Ultrasound-Guided Botulinum Toxin Injections for Hand Spasticity: A Technical Guide for the Dorsal Approach
Abstract
1. Introduction
2. Results
2.1. Ultrasound-Guided Approach for the Thumb-in-Palm Pattern
2.1.1. Adductor Pollicis Muscle
- Anatomy: The AdP presents an oblique and a transverse head. The oblique component originates from the capitate bone, the second and third metacarpal bones, and the flexor carpi radialis tendon sheath; the transverse component originates from the diaphysis of the third metacarpal bone;
- Innervation: Ulnar nerve;
- Function: Adduction of the first metacarpal and flexion of the interphalangeal joint of the thumb;
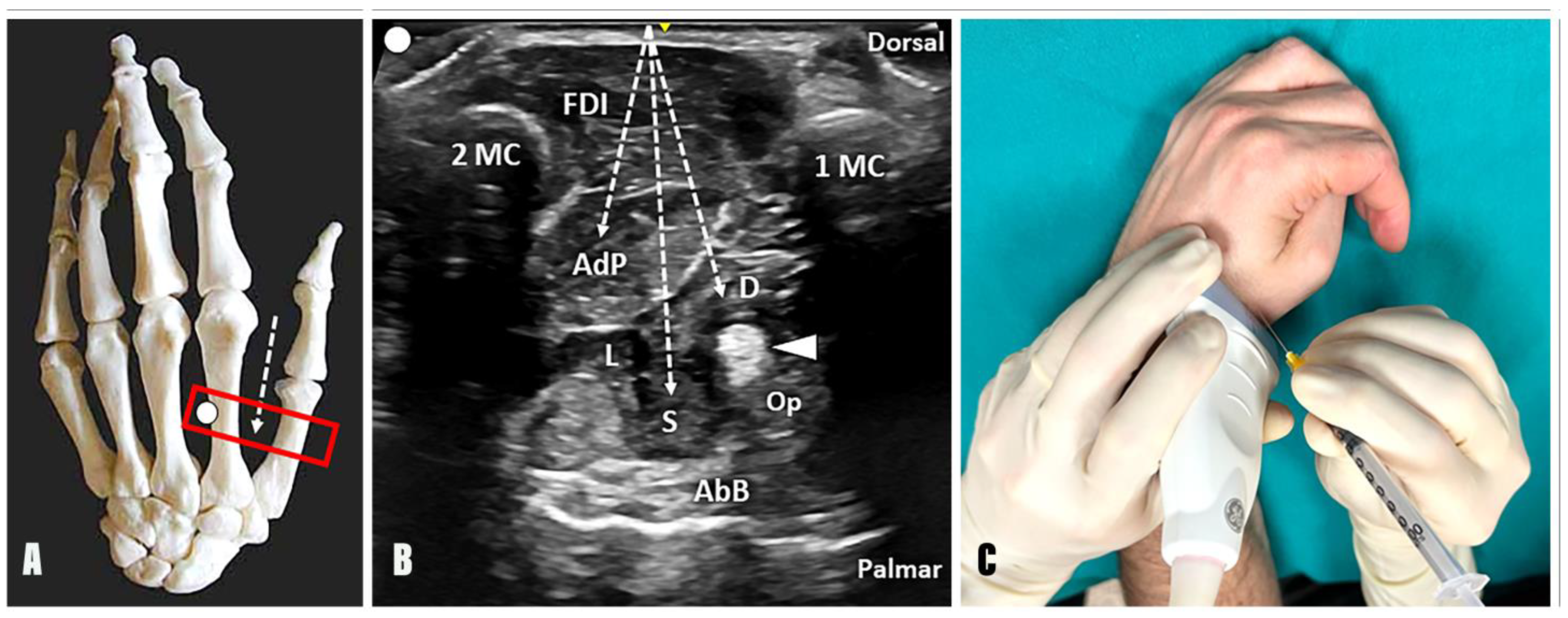
- Technique: Considering the second metacarpal bone as an anatomical landmark, the needle can be advanced using an out-of-plane technique and dorsal-to-palmar approach towards the AdP by crossing the FDI (Figure 2);
- Tips and tricks: The authors suggest slightly tilting the needle in an ulnar direction after the first inoculation point into the oblique muscle belly of the AdP and advancing towards the third metacarpal bone. At this level, a second inoculation can be performed to promote the diffusion of BTX towards the transverse muscular belly of the AdP as well. Of note, a small vascular bundle can be observed between the FDI, the AdP, and the second metacarpal bone using a transverse scan over the dorsal aspect of the first intermetacarpal space. In this sense, before the aforementioned ulnar tilting of the needle to reach and inject the transverse muscular belly of the AdP, a color Doppler assessment of this region should always be performed to ensure a safe interventional procedure (Supplementary Video S1).
2.1.2. Flexor Pollicis Brevis Muscle
- Anatomy: The FPB muscle presents two muscular bellies. The superficial head (sFPB) arises from the distal edge of the flexor retinaculum and the tubercle of the trapezium; the deep head (dFPB) originates from the trapezoid and the capitate bone. Both converge and insert at the base of the proximal phalanx of the thumb through the radial sesamoid bone of the first MCP joint.
- Innervation: Median nerve for the superficial head, ulnar nerve for the deep head.
- Function: Flexion of the thumb proximal phalanx on the first metacarpal, internal rotation of the first metacarpal on the trapezium.
- Technique: Considering the first metacarpal bone as an anatomical landmark, the needle can be advanced using an out-of-plane technique and dorsal-to-palmar approach toward the superficial and deep head of the FPB by crossing the FDI and the adductor pollicis muscle (Figure 2). Of note, using a dorsal approach, the deep head of the FPB is located between the AdP muscle and the FPL tendon; instead, its superficial head can be identified between the FPL tendon and the abductor pollicis brevis (AbB) muscle. In this sense, an excessive advancement of the needle in a palmar direction may be associated with an unintentional release of BTX into the AbB muscle.
- Tips and Tricks: A distal to proximal sonographic tracking of the flexor pollicis brevis may assist in differentiating its two muscular heads. Indeed, the superficial head originates from the flexor retinaculum and the tubercle of the trapezium bone; conversely, the deep head originates more medially from the trapezoid and capitate bones. When using this acoustic window in patients with a “thumb-in-palm” hand deformity, the Op muscle can be identified, in some rare cases, between the FPL tendon and the AbB muscle on the radial side (Figure 1). More commonly, the Op muscle is extremely difficult to recognize, and an unintentional spilling of BTX in the muscle may occur, without compromising the effective release of the thumb flexion [12].
2.2. Ultrasound-Guided Approach for the Intrinsic Plus Pattern
2.2.1. Palmar and Dorsal Interosseus Muscles
- Anatomy: The dorsal (dIO) and palmar (pIO) interossei are seven small muscles arranged in two layers between the metacarpal bones. The dIO muscles emerge from the adjacent sides of two metacarpal bones, while the pIO muscles originate from the palmar surface of the metacarpals. Both muscle groups are inserted at the extensor expansions of the fingers and the base of the proximal phalanx.
- Innervation: Ulnar nerve.
- Function: The interosseus muscles serve a dual role; dIO muscles are abductors of the fingers, whereas pIO muscles are adductors of the fingers. Moreover, IO muscles aid the lumbrical muscles in flexing the MCP joints while extending the interphalangeal.
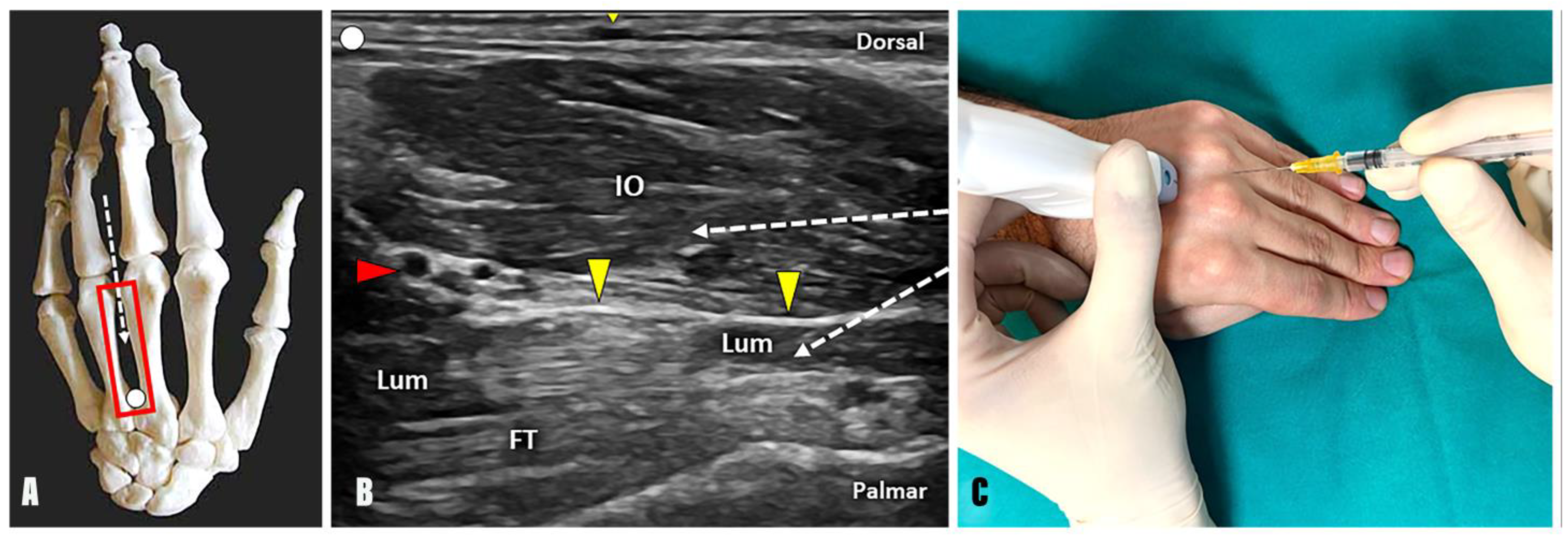
- Technique: Positioning the probe in a longitudinal plane over the dorsal aspect of the intermetacarpal space, the needle can be advanced using an in-plane technique and a distal-to-proximal approach within the most superficial muscular compartment, which represents the dorsal and palmar interosseous muscles (Figure 3).
- Tips and tricks: Interestingly, the anatomical arrangement of the interosseous muscles within the second intermetacarpal space makes the distinction between dorsal and palmar one quite reliable, as they lie parallel to each other in the longitudinal axis. Likewise, their arrangement does not follow the same spatial pattern in the third and fourth intermetacarpal spaces, arranging themselves next to each other or twisting together. Moreover, individual anatomical variability makes the distinction of muscle boundaries even more complicated in such a small anatomical district as the intermetacarpal interval [26]. In the authors’ experience, the BTX injection of the interosseous muscle “complex” ensures accurate management of hand spasticity without the need to distinguish between the dorsal and palmar components.
2.2.2. Lumbrical Muscles
- Anatomy: Lumbricals are four worm-like muscles located in the spaces between the fingers. They emerge from the flexor digitorum profundus tendons and attach to the lateral aspects of the extensor tendons from the second to the fifth fingers.
- Innervation: Median nerve for the two lumbrical muscles on the radial side and ulnar nerve for the two lumbrical muscles on the ulnar side.
- Function: Flexion of the MCP joints while simultaneously extending the interphalangeal joints.
- Technique: Positioning the probe in a longitudinal plane over the dorsal aspect of the intermetacarpal space, the needle can be advanced using an in-plane technique and distal-to-proximal approach within the lumbrical muscles (Figure 3). Interestingly, a thin hyperechoic fibrofatty band is located between the interosseous and lumbrical muscles and can be used as a sonographic landmark to optimize the accuracy of the procedure. Likewise, a dynamic scanning with passive/active mobilization of the fingers can be performed to observe a differential gliding of the interosseous and lumbrical muscles within the intermetacarpal space (Supplementary Video S2).
- Tips and tricks: Using the B-mode, a small vascular bundle can be identified within the hyperechoic fat pad between the interosseous and lumbrical muscles (Figure 3). Using color Doppler, a small vascular bundle is observed between the interosseous and lumbrical muscles within the third intermetacarpal space. Therefore, a color Doppler assessment of the intermetacarpal space should always be performed before the injection to avoid unintentional bleeding and iatrogenic injuries to the hand vasculature (Supplementary Video S3).
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BTX | Botulinum toxin |
| US | Ultrasound |
| FLP | Flexor longus pollicis |
| FBP | Flexor brevis pollicis |
| AdP | Adductor pollicis |
| MCP | Metacarpophalangeal |
| IO | Interosseus muscles |
| FDI | First dorsal interosseous |
References
- Rivelis, Y.; Zafar, N.; Morice, K. Spasticity; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Pandyan, A.; Gregoric, M.; Barnes, M.; Wood, D.; Van Wijck, F.; Burridge, J.; Hermens, H.; Johnson, G. Spasticity: Clinical Perceptions, Neurological Realities and Meaningful Measurement. Disabil. Rehabil. 2005, 27, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.F.; Piscitelli, D.; Khayat, J. Tonic Stretch Reflex Threshold as a Measure of Disordered Motor Control and Spasticity—A Critical Review. Clin. Neurophysiol. 2024, 165, 138–150. [Google Scholar] [CrossRef]
- Facciorusso, S.; Spina, S.; Picelli, A.; Baricich, A.; Francisco, G.E.; Molteni, F.; Wissel, J.; Santamato, A. The Role of Botulinum Toxin Type-A in Spasticity: Research Trends from a Bibliometric Analysis. Toxins 2024, 16, 184. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, T.; Hu, X.; Wang, T. Efficacy and Safety of Botulinum Toxin Type A for Upper Limb Spasticity after Stroke or Traumatic Brain Injury: A Systematic Review with Meta-Analysis and Trial Sequential Analysis. Eur. J. Phys. Rehabil. Med. 2017, 53, 256–267. [Google Scholar] [CrossRef]
- Walker, H.W.; Lee, M.Y.; Bahroo, L.B.; Hedera, P.; Charles, D. Botulinum Toxin Injection Techniques for the Management of Adult Spasticity. PMR 2015, 7, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Kara, M.; Kaymak, B.; Ulaşli, A.M.; Tok, F.; Öztürk, G.T.; Chang, K.-V.; Hsiao, M.-Y.; Hung, C.-Y.; Yağiz On, A.; Özçakar, L. Sonographic Guide for Botulinum Toxin Injections of the Upper Limb: EUROMUSCULUS/USPRM Spasticity Approach. Eur. J. Phys. Rehabil. Med. 2018, 54, 469–485. [Google Scholar] [CrossRef]
- Wissel, J.; Ward, A.; Erztgaard, P.; Bensmail, D.; Hecht, M.; Lejeune, T.; Schnider, P. European Consensus Table on the Use of Botulinum Toxin Type A in Adult Spasticity. J. Rehabil. Med. 2009, 41, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Picasso, R.; Zaottini, F.; Pistoia, F.; Perez, M.M.; MacCiò, M.; Bianco, D.; Rinaldi, S.; Pansecchi, M.; Rossi, G.; Tovt, L.; et al. Ultrasound of the Palmar Aspect of the Hand: Normal Anatomy and Clinical Applications of Intrinsic Muscles Imaging. J. Ultrason. 2023, 23, E122–E130. [Google Scholar] [CrossRef]
- Lagnau, P.; Lo, A.; Sandarage, R.; Alter, K.; Picelli, A.; Wissel, J.; Verduzco-Gutierrez, M.; Suputtitada, A.; Munin, M.C.; Carda, S.; et al. Ergonomic Recommendations in Ultrasound-Guided Botulinum Neurotoxin Chemodenervation for Spasticity: An International Expert Group Opinion. Toxins 2021, 13, 249. [Google Scholar] [CrossRef]
- Carlson, M.G.; Athwal, G.S.; Bueno, R.A. Treatment of the Wrist and Hand in Cerebral Palsy. J. Hand Surg. 2006, 31, 483–490. [Google Scholar] [CrossRef]
- John, J.; Cianca, J.; Chiou-Tan, F.; Pandit, S.; Furr-Stimming, E.; Taber, K.H. Procedure-Oriented Torsional Anatomy of the Hand for Spasticity Injection. J. Comput. Assist. Tomogr. 2017, 41, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Ata, E.; Güler, M.A.; Adıgüzel, E. Ultrasonography-Guided Botulinum Toxin Injection for Lumbrical Muscle Spasticity in a Hemiplegic Patient. Med. Ultrason. 2023, 25, 236. [Google Scholar] [CrossRef] [PubMed]
- Toliopoulos, A. In-Plane Ultrasound-Guided Botulinum Toxin Injection to Lumbrical and Interosseus Upper Limb Muscles: Technical Report. Cureus 2023, 15, e45073. [Google Scholar] [CrossRef]
- Tafti, M.A.; Cramer, S.C.; Gupta, R. Orthopaedic Management of the Upper Extremity of Stroke Patients. J. Am. Acad. Orthop. Surg. 2008, 16, 462–470. [Google Scholar] [CrossRef]
- Botte, M.J.; Keenan, M.A.; Gellman, H.; Garland, D.E.; Waters, R.L. Surgical Management of Spastic Thumb-in-Palm Deformity in Adults with Brain Injury. J. Hand Surg. Am. 1989, 14, 174–182. [Google Scholar] [CrossRef]
- Datta Gupta, A.; Eyre, R. Role of Botulinum Toxin in the Management of Hand Ulceration Due to Post-Stroke Spasticity among Aged Care Residents. Aust. J. Gen. Pract. 2022, 51, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Buyukavci, R.; Akturk, S.; Ersoy, Y. Evaluating the Functional Outcomes of Ultrasound-Guided Botulinum Toxin Type A Injections Using the Euro-Musculus Approach for Upper Limb Spasticity Treatment in Post-Stroke Patients: An Observational Study. Eur. J. Phys. Rehabil. Med. 2018, 54, 738–744. [Google Scholar] [CrossRef]
- Wu, W.T.; Chang, K.V.; Hsu, Y.C.; Tsai, Y.Y.; Mezian, K.; Ricci, V.; Özçakar, L. Ultrasound Imaging and Guidance for Distal Peripheral Nerve Pathologies at the Wrist/Hand. Diagnostics 2023, 13, 1928. [Google Scholar] [CrossRef]
- Bianchi, S.; Martinoli, C. Ultrasound of the Musculoskeletal System; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-42267-9. [Google Scholar]
- Abdulsalam, A.J.; Mezian, K.; Ricci, V.; Sobotova, K.; Alkandari, S.A.; Al-Mejalhem, A.Y.; Albarazi, N.B.; Özçakar, L. Interdigital Approach to Trigger Finger Injection Using Ultrasound Guidance. Pain Med. 2019, 20, 2607–2610. [Google Scholar] [CrossRef]
- Heest, A.E.V. Surgical Management of Wrist and Finger Deformity. Hand Clin. 2003, 19, 657–665. [Google Scholar] [CrossRef]
- Carlson, M.G.; Gallagher, K.; Spirtos, M. Surgical Treatment of Swan-Neck Deformity in Hemiplegic Cerebral Palsy. J. Hand Surg. Am. 2007, 32, 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- Maas, H.; Veeger, H.E.J.; Stegeman, D.F. Understanding the Constraints of Finger Motor Control. J. Electromyogr. Kinesiol. 2018, 38, 182–186. [Google Scholar] [CrossRef]
- Vargas, A.; Chiapas-Gasca, K.; Hernández-Díaz, C.; Canoso, J.J.; Saavedra, M.Á.; Navarro-Zarza, J.E.; Villaseñor-Ovies, P.; Kalish, R.A. Clinical Anatomy of the Hand. Reum. Clin. 2012, 8, 25–32. [Google Scholar] [CrossRef]
- Eladoumikdachi, F.; Valkov, P.L.; Thomas, J.; Netscher, D.T. Anatomy of the Intrinsic Hand Muscles Revisited: Part I. Interossei. Plast. Reconstr. Surg. 2002, 110, 1211–1224. [Google Scholar] [CrossRef]
- Mezian, K.; Ricci, V.; Güvener, O.; Jačisko, J.; Novotny, T.; Kara, M.; Ata, A.M.; Wu, W.-T.; Chang, K.-V.; Stecco, C.; et al. EURO-MUSCULUS/USPRM Dynamic Ultrasound Protocols for Wrist and Hand. Am. J. Phys. Med. Rehabil. 2022, 101, e132–e138. [Google Scholar] [CrossRef] [PubMed]
- Tok, F.; Özçakar, L.; Safaz, I.; Alaca, R. Effects of Botulinum Toxin-A on the Muscle Architecture of Stroke Patients: An Ultrasonographic Study. J. Rehabil. Med. 2011, 43, 1016–1019. [Google Scholar] [CrossRef]
- Eladoumikdachi, F.; Valkov, P.L.; Thomas, J.; Netscher, D.T. Anatomy of the Intrinsic Hand Muscles Revisited: Part II. Lumbricals. Plast. Reconstr. Surg. 2002, 110, 1225–1231. [Google Scholar] [CrossRef]
- Ashford, S.A.; Morris, G.; Smith, M.J. Ultrasound Image Guided Injection of Botulinum Toxin for the Management of Spasticity: A Delphi Study to Develop Recommendations for a Scope of Practice, Competency, and Governance Framework. Arch. Rehabil. Res. Clin. Transl. 2023, 5, 100299. [Google Scholar] [CrossRef] [PubMed]
- Suputtitada, A.; Chatromyen, S.; Chen, C.P.C.; Simpson, D.M. Best Practice Guidelines for the Management of Patients with Post-Stroke Spasticity: A Modified Scoping Review. Toxins 2024, 16, 98. [Google Scholar] [CrossRef]
- De Muynck, M.; Parlevliet, T.; De Cock, K.; Vanden Bossche, L.; Vanderstraeten, G.; Özçakar, L. Musculoskeletal Ultrasound for Interventional Physiatry. Eur. J. Phys. Rehabil. Med. 2012, 48, 675–687. [Google Scholar]
- Alter, K.; Karp, B. Ultrasound Guidance for Botulinum Neurotoxin Chemodenervation Procedures. Toxins 2017, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Asimakidou, E.; Sidiropoulos, C. A Bayesian Network Meta-Analysis and Systematic Review of Guidance Techniques in Botulinum Toxin Injections and Their Hierarchy in the Treatment of Limb Spasticity. Toxins 2023, 15, 256. [Google Scholar] [CrossRef] [PubMed]
- Francisco, G.E.; Balbert, A.; Bavikatte, G.; Bensmail, D.; Carda, S.; Deltombe, T.; Draulans, N.; Escaldi, S.; Gross, R.; Jacinto, J.; et al. A Practical Guide to Optimizing the Benefits of Post-Stroke Spasticity Interventions with Botulinum Toxin A: An International Group Consensus. J. Rehabil. Med. 2021, 53, jrm00134. [Google Scholar] [CrossRef] [PubMed]
- Kinnear, B.Z.; Lannin, N.A.; Cusick, A.; Harvey, L.A.; Rawicki, B. Rehabilitation Therapies After Botulinum Toxin-A Injection to Manage Limb Spasticity: A Systematic Review. Phys. Ther. 2014, 94, 1569–1581. [Google Scholar] [CrossRef]
- Andringa, A.; van de Port, I.; van Wegen, E.; Ket, J.; Meskers, C.; Kwakkel, G. Effectiveness of Botulinum Toxin Treatment for Upper Limb Spasticity Poststroke Over Different ICF Domains: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2019, 100, 1703–1725. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malfitano, C.; Robecchi Majnardi, A.; Pesaresi, A.; Ricci, V. Ultrasound-Guided Botulinum Toxin Injections for Hand Spasticity: A Technical Guide for the Dorsal Approach. Toxins 2025, 17, 225. https://doi.org/10.3390/toxins17050225
Malfitano C, Robecchi Majnardi A, Pesaresi A, Ricci V. Ultrasound-Guided Botulinum Toxin Injections for Hand Spasticity: A Technical Guide for the Dorsal Approach. Toxins. 2025; 17(5):225. https://doi.org/10.3390/toxins17050225
Chicago/Turabian StyleMalfitano, Calogero, Antonio Robecchi Majnardi, Arianna Pesaresi, and Vincenzo Ricci. 2025. "Ultrasound-Guided Botulinum Toxin Injections for Hand Spasticity: A Technical Guide for the Dorsal Approach" Toxins 17, no. 5: 225. https://doi.org/10.3390/toxins17050225
APA StyleMalfitano, C., Robecchi Majnardi, A., Pesaresi, A., & Ricci, V. (2025). Ultrasound-Guided Botulinum Toxin Injections for Hand Spasticity: A Technical Guide for the Dorsal Approach. Toxins, 17(5), 225. https://doi.org/10.3390/toxins17050225





