene disruption of trichodiene synthase on the virulence of Gibberella pulicaris. Mol. Plant Microbe Interact. 1992, 5, 214–222. [Google Scholar] [CrossRef]
- Li, X.; Shin, S.; Heinen, S.; Dill-Macky, R.; Berthiller, F.; Nersesian, N.; Clemente, T.; McCormick, S.; Muehlbauer, G.J. Transgenic wheat expressing a barley UDP-glucosyltransferase detoxifies deoxynivalenol and provides high levels of resistance to Fusarium graminearum. Mol. Plant Microbe Interact. 2015, 28, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Mandalà, G.; Tundo, S.; Francesconi, S.; Gevi, F.; Zolla, L.; Ceoloni, C.; D’Ovidio, R. Deoxynivalenol detoxification in transgenic wheat confers resistance to Fusarium head blight and crown rot diseases. Mol. Plant Microbe Interact. 2019, 32, 583–592. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, L.; Liu, X.; Jiang, P.; Yu, L.; Qiu, J.; Wang, G.; Zhang, X.; Ma, H. TaUGT6, a novel UDP-glycosyltransferase gene enhances the resistance to FHB and DON accumulation in wheat. Front. Plant Sci. 2020, 11, 574775. [Google Scholar] [CrossRef]
- He, W.-J.; Yang, P.; Huang, T.; Liu, Y.-F.; Zhang, Y.-W.; Zhang, W.-M.; Zhang, T.-T.; Zheng, M.-R.; Ma, L.; Zhao, C.-X.; et al. Detoxifying bacterial genes for deoxynivalenol epimerization confer durable resistance to Fusarium head blight in wheat. Plant Biotechnol. J. 2024, 22, 2395–2409. [Google Scholar] [CrossRef]
- Proctor, R.H.; Hohn, T.M.; McCormick, S.P. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol. Plant Microbe Interact. 1995, 8, 593–601. [Google Scholar] [CrossRef]
- Bai, G.-H.; Desjardins, A.E.; Plattner, R.D. Deoxynivalenol-nonproducing Fusarium graminearum causes initial infection, but does not cause disease spread in wheat spikes. Mycopathologia 2002, 153, 91–98. [Google Scholar] [CrossRef]
- Maier, F.J.; Miedaner, T.; Hadeler, B.; Felk, A.; Salomon, S.; Lemmens, M.; Kassner, H.; Schäfer, W. Involvement of trichothecenes in fusarioses of wheat, barley and maize evaluated by gene disruption of the trichodiene synthase (Tri5) gene in three field isolates of different chemotype and virulence. Mol. Plant Pathol. 2006, 7, 449–461. [Google Scholar] [CrossRef]
- Beccari, G.; Tini, F.; Foroud, N.A.; Ederli, L.; Gardiner, D.M.; Benfield, A.H.; Harris, L.J.; Sulyok, M.; Romani, R.; Bellezza, I.; et al. A comparison between the role of enniatins and deoxynivalenol in Fusarium virulence on different tissues of common wheat. BMC Plant Biol. 2024, 24, 463. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K.; Gardiner, D.M.; Manners, J.M. On the trail of a cereal killer: Recent advances in Fusarium graminearum pathogenomics and host resistance. Mol. Plant Pathol. 2012, 13, 399–413. [Google Scholar] [CrossRef]
- Arunpanichlert, J.; Rukachaisirikul, V.; Sukpondma, Y.; Phongpaichit, S.; Supaphon, O.; Sakayaroj, J. A β-resorcylic macrolide from the seagrass-derived fungus Fusarium sp. PSU-ES73. Arch. Pharm. Res. 2011, 34, 1633–1637. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Tan, Y.; Yan, Z.; Liao, Y.; Chen, J.; De Boevre, M.; De Saeger, S.; Wu, A. Antagonistic and detoxification potentials of Trichoderma isolates for control of zearalenone (ZEN) producing Fusarium graminearum. Front. Microbiol. 2018, 8, 2710. [Google Scholar] [CrossRef]
- Rajasekharan, S.K.; Lee, J.-H.; Zhao, Y.; Lee, J. The mycotoxin zearalenone hinders Candida albicans biofilm formation and hyphal morphogenesis. Indian J. Microbiol. 2018, 58, 19–27. [Google Scholar] [CrossRef]
- Bzducha-Wróbel, A.; Janowicz, M.; Bryła, M.; Grzesiuk, I. Adsorption of zearalenone by Aureobasidium pullulans autolyzed biomass preparation and its detoxification properties in cultures of Saccharomyces cerevisiae yeast. Toxins 2024, 16, 105. [Google Scholar] [CrossRef]
- Boeira, L.S.; Bryce, J.H.; Stewart, G.G.; Flannigan, B. The effect of combinations of Fusarium mycotoxins (deoxynivalenol, zearalenone and fumonisin B1) on growth of brewing yeasts. J. Appl. Microbiol. 2000, 88, 388–403. [Google Scholar] [CrossRef] [PubMed]
- Dawidziuk, A.; Koczyk, G.; Popiel, D. Adaptation and response to mycotoxin presence in pathogen-pathogen interactions within the Fusarium genus. World Mycotoxin J. 2016, 9, 565–576. [Google Scholar] [CrossRef]
- Armando, M.R.; Dogi, C.A.; Poloni, V.; Rosa, C.a.R.; Dalcero, A.M.; Cavaglieri, L.R. In vitro study on the effect of Saccharomyces cerevisiae strains on growth and mycotoxin production by Aspergillus carbonarius and Fusarium graminearum. Int. J. Food Microbiol. 2013, 161, 182–188. [Google Scholar] [CrossRef]
- Milles, J.; Kraemer, J.; Prange, A. In vitro competitive interactions of Fusarium graminearum with Aspergillus ochraceus and Penicillium verrucosum with regard to mycotoxin production. J. Food Agric. Environ. 2007, 5, 384–388. [Google Scholar]
- Gromadzka, K.; Chelkowski, J.; Popiel, D.; Kachlicki, P.; Kostecki, M.; Golinski, P. Solid substrate bioassay to evaluate the effect of Trichoderma and Clonostachys on the production of zearalenone by Fusarium species. World Mycotoxin J. 2009, 2, 45–52. [Google Scholar] [CrossRef]
- Müller, M.E.H.; Steier, I.; Köppen, R.; Siegel, D.; Proske, M.; Korn, U.; Koch, M. Cocultivation of phytopathogenic Fusarium and Alternaria strains affects fungal growth and mycotoxin production. J. Appl. Microbiol. 2012, 113, 874–887. [Google Scholar] [CrossRef]
- Sass, V.; Milles, J.; Kraemer, J.; Prange, A. Competitive interactions of Fusarium graminearum and Alternaria alternata in vitro in relation to deoxynivalenol and zearalenone production. J. Food Agric. Environ. 2007, 5, 257–261. [Google Scholar]
- Müller, M.E.H.; Urban, K.; Köppen, R.; Siegel, D.; Korn, U.; Koch, M. Mycotoxins as antagonistic or supporting agents in the interaction between phytopathogenic Fusarium and Alternaria fungi. World Mycotoxin J. 2015, 8, 311–322. [Google Scholar] [CrossRef]
- Kirsch, N. Rolle von Mykotoxinen in der Interaktion von Fusarium Graminearum Mit Gliocladium Roseum und Fusarium Verticillioides; University of Göttingen: Göttingen, Germany, 2013. [Google Scholar]
- Velluti, A.; Marín, S.; Gonzalez, R.; Ramos, A.J.; Sanchis, V. Fumonisin B1, zearalenone and deoxynivalenol production by Fusarium moniliforme, F proliferatum and F graminearum in mixed cultures on irradiated maize kernels. J. Sci. Food Agric. 2001, 81, 88–94. [Google Scholar] [CrossRef]
- Velluti, A.; Marín, S.; Bettucci, L.; Ramos, A.J.; Sanchis, V. The effect of fungal competition on colonization of maize grain by Fusarium moniliforme, F. proliferatum and F. graminearum and on fumonisin B1 and zearalenone formation. Int. J. Food Microbiol. 2000, 59, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Sherif, M.; Kirsch, N.; Splivallo, R.; Pfohl, K.; Karlovsky, P. The role of mycotoxins in interactions between Fusarium graminearum and F. verticillioides growing in saprophytic cultures and co-infecting maize plants. Toxins 2023, 15, 575. [Google Scholar] [CrossRef]
- Hoffmann, A.; Lischeid, G.; Koch, M.; Lentzsch, P.; Sommerfeld, T.; Müller, M.E.H. Co-cultivation of Fusarium, Alternaria, and Pseudomonas on wheat ears affects microbial growth and mycotoxin production. Microorganisms 2021, 9, 443. [Google Scholar] [CrossRef]
- El-Sharkawy, S.; Abul-Hajj, Y.J. Microbial cleavage of zearalenone. Xenobiotica 1988, 18, 365–371. [Google Scholar] [CrossRef]
- Karlovsky, P. Biological detoxification of fungal toxins and its use in plant breeding, feed and food production. Nat. Toxins 1999, 7, 1–23. [Google Scholar] [CrossRef]
- Woerfel, G.; Karlovsky, P. Hydrolyse von Zearalenon Durch Gliocladium Roseum. In Proceedings of the 20th Mycotoxin Workshop; Gesellschaft für Mykotoxinforschung: Detmold, Germany, 1998; pp. 189–192. [Google Scholar]
- Shimkets, R.A.; Lowe, D.G.; Tai, J.T.-N.; Sehl, P.; Jin, H.; Yang, R.; Predki, P.F.; Rothberg, B.E.G.; Murtha, M.T.; Roth, M.E.; et al. Gene expression analysis by transcript profiling coupled to a gene database query. Nat. Biotechnol. 1999, 17, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Crane, E.; Gilliam, J.T.; Karlovsky, P.; Maddox, J.R. Composition and Methods for Zearalenone Detoxification. PCT Application WO02/076205, 3 October 2002. [Google Scholar]
- Karlovsky, P.; Crane, E.; Gilliam, J.T.; Maddox, J.R. Composition and Methods of Zearalenone Detoxification. U.S. Patent No. 6,812,380, 2 November 2004. [Google Scholar]
- Kakeya, H.; Takahashi-Ando, N.; Kimura, M.; Onose, R.; Yamaguchi, I.; Osada, H. Biotransformation of the mycotoxin, zearalenone, to a non-estrogenic compound by a fungal strain of Clonostachys sp. Biosci. Biotechnol. Biochem. 2002, 66, 2723–2726. [Google Scholar] [CrossRef]
- Takahashi-Ando, N.; Kimura, M.; Kakeya, H.; Osada, H.; Yamaguchi, I. A novel lactonohydrolase responsible for the detoxification of zearalenone: Enzyme purification and gene cloning. Biochem. J. 2002, 365, 1–6. [Google Scholar] [CrossRef]
- Takahashi-Ando, N.; Ohsato, S.; Shibata, T.; Hamamoto, H.; Yamaguchi, I.; Kimura, M. Metabolism of zearalenone by genetically modified organisms expressing the detoxification gene from Clonostachys rosea. Appl. Environ. Microbiol. 2004, 70, 3239–3245. [Google Scholar] [CrossRef]
- Takahashi-Ando, N.; Tokai, T.; Hamamoto, H.; Yamaguchi, I.; Kimura, M. Efficient decontamination of zearalenone, the mycotoxin of cereal pathogen, by transgenic yeasts through the expression of a synthetic lactonohydrolase gene. Appl. Microbiol. Biotechnol. 2005, 67, 838–844. [Google Scholar] [CrossRef]
- Yamaguchi, I.; Kimura, M.; Ando, N.; Nishiyama, A.; Fukuda, T.; Kakeya, H.; Osada, H. Zearalenone-Detoxifying Enzyme Gene and Transformant Having the Gene Transferred Thereinto. PCT Application No. WO2003080842, 25 March 2003. [Google Scholar]
- Shigo, A.L. Fungi isolated from oak-wilt trees and their effects on Ceratocystis fagacearum. Mycologia 1958, 50, 757–769. [Google Scholar] [CrossRef]
- Teperi, E.; Keskinen, M.; Ketoja, E.; Tahvonen, R. Screening for fungal antagonists of seed-borne Fusarium culmorum on wheat using in vivo tests. Eur. J. Plant Pathol. 1998, 104, 243–251. [Google Scholar] [CrossRef]
- Hue, A.G.; Voldeng, H.D.; Savard, M.E.; Fedak, G.; Tian, X.; Hsiang, T. Biological control of Fusarium head blight of wheat with Clonostachys rosea strain ACM941. Can. J. Plant Pathol. 2009, 31, 169–179. [Google Scholar] [CrossRef]
- Popiel, D.; Koczyk, G.; Dawidziuk, A.; Gromadzka, K.; Blaszczyk, L.; Chelkowski, J. Zearalenone lactonohydrolase activity in Hypocreales and its evolutionary relationships within the epoxide hydrolase subset of a/b-hydrolases. BMC Microbiol. 2014, 14, 82. [Google Scholar] [CrossRef]
- Chen, S.; Pan, L.; Liu, S.; Pan, L.; Li, X.; Wang, B. Recombinant expression and surface display of a zearalenone lactonohydrolase from Trichoderma aggressivum in Escherichia coli. Protein Expr. Purif. 2021, 187, 105933. [Google Scholar] [CrossRef]
- Błaszczyk, L.; Ćwiek-Kupczyńska, H.; Hoppe Gromadzka, K.; Basińska-Barczak, A.; Stępień, Ł.; Kaczmarek, J.; Lenc, L. Containment of Fusarium culmorum and its mycotoxins in various biological systems by antagonistic Trichoderma and Clonostachys strains. J. Fungi 2023, 9, 289. [Google Scholar] [CrossRef] [PubMed]
- el-Sharkawy, S.; Abul-Hajj, Y. Microbial transformation of zearalenone, I. Formation of zearalenone-4-O-β-glucoside. J. Nat. Prod. 1987, 50, 520–521. [Google Scholar] [CrossRef]
- Nowak, M.; Soboń, A.; Bernat, P.; Różalska, S. Entomopathogenic fungi of the genus Cordyceps biotransform zearalenone—Metabolomic and proteomic backgrounds. Int. Biodeterior. Biodegrad. 2023, 179, 105572. [Google Scholar] [CrossRef]
- Borzekowski, A.; Drewitz, T.; Keller, J.; Pfeifer, D.; Kunte, H.-J.; Koch, M.; Rohn, S.; Maul, R. Biosynthesis and characterization of zearalenone-14-sulfate, zearalenone-14-glucoside and zearalenone-16-glucoside using common fungal strains. Toxins 2018, 10, 104. [Google Scholar] [CrossRef]
- Kamimura, H. Conversion of zearalenone to zearalenone glycoside by Rhizopus sp. Appl. Environ. Microbiol. 1986, 52, 515–519. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.P. Microbial detoxification of mycotoxins. J. Chem. Ecol. 2013, 39, 907–918. [Google Scholar] [CrossRef]
- Vero, S.; Garmendia, G.; Allori, E.; Sanz, J.M.; Gonda, M.; Alconada, T.; Cavello, I.; Dib, J.R.; Diaz, M.A.; Nally, C.; et al. Microbial biopesticides: Diversity, scope, and mechanisms involved in plant disease control. Diversity 2023, 15, 457. [Google Scholar] [CrossRef]
- Schiwek, S.; Slonka, M.; Alhussein, M.; Knierim, D.; Margaria, P.; Rose, H.; Richert-Pöggeler, K.R.; Rostás, M.; Karlovsky, P. Mycoviruses increase the attractiveness of Fusarium graminearum for fungivores and suppress production of the mycotoxin deoxynivalenol. Toxins 2024, 16, 131. [Google Scholar] [CrossRef]
- Gimeno, A.; Leimgruber, M.; Kägi, A.; Jenny, E.; Vogelgsang, S. UV protection and shelf life of the biological control agent Clonostachys rosea against Fusarium graminearum. Biological Control 2021, 158, 104600. [Google Scholar] [CrossRef]
- Kosawang, C.; Karlsson, M.; Vélëz, H.; Rasmussen, P.H.; Collinge, D.B.; Jensen, B.; Jensen, D.F. Zearalenone detoxification by zearalenone hydrolase is important for the antagonistic ability of Clonostachys rosea against mycotoxigenic Fusarium graminearum. Fungal Biol. 2014, 118, 364–373. [Google Scholar] [CrossRef]
- Lee, J.; Son, H.; Lee, S.; Park, A.R.; Lee, Y.-W. Development of a conditional gene expression system using a zearalenone-inducible promoter for the ascomycete fungus Gibberella zeae. Appl. Environ. Microbiol. 2010, 76, 3089–3096. [Google Scholar] [CrossRef] [PubMed]
- Bui, D.-C.; Lee, Y.; Lim, J.Y.; Fu, M.; Kim, J.-C.; Choi, G.J.; Son, H.; Lee, Y.-W. Heat shock protein 90 is required for sexual and asexual development, virulence, and heat shock response in Fusarium graminearum. Sci. Rep. 2016, 6, 28154. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Mao, H.; Huang, Q.; Dong, J. Benzenediol lactones: A class of fungal metabolites with diverse structural features and biological activities. Eur. J. Med. Chem. 2015, 97, 747–777. [Google Scholar] [CrossRef] [PubMed]
- Ayer, W.A.; Lee, S.P.; Tsuneda, A.; Hiratsuka, Y. The isolation, identification, and bioassay of the antifungal metabolites produced by Monocillium nordinii. Can. J. Microbiol. 1980, 26, 766–773. [Google Scholar] [CrossRef]
- Wicklow, D.T.; Joshi, B.K.; Gamble, W.R.; Gloer, J.B.; Dowd, P.F. Antifungal metabolites (monorden, monocillin IV, and cerebrosides) from Humicola fuscoatra Traaen NRRL 22980, a mycoparasite of Aspergillus flavus sclerotia. Appl. Environ. Microbiol. 1998, 64, 4482–4484. [Google Scholar] [CrossRef]
- Piper, P.W.; Millson, S.H. Spotlight on the microbes that produce heat shock protein 90-targeting antibiotics. Open Biol. 2012, 2, 120138. [Google Scholar] [CrossRef]
- Poling, S.M.; Wicklow, D.T.; Rogers, K.D.; Gloer, J.B. Acremonium zeae, a protective endophyte of maize, produces dihydroresorcylide and 7-hydroxydihydroresorcylides. J. Agric. Food Chem. 2008, 56, 3006–3009. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, S.; Zhou, T.; Zhan, J. Three new resorcylic acid derivatives from Sporotrichum laxum. Bioorg. Med. Chem. Lett. 2013, 23, 5806–5809. [Google Scholar] [CrossRef]
- Tomoko, A.; Yoji, K.; Haruaki, Y.; Hiroshi, A.; Hekibai, K.; Sosho, C. Isobenzofuranone Compounds and Their Analogs. Japanese Patent No. 09-110781, 28 April 1997. [Google Scholar]
- Arnone, A.; Assante, G.; Nasini, G.; De Pava, O.V. Phanerosporic acid, a β-resorcylate obtained from Phanerochaete chrysosporium. Phytochemistry 1989, 28, 2803–2806. [Google Scholar] [CrossRef]
- Wang, X.; Radwan, M.M.; Taráwneh, A.H.; Gao, J.; Wedge, D.E.; Rosa, L.H.; Cutler, H.G.; Cutler, S.J. Antifungal activity against plant pathogens of metabolites from the endophytic fungus Cladosporium cladosporioides. J. Agric. Food Chem. 2013, 61, 4551–4555. [Google Scholar] [CrossRef]
- Scott, P.M.; Van Walbeek, W.; MacLean, W.M. Cladosporin, a new antifungal metabolite from Cladosporium cladosporioides. J. Antibiot. 1971, 24, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Brady, S.F.; Wagenaar, M.M.; Singh, M.P.; Janso, J.E.; Clardy, J. The cytosporones, new octaketide antibiotics isolated from an endophytic fungus. Org. Lett. 2000, 2, 4043–4046. [Google Scholar] [CrossRef]
- Delmotte, P.; Delmotte-Plaquee, J. A new antifungal substance of fungal origin. Nature 1953, 171, 344. [Google Scholar] [CrossRef] [PubMed]
- McCapra, F.; Scott, A.I.; Delmotte, P.; Delmotte-Plaquée, J.; Bhacca, N.S. The constitution of monorden, an antibiotic with tranquilising action. Tetrahedron Lett. 1964, 5, 869–875. [Google Scholar] [CrossRef]
- Mirrington, R.N.; Ritchie, E.; Shoppee, C.W.; Taylor, W.C.; Sternhell, S. The constitution of radicicol. Tetrahedron Lett. 1964, 5, 365–370. [Google Scholar] [CrossRef]
- Wicklow, D.T.; Jordan, A.M.; Gloer, J.B. Antifungal metabolites (monorden, monocillins I, II, III) from Colletotrichum graminicola, a systemic vascular pathogen of maize. Mycol. Res. 2009, 113, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, K.; Nakajima, S. Isolation of radicicol from Penicillium luteo-aurantium, and meleagrin, a new metabolite from Penicillium meleagrinum. J. Nat. Prod. 1979, 42, 374–377. [Google Scholar] [CrossRef]
- Cutler, H.G.; Arrendale, R.F.; Springer, J.P.; Cole, P.D.; Roberts, R.G.; Hanlin, R.T. Monorden from a novel source, Neocosmospora tenuicristata: Stereochemistry and plant growth regulatory properties. Agric. Biol. Chem. 1987, 51, 3331–3338. [Google Scholar] [CrossRef]
- Tanney, J.B.; Di Stefano, J.; Miller, J.D.; McMullin, D.R. Natural products from the Picea foliar endophytes Niesslia endophytica sp. nov. and Strasseria geniculata. Mycol. Prog. 2023, 22, 1–13. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Choi, S.; Kim, S.; Lee, J.-H.; Park, A.R.; Yu, N.H.; Yoon, H.; Bae, C.-H.; Yeo, J.H.; Choi, G.J.; et al. The Hsp90 inhibitor, monorden, is a promising lead compound for the development of novel fungicides. Front. Plant Sci. 2020, 11, 371. [Google Scholar] [CrossRef]
- Horáková, K.; Betina, V. Cytotoxic activity of macrocyclic metabolites from fungi. Neoplasma 1977, 24, 21–27. [Google Scholar]
- Kwon, H.J.; Yoshida, M.; Fukui, Y.; Horinouchi, S.; Beppu, T. Potent and specific inhibition of p60v-src protein kinase both in vivo and in vitro by radicicol. Cancer Res. 1992, 52, 6926–6930. [Google Scholar]
- Schulte, T.W.; Akinaga, S.; Soga, S.; Sullivan, W.; Stensgard, B.; Toft, D.; Neckers, L.M. Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin. Cell Stress Chaperones 1998, 3, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Schulte, T.W.; Akinaga, S.; Murakata, T.; Agatsuma, T.; Sugimoto, S.; Nakano, H.; Lee, Y.S.; Simen, B.B.; Argon, Y.; Felts, S.; et al. Interaction of radicicol with members of the heat shock protein 90 family of molecular chaperones. Mol. Endocrinol. 1999, 13, 1435–1448. [Google Scholar] [CrossRef] [PubMed]
- McLellan, C.A.; Turbyville, T.J.; Wijeratne, E.M.K.; Kerschen, A.; Vierling, E.; Queitsch, C.; Whitesell, L.; Gunatilaka, A.A.L. A rhizosphere fungus enhances Arabidopsis thermotolerance through production of an HSP90 inhibitor. Plant Physiol. 2007, 145, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Rink, C.; Sasse, F.; Zubrienė, A.; Matulis, D.; Maier, M.E. Probing the influence of an allylic methyl group in zearalenone analogues on binding to Hsp90. Chem. Eur. J. 2010, 16, 14469–14478. [Google Scholar] [CrossRef]
- Talontsi, F.M.; Facey, P.; Tatong, M.D.K.; Tofazzal Islam, M.; Frauendorf, H.; Draeger, S.; von Tiedemann, A.; Laatsch, H. Zoosporicidal metabolites from an endophytic fungus Cryptosporiopsis sp. of Zanthoxylum leprieurii. Phytochemistry 2012, 83, 87–94. [Google Scholar] [CrossRef]
- Almassi, F.; Ghisalberti, E.L.; Skelton, B.W.; White, A.H. Structural study of dehydrocurvularin, an inhibitor of microtubule assembly. Aust. J. Chem. 1994, 47, 1193–1197. [Google Scholar] [CrossRef]
- Ghisalberti, E.L.; Hockless, D.C.R.; Rowland, C.Y.; White, A.H. Structural study of curvularin, a cell division inhibitor. Aust. J. Chem. 1993, 46, 571–575. [Google Scholar] [CrossRef]
- Huo, R.-Y.; Zhang, J.-X.; Jia, J.; Bi, H.-K.; Liu, L. Alternarialone A, a new curvularin-type metabolite from the mangrove-derived fungus Alternaria longipes. J. Asian Nat. Prod. Res. 2023, 25, 610–616. [Google Scholar] [CrossRef]
- Musgrave, O.C. 828. Curvularin. Part I. Isolation and partial characterisation of a metabolic product from a new species of Curvularia. J. Chem. Soc. 1956, 1956, 4301–4305. [Google Scholar] [CrossRef]
- Kamauchi, H.; Furukawa, M.; Kiba, Y.; Kitamura, M.; Usui, K.; Katakura, M.; Takao, K.; Sugita, Y. Antifungal activity of dehydrocurvularin for Candida spp. through the inhibition of adhesion to human adenocarcinoma cells. J. Antibiot. 2022, 75, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Duan, F.-F.; Liu, L.; Peng, X.-G.; Meng, X.-G.; Ruan, H.-L. Hypothemycin-type resorcylic acid lactones with immunosuppressive activities from a Podospora sp. J. Nat. Prod. 2021, 84, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Reeves, C.D.; Hu, Z.; Reid, R.; Kealey, J.T. Genes for the biosynthesis of the fungal polyketides hypothemycin from Hypomyces subiculosus and radicicol from Pochonia chlamydosporia. Appl. Environ. Microbiol. 2008, 74, 5121–5129. [Google Scholar] [CrossRef]
- Wee, J.L.; Sundermann, K.; Licari, P.; Galazzo, J. Cytotoxic hypothemycin analogues from Hypomyces subiculosus. J. Nat. Prod. 2006, 69, 1456–1459. [Google Scholar] [CrossRef]
- Xu, L.; Xue, J.; Wu, P.; Wang, D.; Lin, L.; Jiang, Y.; Duan, X.; Wei, X. Antifungal activity of hypothemycin against Peronophythora litchii in vitro and in vivo. J. Agric. Food Chem. 2013, 61, 10091–10095. [Google Scholar] [CrossRef]
- Luo, H.; Meng, S.; Deng, Y.; Deng, Z.; Shi, H. In vitro antifungal activity of lasiodiplodin, isolated from endophytic fungus Lasiodiplodia pseudotheobromae J-10 associated with Sarcandra glabra and optimization of culture conditions for lasiodiplodin production. Arch. Microbiol. 2023, 205, 140. [Google Scholar] [CrossRef]
- Xu, L.; Xue, J.; Zou, Y.; He, S.; Wei, X. Three new β-resorcylic acid lactones from Paecilomyces sp. SC0924. Chin. J. Chem. 2012, 30, 1273–1277. [Google Scholar] [CrossRef]
- Xu, L.-X.; Wu, P.; Wei, H.-H.; Xue, J.-H.; Hu, X.-P.; Wei, X.-Y. Paecilomycins J–M, four new β-resorcylic acid lactones from Paecilomyces sp. SC0924. Tetrahedron Lett. 2013, 54, 2648–2650. [Google Scholar] [CrossRef]
- Xu, L.; Wu, P.; Xue, J.; Molnar, I.; Wei, X. Antifungal and cytotoxic β-resorcylic acid lactones from a Paecilomyces species. J. Nat. Prod. 2017, 80, 2215–2223. [Google Scholar] [CrossRef]
- Hoshino, Y.; Ivanova, V.B.; Yazawa, K.; Ando, A.; Mikami, Y.; Zaki, S.M.; Karam, A.-Z.A.; Youssef, Y.A.; Gräfe, U. Queenslandon, a new antifungal compound produced by Chrysosporium queenslandicum: Production, isolation and structure elucidation. J. Antibiot. 2002, 55, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.; White, N.H.; Evans, G.; White, N.H. Radicicolin and radicicol, two new antibiotics produced by Cylindrocarpon radicicola. Brit. Mycol. Soc. Trans. 1966, 49, 563–576. [Google Scholar] [CrossRef]
- Patocka, J.; Soukup, O.; Kuca, K. Resorcylic acid lactones as the protein kinase inhibitors, naturally occuring toxins. Mini Rev. Med. Chem. 2013, 13, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Kuttikrishnan, S.; Prabhu, K.S.; Al Sharie, A.H.; Al Zu’bi, Y.O.; Alali, F.Q.; Oberlies, N.H.; Ahmad, A.; El-Elimat, T.; Uddin, S. Natural resorcylic acid lactones: A chemical biology approach for anticancer activity. Drug Discov. Today 2022, 27, 547–557. [Google Scholar] [CrossRef]
- Chanda, A.; Roze, L.V.; Kang, S.; Artymovich, K.A.; Hicks, G.R.; Raikhel, N.V.; Calvo, A.M.; Linz, J.E. A key role for vesicles in fungal secondary metabolism. Proc. Natl. Acad. Sci. USA 2009, 106, 19533–19538. [Google Scholar] [CrossRef]
- Kang, Y.; Lee, S.-H.; Lee, J. Development of a selective medium for the fungal pathogen Cylindrocarpon destructans using radicicol. Plant Pathol. J. 2014, 30, 432–436. [Google Scholar] [CrossRef]
- Prodromou, C.; Nuttall, J.M.; Millson, S.H.; Roe, S.M.; Sim, T.-S.; Tan, D.; Workman, P.; Pearl, L.H.; Piper, P.W. Structural basis of the radicicol resistance displayed by a fungal Hsp90. ACS Chem. Biol. 2009, 4, 289–297. [Google Scholar] [CrossRef]
- Adam, G.; Torres Acosta, J.; Berthiller, F.; Wiesenberger, G.; Mitterbauer, R.; Krska, R. Mechanisms of Self Resistance to Zearalenone in Fusarium graminearum. In Proceedings of the 26th Fungal Genetics Conference, Pacific Grove, CA, USA, 15–20 March 2011; Fungal Genetics Reports. Genetics Society of America: Asilomar, CA, USA, 2011; Volume 58, p. 207. [Google Scholar]
- Plasencia, J.; Mirocha, C.J. Isolation and characterization of zearalenone sulfate produced by Fusarium spp. Appl. Environ. Microbiol. 1991, 57, 146–150. [Google Scholar] [CrossRef]
- Xu, Y.; Vinas, M.; Alsarrag, A.; Su, L.; Pfohl, K.; Rohlfs, M.; Schäfer, W.; Chen, W.; Karlovsky, P. Bis-naphthopyrone pigments protect filamentous ascomycetes from a wide range of predators. Nat. Commun. 2019, 10, 3579. [Google Scholar] [CrossRef]
- Brown, D.W.; Butchko, R.A.E.; Busman, M.; Proctor, R.H. The Fusarium verticillioides FUM gene cluster encodes a zn(II)2Cys6 protein that affects FUM gene expression and fumonisin production. Eukaryot. Cell 2007, 6, 1210–1218. [Google Scholar] [CrossRef]
- Montis, V.; Pasquali, M.; Visentin, I.; Karlovsky, P.; Cardinale, F. Identification of a cis-acting factor modulating the transcription of FUM1, a key fumonisin-biosynthetic gene in the fungal maize pathogen Fusarium verticillioides. Fungal Genet. Biol. 2013, 51, 42–49. [Google Scholar] [CrossRef]
- Visentin, I.; Montis, V.; Döll, K.; Alabouvette, C.; Tamietti, G.; Karlovsky, P.; Cardinale, F. Transcription of genes in the biosynthetic pathway for fumonisin mycotoxins is epigenetically and differentially regulated in the fungal maize pathogen Fusarium verticillioides. Eukaryot. Cell 2012, 11, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Myung, K.; Li, S.; Butchko, R.A.E.; Busman, M.; Proctor, R.H.; Abbas, H.K.; Calvo, A.M. FvVE1 regulates biosynthesis of the mycotoxins fumonisins and fusarins in Fusarium verticillioides. J. Agric. Food Chem. 2009, 57, 5089–5094. [Google Scholar] [CrossRef]
- Rösler, S.M.; Sieber, C.M.K.; Humpf, H.-U.; Tudzynski, B. Interplay between pathway-specific and global regulation of the fumonisin gene cluster in the rice pathogen Fusarium fujikuroi. Appl. Microbiol. Biotechnol. 2016, 100, 5869–5882. [Google Scholar] [CrossRef]
- Xie, L.; Feng, L.; Ren, Y.; Yang, Q.; Qu, H.; Li, T.; Jiang, Y. Transcriptome-wide N6-methyladenosinem modifications analysis of growth and fumonisins production in Fusarium proliferatum causing banana crown rot. Int. J. Biol. Macromol. 2025, 300, 140385. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, X.; Gao, J.; Zhao, Y.; Liu, L.; Hou, Y.; Wang, L.; Huang, S. Effects of disruption of five FUM genes on fumonisin biosynthesis and pathogenicity in Fusarium proliferatum. Toxins 2019, 11, 327. [Google Scholar] [CrossRef]
- Dastjerdi, R.; Karlovsky, P. Systemic infection of maize, sorghum, rice, and beet seedlings with fumonisin-producing and nonproducing Fusarium verticillioides strains. Plant Pathol. J. 2015, 31, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Glenn, A.E.; Zitomer, N.C.; Zimeri, A.M.; Williams, L.D.; Riley, R.T.; Proctor, R.H. Transformation-mediated complementation of a FUM gene cluster deletion in Fusarium verticillioides restores both fumonisin production and pathogenicity on maize seedlings. Mol. Plant Microbe Interact. 2008, 21, 87–97. [Google Scholar] [CrossRef]
- Proctor, R.H.; Desjardins, A.E.; McCormick, S.P.; Plattner, R.D.; Alexander, N.J.; Brown, D.W. Genetic analysis of the role of trichothecene and fumonisin mycotoxins in the virulence of Fusarium. Eur. J. Plant Pathol. 2002, 108, 691–698. [Google Scholar] [CrossRef]
- Desjardins, A.E.; Munkvold, G.P.; Plattner, R.D.; Proctor, R.H. FUM1--a gene required for fumonisin biosynthesis but not for maize ear rot and ear infection by Gibberella moniliformis in field tests. Mol. Plant-Microbe Interact. MPMI 2002, 15, 1157–1164. [Google Scholar] [CrossRef]
- Desjardins, A.E.; Plattner, R.D. Fumonisin B(1)-nonproducing strains of Fusarium verticillioides cause maize (Zea mays) ear infection and ear rot. J. Agric. Food Chem. 2000, 48, 5773–5780. [Google Scholar] [CrossRef]
- Warfield, C.Y.; Gilchrist, D.G. Influence of kernel age on fumonisin B1 production in maize by Fusarium moniliforme. Appl. Environ. Microbiol. 1999, 65, 2853–2856. [Google Scholar] [CrossRef] [PubMed]
- Nygren, K.; Dubey, M.; Zapparata, A.; Iqbal, M.; Tzelepis, G.D.; Durling, M.B.; Jensen, D.F.; Karlsson, M. The mycoparasitic fungus Clonostachys rosea responds with both common and specific gene expression during interspecific interactions with fungal prey. Evol. Appl. 2018, 11, 931–949. [Google Scholar] [CrossRef]
- Stange, P.; Kersting, J.; Sivaprakasam Padmanaban, P.B.; Schnitzler, J.-P.; Rosenkranz, M.; Karl, T.; Benz, J.P. The decision for or against mycoparasitic attack by Trichoderma spp. is taken already at a distance in a prey-specific manner and benefits plant-beneficial interactions. Fungal Biol. Biotechnol. 2024, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Kim, J.-E.; Yun, S.-H.; Lee, Y.-W. Possible negative effect of pigmentation on biosynthesis of polyketide mycotoxin zearalenone in Gibberella zeae. J. Microbiol. Biotechnol. 2006, 16, 1392–1398. [Google Scholar]
- Audenaert, K.; Vanheule, A.; Höfte, M.; Haesaert, G. Deoxynivalenol: A major player in the multifaceted response of Fusarium to its environment. Toxins 2014, 6, 1–19. [Google Scholar] [CrossRef]
- Shiono, Y.; Tsuchinari, M.; Shimanuki, K.; Miyajima, T.; Murayama, T.; Koseki, T.; Laatsch, H.; Funakoshi, T.; Takanami, K.; Suzuki, K. Fusaristatins a and B, two new cyclic lipopeptides from an endophytic Fusarium sp. J. Antibiot. 2007, 60, 309–316. [Google Scholar] [CrossRef]
- Sørensen, J.L.; Sondergaard, T.E.; Covarelli, L.; Fuertes, P.R.; Hansen, F.T.; Frandsen, R.J.N.; Saei, W.; Lukassen, M.B.; Wimmer, R.; Nielsen, K.F.; et al. Identification of the biosynthetic gene clusters for the lipopeptides fusaristatin a and W493 B in Fusarium graminearum and F. pseudograminearum. J. Nat. Prod. 2014, 77, 2619–2625. [Google Scholar] [CrossRef]
- Wollenberg, R.D.; Sondergaard, T.E.; Nielsen, M.R.; Knutsson, S.; Pedersen, T.B.; Westphal, K.R.; Wimmer, R.; Gardiner, D.M.; Sørensen, J.L. There it is! Fusarium pseudograminearum did not lose the fusaristatin gene cluster after all. Fungal Biol. 2019, 123, 10–17. [Google Scholar] [CrossRef]
- Khudhair, M.; Kazan, K.; Thatcher, L.F.; Obanor, F.; Rusu, A.; Sørensen, J.L.; Wollenberg, R.D.; McKay, A.; Giblot-Ducray, D.; Simpfendorfer, S.; et al. Fusaristatin A production negatively affects the growth and aggressiveness of the wheat pathogen Fusarium pseudograminearum. Fungal Genet. Biol. 2020, 136, 103314. [Google Scholar] [CrossRef]
- Fitzpatrick, D.W.; Picken, C.A.; Murphy, L.C.; Buhr, M.M. Measurement of the relative binding affinity of zearalenone, alpha-zearalenol and beta-zearalenol for uterine and oviduct estrogen receptors in swine, rats and chickens: An indicator of estrogenic potencies. Comp. Biochem. Physiol. C 1989, 94, 691–694. [Google Scholar] [CrossRef] [PubMed]
- EFSA CONTAM Panel. Appropriateness to set a group health-based guidance value for zearalenone and its modified forms. EFSA J. 2016, 14, e04425. [Google Scholar] [CrossRef]
- Bennett, G.A.; Shotwell, O.L.; Kwolek, W.F. Liquid chromatographic determination of alpha-zearalenol and zearalenone in corn: Collaborative study. J. Assoc. Off. Anal. Chem. 1985, 68, 958–961. [Google Scholar]
- Müller, H.-M.; Schwadorf, K. A survey of the natural occurrence of Fusarium toxins in wheat grown ina southwestern area of Germany. Mycopathologia 1993, 121, 115–121. [Google Scholar] [CrossRef]
- Dall’Asta, C.; Faccini, A.; Galaverna, G.; Corradini, R.; Dossena, A.; Marchelli, R. Complexation of zearalenone and zearalenols with native and modified β-cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2009, 64, 331–340. [Google Scholar] [CrossRef]
- Berthiller, F.; Werner, U.; Sulyok, M.; Krska, R.; Hauser, M.-T.; Schuhmacher, R. Liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) determination of phase II metabolites of the mycotoxin zearalenone in the model plant Arabidopsis thaliana. Food Addit. Contam. 2006, 23, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Adejumo, T.O.; Hettwer, U.; Karlovsky, P. Survey of maize from south-western Nigeria for zearalenone, alpha- and beta-zearalenols, fumonisin B-1 and enniatins produced by Fusarium species. Food Addit. Contam. 2007, 24, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- De Boevre, M.; Di Mavungu, J.D.; Maene, P.; Audenaert, K.; Deforce, D.; Haesaert, G.; Eeckhout, M.; Callebaut, A.; Berthiller, F.; Van Peteghem, C.; et al. Development and validation of an LC-MS/MS method for the simultaneous determination of deoxynivalenol, zearalenone, T-2-toxin and some masked metabolites in different cereals and cereal-derived food. Food Addit. Contam. 2012, 29, 819–835. [Google Scholar] [CrossRef]
- Adetunji, M.; Atanda, O.; Ezekiel, C.N.; Sulyok, M.; Warth, B.; Beltrán, E.; Krska, R.; Obadina, O.; Bakare, A.; Chilaka, C.A. Fungal and bacterial metabolites of stored maize (Zea mays L.) from five agro-ecological zones of Nigeria. Mycotoxin Res. 2014, 30, 89–102. [Google Scholar] [CrossRef]
- Vanheule, A.; Audenaert, K.; De Boevre, M.; Landschoot, S.; Bekaert, B.; Munaut, F.; Eeckhout, M.; Höfte, M.; De Saeger, S.; Haesaert, G. The compositional mosaic of Fusarium species and their mycotoxins in unprocessed cereals, food and feed products in Belgium. Int. J. Food Microbiol. 2014, 181, 28–36. [Google Scholar] [CrossRef]
- Nathanail, A.V.; Syvähuoko, J.; Malachová, A.; Jestoi, M.; Varga, E.; Michlmayr, H.; Adam, G.; Sieviläinen, E.; Berthiller, F.; Peltonen, K. Simultaneous determination of major type A and B trichothecenes, zearalenone and certain modified metabolites in Finnish cereal grains with a novel liquid chromatography-tandem mass spectrometric method. Anal. Bioanal. Chem. 2015, 407, 4745–4755. [Google Scholar] [CrossRef] [PubMed]
- Bryła, M.; Waśkiewicz, A.; Podolska, G.; Szymczyk, K.; Jędrzejczak, R.; Damaziak, K.; Sułek, A. Occurrence of 26 mycotoxins in the grain of cereals cultivated in Poland. Toxins 2016, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Chilaka, C.A.; De Boevre, M.; Atanda, O.O.; De Saeger, S. Occurrence of Fusarium mycotoxins in cereal crops and processed products (ogi) from Nigeria. Toxins 2016, 8, 342. [Google Scholar] [CrossRef]
- Olopade, B.K.; Oranusi, S.U.; Nwinyi, O.C.; Gbashi, S.; Njobeh, P.B. Occurrences of deoxynivalenol, zearalenone and some of their masked forms in selected cereals from Southwest Nigeria. NFS J. 2021, 23, 24–29. [Google Scholar] [CrossRef]
- Suchowilska, E.; Wiwart, M.; Sulyok, M.; Kandler, W.; Krska, R. Mycotoxin profiles and plumpness of Tritordeum grain after artificial spike inoculation with Fusarium culmorum W.G. Smith. Int. J. Food Microbiol. 2025, 427, 110963. [Google Scholar] [CrossRef]
- Sulyok, M.; Stadler, D.; Steiner, D.; Krska, R. Validation of an LC-MS/MS-based dilute-and-shoot approach for the quantification of >500 mycotoxins and other secondary metabolites in food crops: Challenges and solutions. Anal. Bioanal. Chem. 2020, 412, 2607–2620. [Google Scholar] [CrossRef]
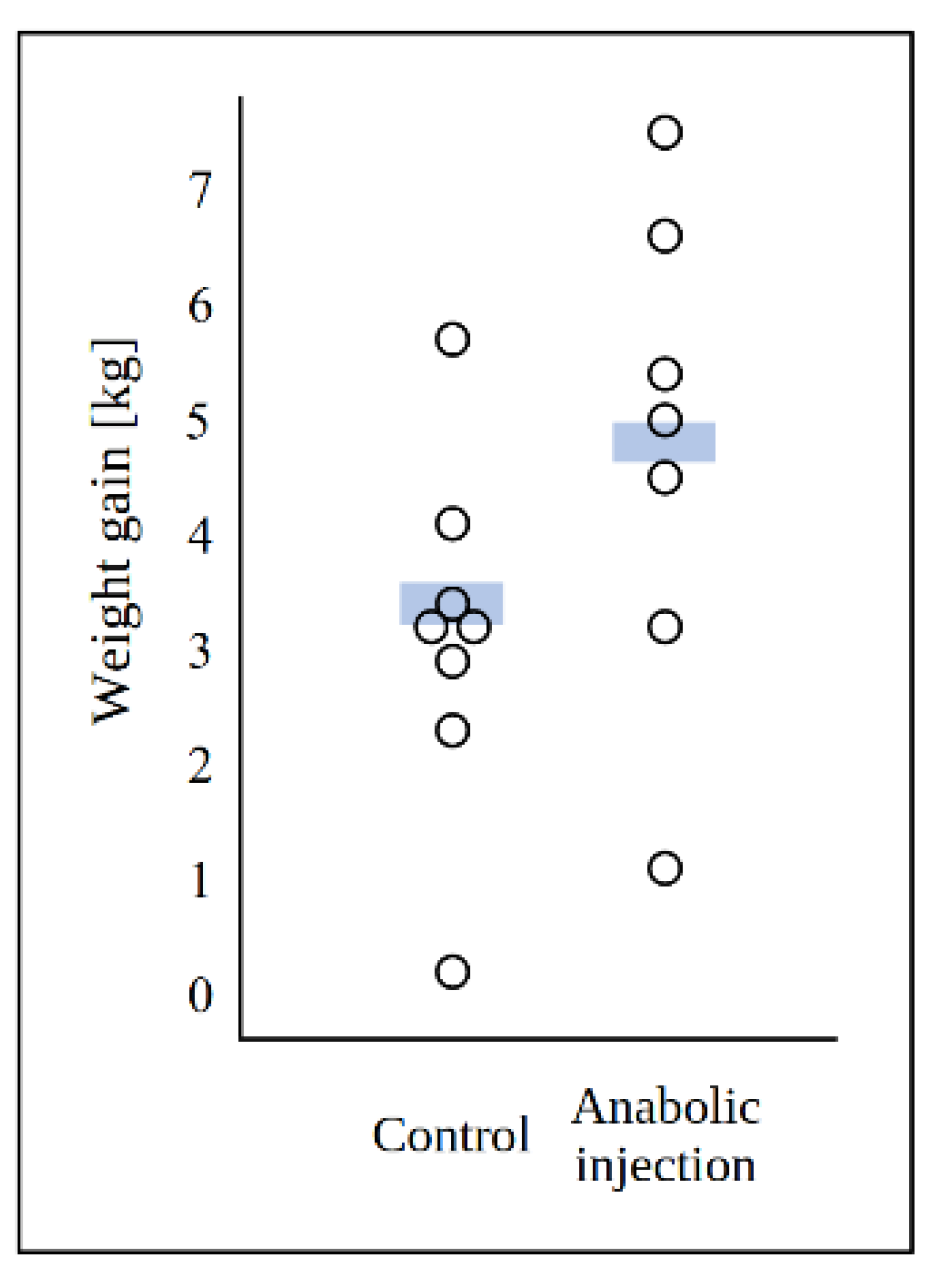
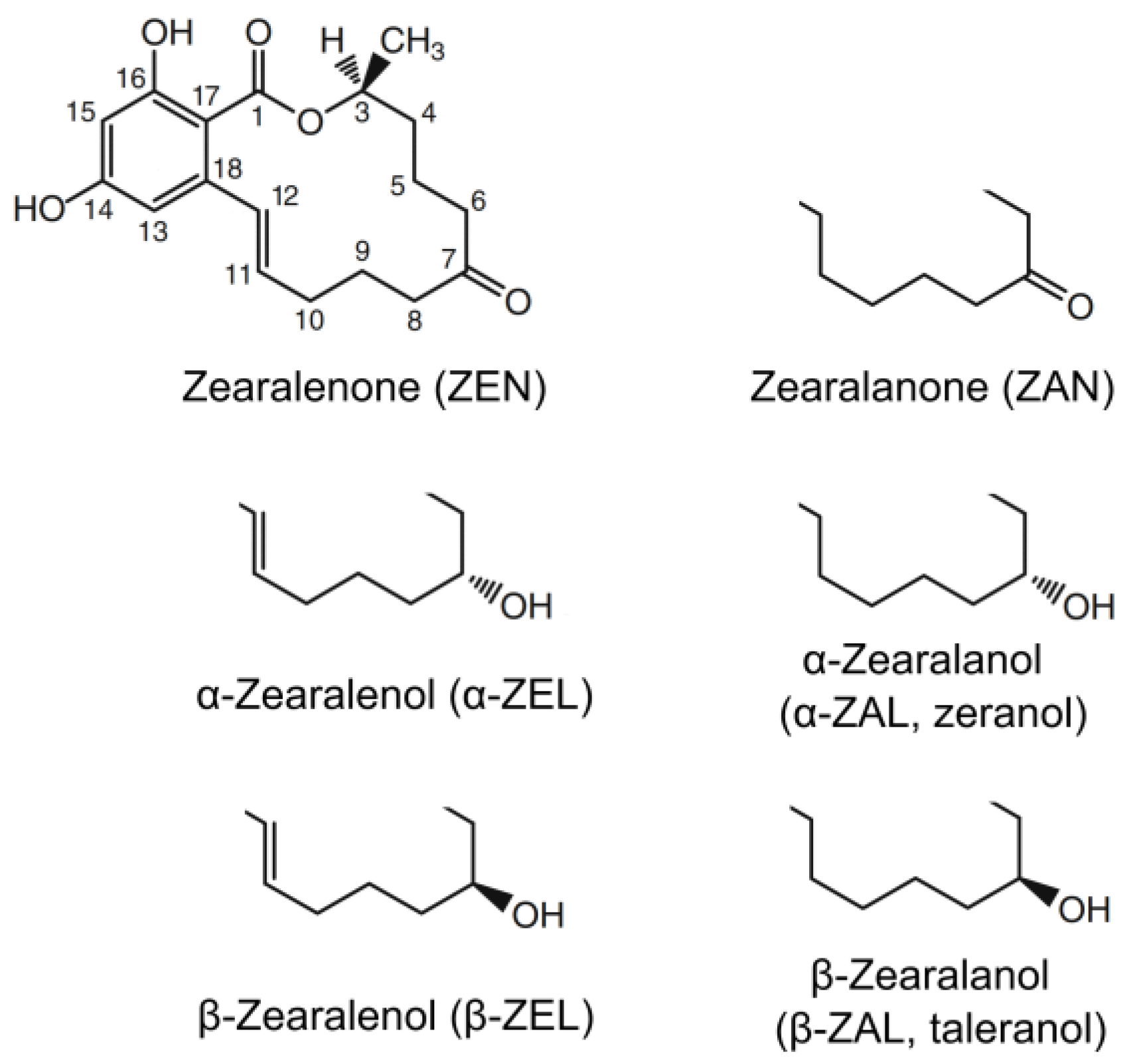
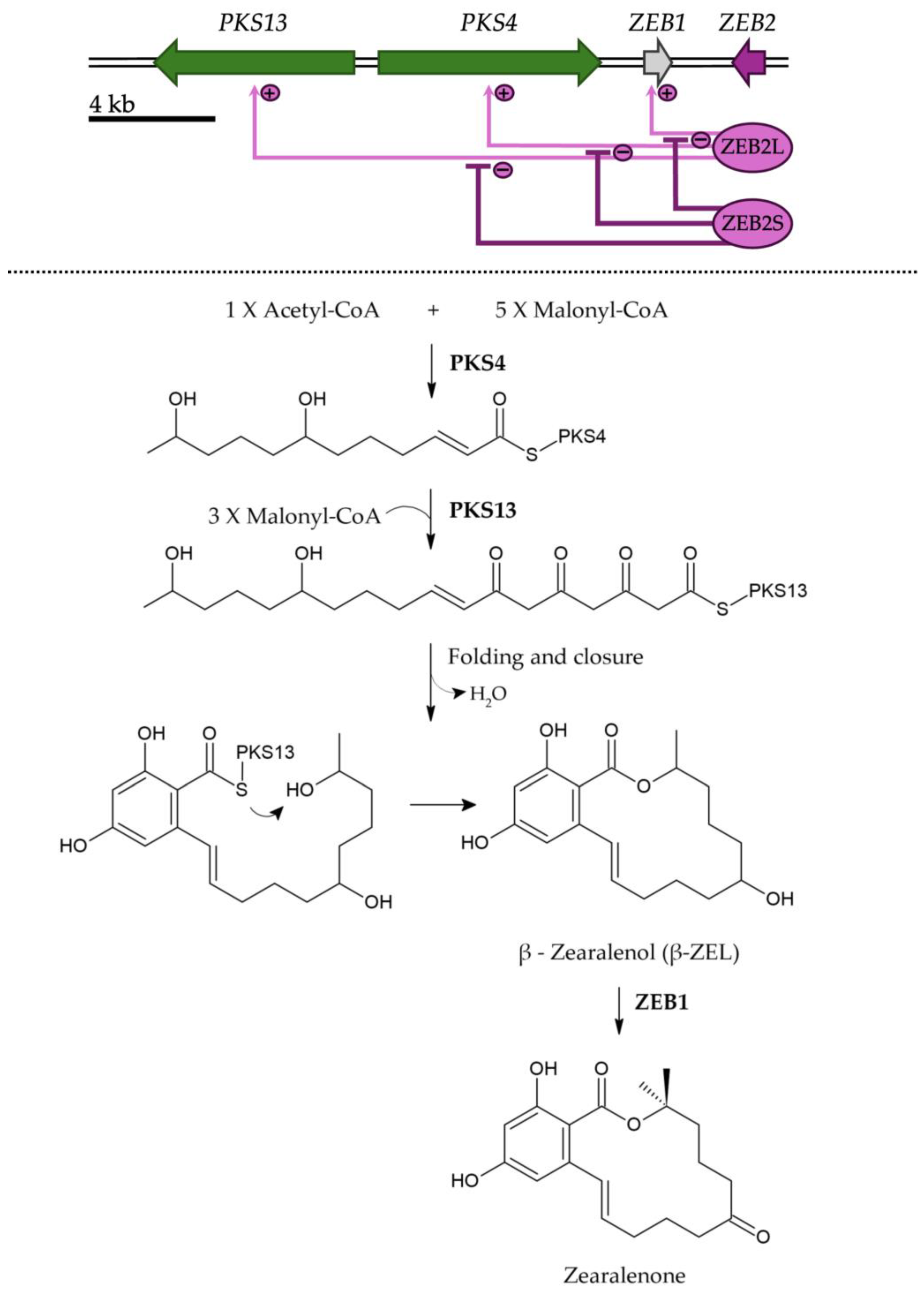

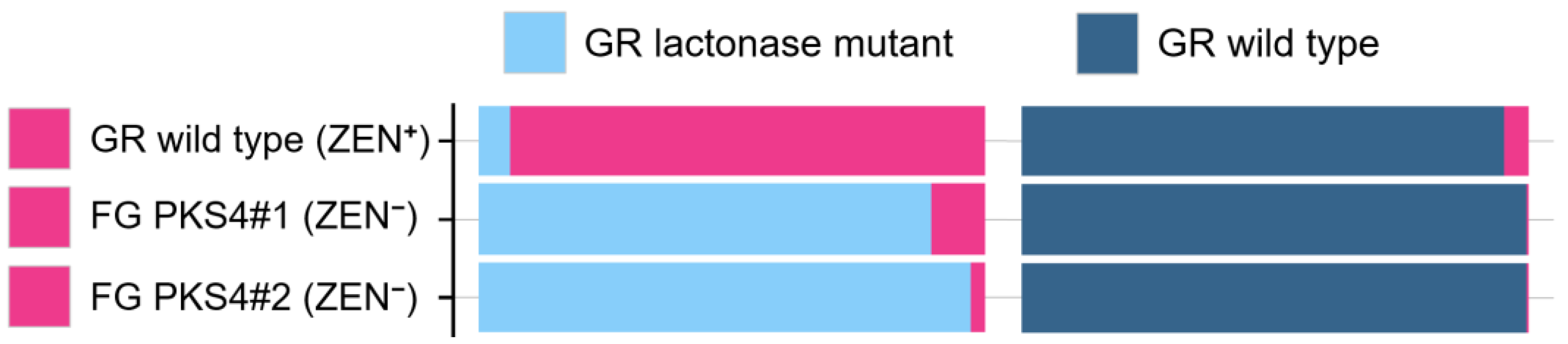
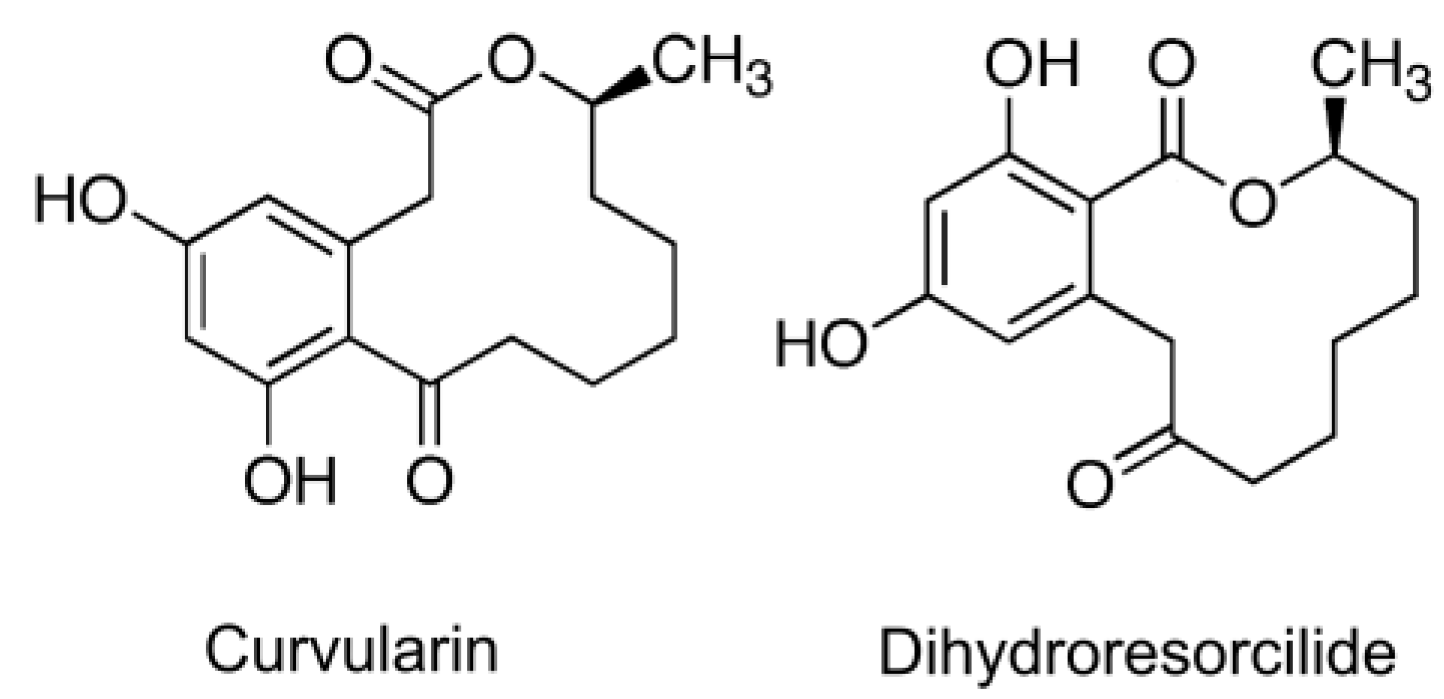
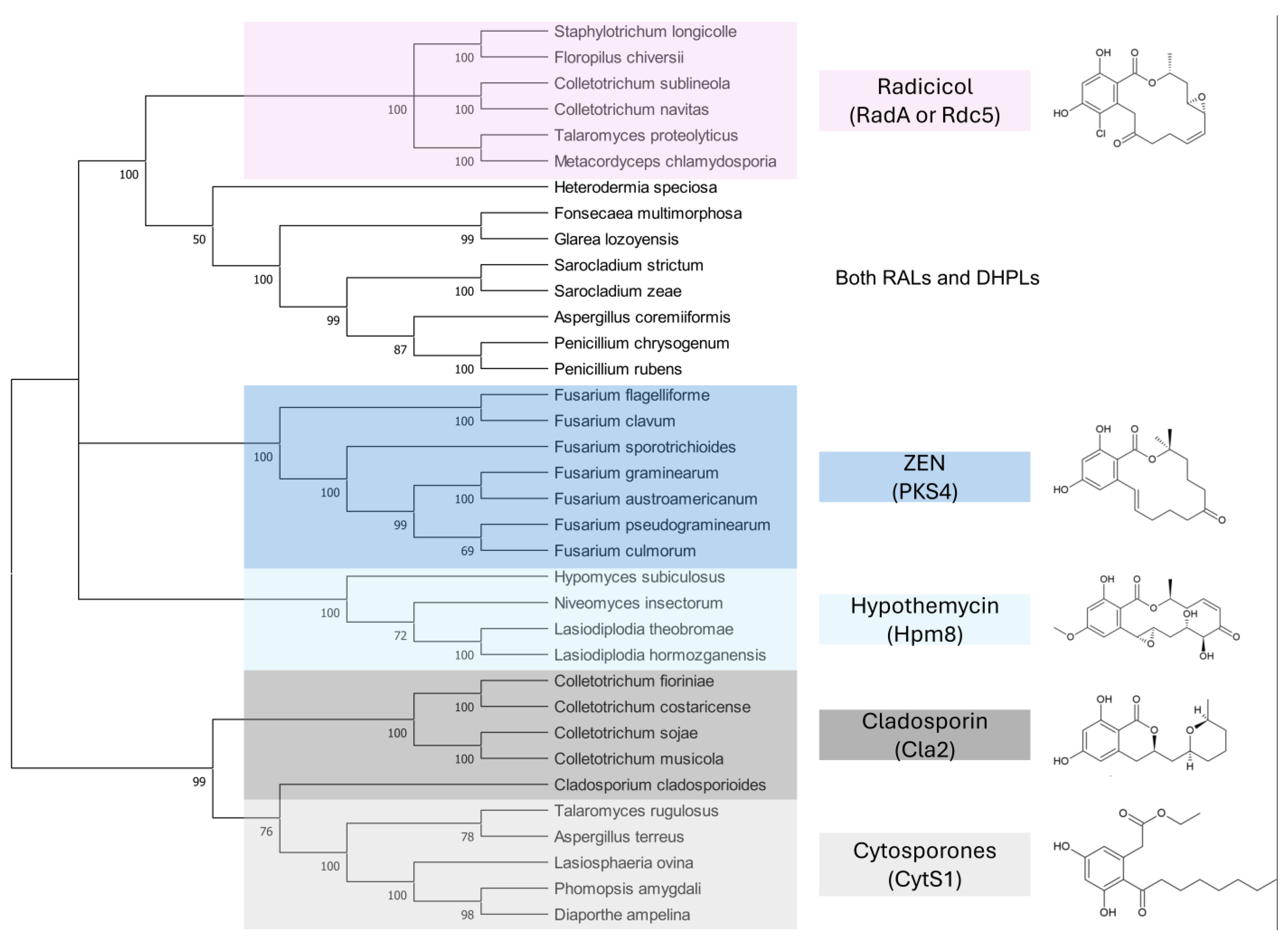
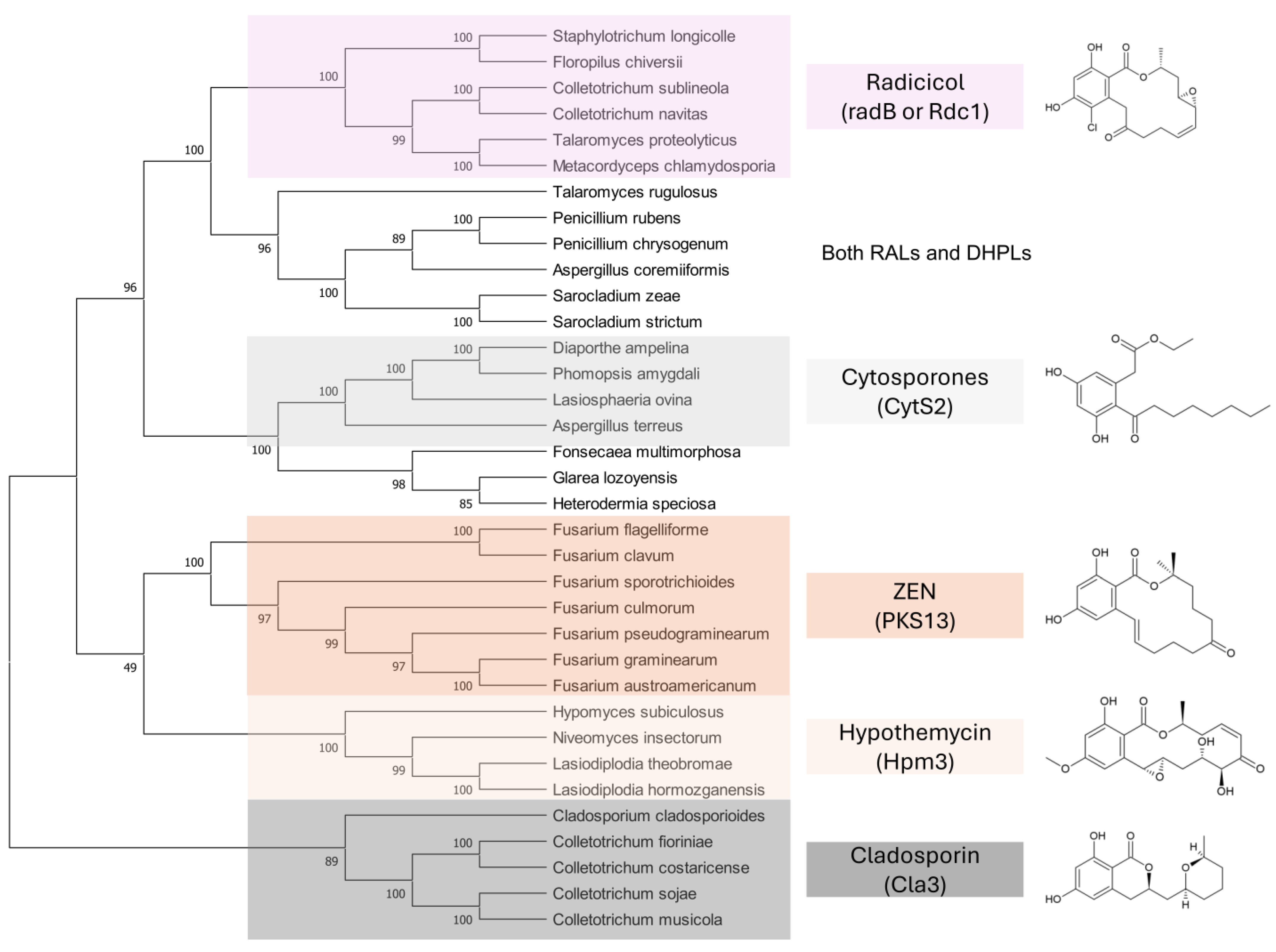

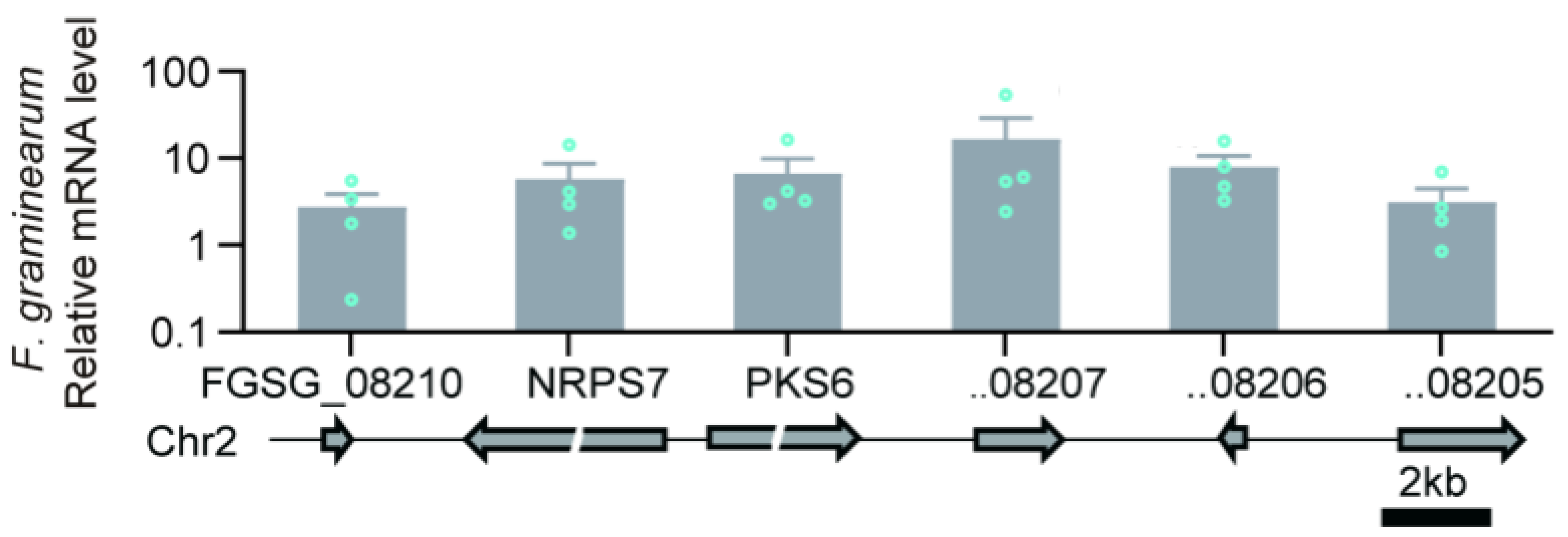
| Species Name | Reference Genome Assembly | PKS13 | PKS4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Identity (%) 1 | Coverage (%) 2 | Length (bp) | Accession No. | Identity (%) 1 | Coverage (%) 2 | Length (bp) | Accession No. | ||
| Fusarium graminearum | ASM24013 v3 | 100 | 100 | 6322 | FGSG_02395 | 100 | 100 | 6876 | FGSG_12126 |
| Fusarium austroamericanum | ASM1765703v1 | 99 | 100 | 6323 | JAGDVG010000009 1239521… 1235114 | 99 | 100 | 6875 | JAGDVG010000009 1233581… 1226706 |
| Fusarium pseudo-graminearum | FP7 | 95 | 100 | 6111 | FPSE_06864 | 97 | 100 | 6875 | NC_031951 7813499… 7806624 |
| Fusarium culmorum | ASM1695235v1 | 95 | 100 | 6329 | CP064750 7855407… 7849078 | 97 | 100 | 6875 | CP064750 7863815… 7856940 |
| Fusarium sporotrichioides | ASM1905464v1 | 93 | 98 | 6311 | JAHKNS010000005 1036321… 1030010 | 94 | 100 | 6874 | JAHKNS010000005 1028384… 1021510 |
| Fusarium flagelliforme | Fuseq1 | 86 | 94 | 6149 | XM_046132901 | 88 | 99 | 6869 | NW_025763510 300333… 293464 |
| Fusarium clavum | ASM4464674 v1 | 88 | 88 | 6317 | JAVTNS010000027 434237… 427920 | 88 | 99 | 6871 | JAVTNS010000027 426204… 419333 |
| Fusarium semitectum (F. incarnatum) | ASM370943 v1 | 86 | 87 | 5959 | RBJE01000009 347467… 341508 | 87 | 99 | 6866 | RBJE01000009 356373… 349507 |
| Fusarium equiseti | ASM331317 v1 | 87 | 82 | 5185 | QOHM01000007 280762… 275577 | 88 | 99 | 6871 | QOHM01000007 290435… 283564 |
| Fusarium verticillioides | ASM14955 v1 | — | — | — | — | — | — | — | — |
| Fusarium oxysporum | ASM1308505 v1 | — | — | — | — | — | — | — | — |
| Fusarium solani | NDSU_Fsol_1.0 | — | — | — | — | — | — | — | — |
| Year | Plant | Note | Ref. |
|---|---|---|---|
| 1986 | Winter wheat and carrot | ZEN-like metabolite and its role in vernalization | [213] |
| 1986 | Brassica campestris | ZEN-like metabolite and its role in vernalization | [214] |
| 1989 | Winter wheat | Isolation of ZEN from shoot apices | [215] |
| 1989 | Apium graveoleus | Isolation of ZEN from wild celery | [216] |
| 1990 | Winter wheat and cotton | Variation of ZEN content during development | [217] |
| 1990 | Winter wheat | Variation of ZEN content during vernalization | [218] |
| 1992 | Wheat, Lemna aequinoctialis | ZEN appears to control plant development | [219] |
| 1994 | Winter wheat | Variation of ZEN content during development | [220] |
| 1995 | Nicotiana tabacum | ZEN appears to play a role in floral development | [221] |
| 1996 | - | Review | [222] |
| 1997 | Maize and wheat | ZEN conjugates with seed proteins | [223] |
| 1998 | Cannabis sativa | ZEN content in flower primordia | [224] |
| 1999 | Sweet corn | ZEN content in tassels | [225] |
| 2000 | Nicotiana tabacum | ZEN content correlated with flower development | [212] |
| 2001 | - | Review | [226] |
| 2010 | - | Review | [176] |
| Examination or Experiment | Observation or Result Expected for VFs | ZEN | DON |
|---|---|---|---|
| Kinetics and spatial location of production during infection | Produced at early stages and at the front of infection | No [249,250,251] | Yes [252,253,254,255] |
| Treatment of host plants with purified metabolite | Causes similar symptoms to the infection | No (Table 3, [191,228]) | Yes [256] |
| Comparison of field isolates | Production of a VF correlates with virulence | No 1 [257,258,259,260] | Yes 2 [261,262] |
| Biological function of similar metabolites | Similar metabolites have been proven to be VFs of their producers | No | Yes 3 [263,264,265] |
| Equip host plants with detoxification ability | Plants detoxifying VFs became more resistant to infection | ? | Yes [266,267,268,269] |
| Gene inactivation | Disruption of biosynthesis reduced virulence | No 4 [58,149] | Yes [270,271,272,273] |
| Gene | Treatment | Expression 1 | Fold Change | p-Value |
|---|---|---|---|---|
| PKS13 | Control | 0.042 | 0.69 | NS |
| Predation | 0.029 | |||
| PKS4 | Control | 0.053 | 0.17 | NS |
| Predation | 0.009 | |||
| ZEB1 | Control | 133 | 0.60 | <0.001 |
| Predation | 79 | |||
| ZEB2 | Control | 34 | 0.57 | <0.05 |
| Predation | 19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viñas, M.; Karlovsky, P. A Comprehensive Review of Hypotheses About the Biological Function of Zearalenone, and a New Hypothesis for the Function of Resorcylic and Dihydroxyphenylacetic Macrolactones in Fungi. Toxins 2025, 17, 226. https://doi.org/10.3390/toxins17050226
Viñas M, Karlovsky P. A Comprehensive Review of Hypotheses About the Biological Function of Zearalenone, and a New Hypothesis for the Function of Resorcylic and Dihydroxyphenylacetic Macrolactones in Fungi. Toxins. 2025; 17(5):226. https://doi.org/10.3390/toxins17050226
Chicago/Turabian StyleViñas, María, and Petr Karlovsky. 2025. "A Comprehensive Review of Hypotheses About the Biological Function of Zearalenone, and a New Hypothesis for the Function of Resorcylic and Dihydroxyphenylacetic Macrolactones in Fungi" Toxins 17, no. 5: 226. https://doi.org/10.3390/toxins17050226
APA StyleViñas, M., & Karlovsky, P. (2025). A Comprehensive Review of Hypotheses About the Biological Function of Zearalenone, and a New Hypothesis for the Function of Resorcylic and Dihydroxyphenylacetic Macrolactones in Fungi. Toxins, 17(5), 226. https://doi.org/10.3390/toxins17050226






