Against Clostridioides difficile Infection: An Update on Vaccine Development
Abstract
1. Introduction
2. Biology of C. difficile Toxins
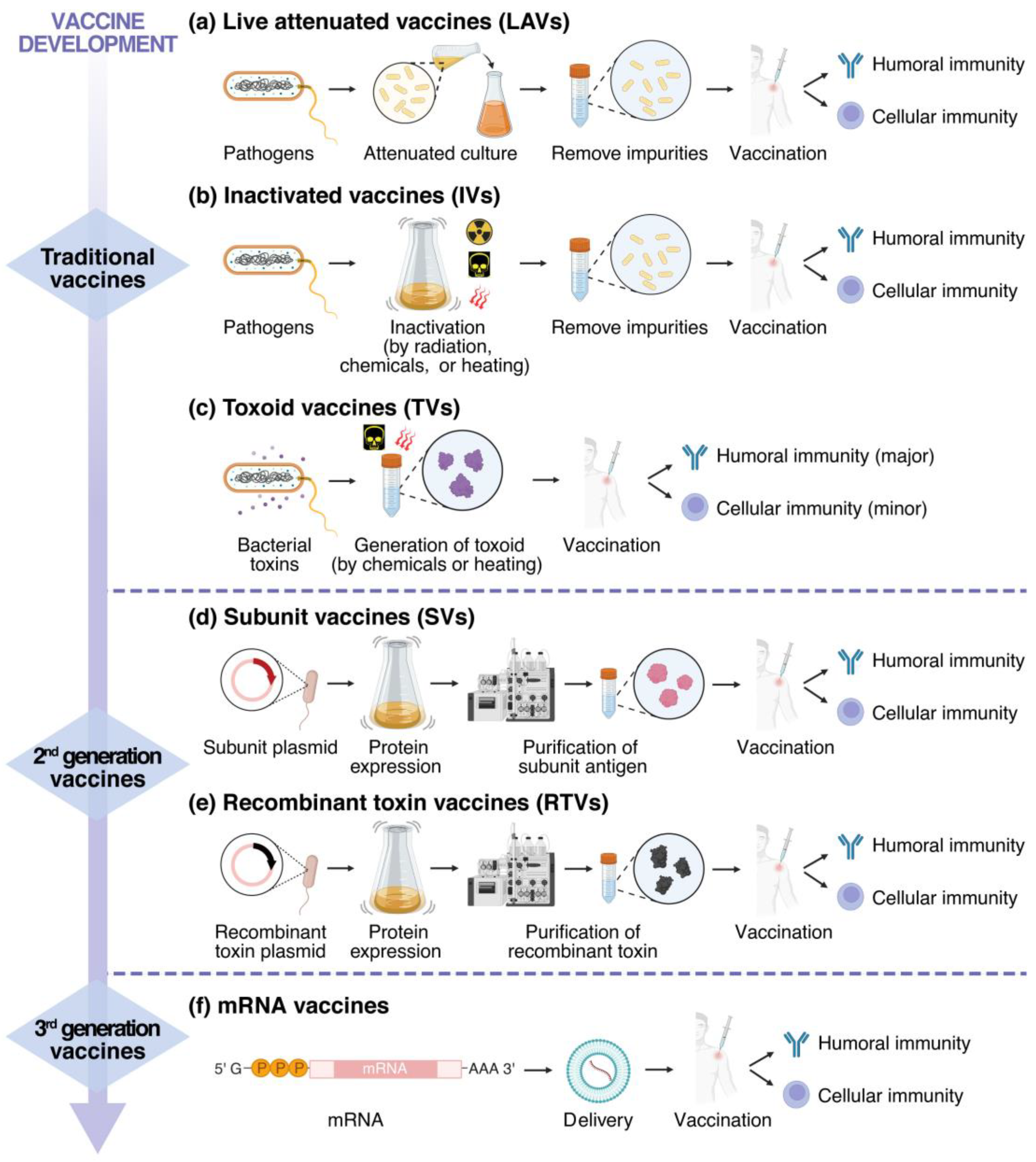
3. Strategies for Vaccine Development
3.1. Traditional Vaccines
3.2. Second-Generation Vaccines
3.3. Third-Generation Vaccines
4. C. difficile Vaccines Studies Based on TcdA and TcdB
4.1. Vaccines in Clinical Trials
4.1.1. CdiffenseTM Vaccine
4.1.2. PF-06425090 Vaccine
4.1.3. VLA84 Vaccine
| Vaccine Candidate | R&D Company | Status | Vaccine Type | Contents | Results |
|---|---|---|---|---|---|
| Cdiffense | Sanofi | Phase Ⅲ (terminated) | TV |
|
|
| PF-06425090 | Pfizer | Phase Ⅲ | RTV |
| |
| VLA84 | Valneva | Phase Ⅱ | SV |
|
|
4.2. Preclinical Studies
5. Preclinical Studies Based on Other Antigens
5.1. Using CDT as Antigen
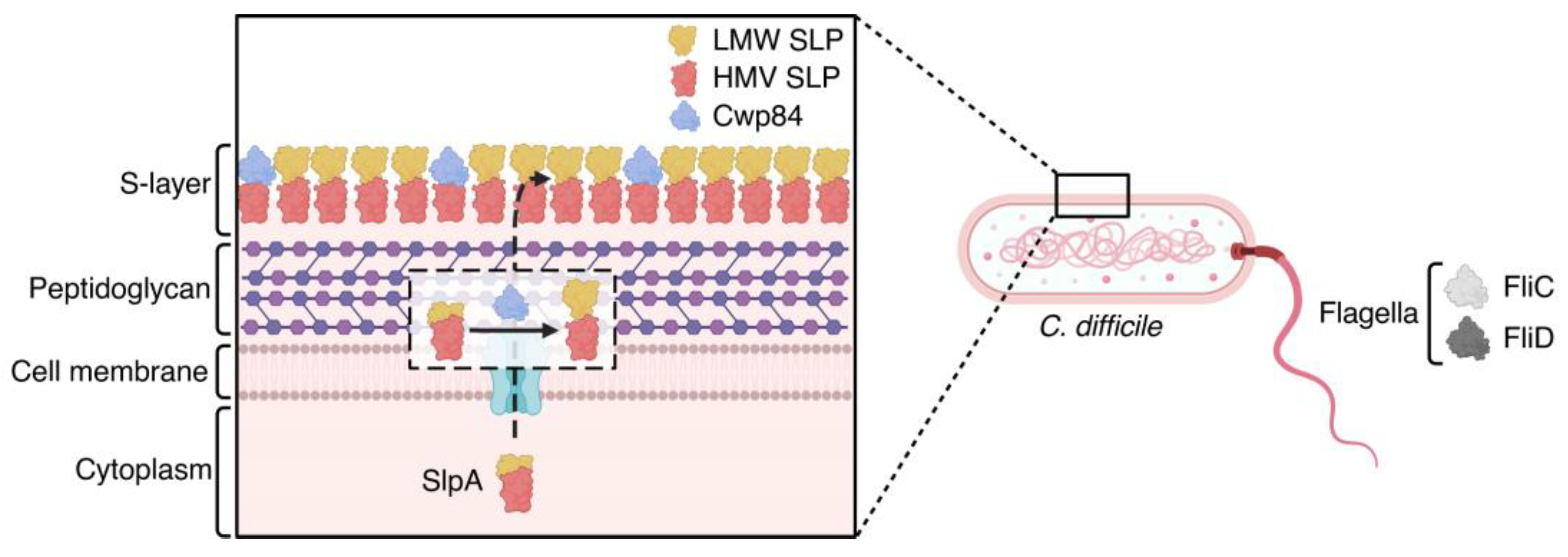
5.2. Antigens Involved in C. difficile Early Colonization
5.3. Phosphorylated Polysaccharides
5.4. Spore Coat Proteins
| Antigen Types | Antigens | Results |
|---|---|---|
| Toxin | TcdA and TcdB | |
| CDT |
| |
| Surface antigens | SlpA | |
| Cwp84 |
| |
| Flagella | FliC | |
| FliD | ||
| Spore coat antigens | BclA1, BclA2, and BclA3 |
|
| CdeC and CdeM |
| |
| CotA | ||
| Phosphorylated polysaccharides | PS-I, PS-II, and PS-III |
6. Discussion and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDC | Centers for Disease Control and Prevention |
| CDI | Clostridioides difficile infection |
| CDT | Clostridioides difficile transferase; or binary toxin |
| CPD | cysteine protease domain |
| CROPs | combined repetitive oligopeptides |
| CSPG4 | chondroitin sulfate proteoglycan 4 |
| Cwp84 | cysteine protease (encoded by cwp84) |
| DRBD | delivery and receptor-binding domain |
| ELISA | enzyme-linked immunosorbent assay |
| FliC | flagellar structural subunit |
| FliD | flagellar cap protein |
| FMT | Fecal microbiota transplantation |
| FZDs | frizzled receptors |
| Gp96 | Glycoprotein 96 |
| GTD | glucosyltransferase domain |
| GTPases | guanosine triphosphatases |
| InsP6 | inositol hexakisphosphate |
| IVs | inactivated vaccines |
| KLH | keyhole limpet hemocyanin |
| LAVs | live attenuated vaccines |
| LDLR | low-density lipoprotein receptor |
| LNPs | lipid nanoparticles |
| LRP1 | low-density lipoprotein receptor-related protein 1 |
| PaLoc | pathogenicity locus |
| PS | polysaccharide |
| PVRL3 | poliovirus receptor-like protein 3 |
| RTVs | recombinant toxin vaccines |
| sGAGs | sulfated glycosaminoglycans |
| SLP | surface layer proteins |
| SVs | subunit vaccines |
| TcdA | Clostridioides difficile toxin A |
| TcdB | Clostridioides difficile toxin B |
| TFPI | tissue factor pathway inhibitor |
| TVs | toxoid vaccines |
References
- Czepiel, J.; Drózdz, M.; Pituch, H.; Kuijper, E.J.; Perucki, W.; Mielimonka, A.; Goldman, S.; Wultanska, D.; Garlicki, A.; Biesiada, G. Clostridium difficile Infection: Review. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T. Biofilm Formation of Clostridioides difficile, Toxin Production and Alternatives to Conventional Antibiotics in the Treatment of Cdi. Microorganisms 2023, 11, 2161. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.A.; Chen, L.F.; Sexton, D.J.; Anderson, D.J. Comparison of the Burdens of Hospital-Onset, Healthcare Facility-Associated Clostridium difficile Infection and of Healthcare-Associated Infection Due to Methicillin-Resistant Staphylococcus aureus in Community Hospitals. Infect. Control Hosp. Epidemiol. 2011, 32, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Guh, A.Y.; Mu, Y.; Winston, L.G.; Johnston, H.; Olson, D.; Farley, M.M.; Wilson, L.E.; Holzbauer, S.M.; Phipps, E.C.; Dumyati, G.K.; et al. Trends in US Burden of Clostridioides difficile Infection and Outcomes. N. Engl. J. Med. 2020, 382, 1320–1330. [Google Scholar] [CrossRef] [PubMed]
- Berry, P.; Khanna, S. Recurrent Clostridioides difficile Infection: Current Clinical Management and Microbiome-Based Therapies. Biodrugs 2023, 37, 757–773. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Monaghan, T.; Yadegar, A.; Louie, T.; Kao, D. Insights into the Evolving Epidemiology of Clostridioides difficile Infection and Treatment: A Global Perspective. Antibiotics 2023, 12, 1141. [Google Scholar] [CrossRef]
- Skjot-Arkil, H.; Nanthan, K.R.; Chen, M.; Rosenvinge, F.S. Carrier Prevalence of Clostridioides difficile in Emergency Departments and the Association of Prior Antibiotic Consumption: A Combined Cross-Sectional and Nested Case-Control Study. J. Antimicrob. Chemother. 2023, 78, 2089–2096. [Google Scholar] [CrossRef] [PubMed]
- Smits, W.K.; Lyras, D.; Lacy, D.B.; Wilcox, M.H.; Kuijper, E.J. Clostridium difficile Infection. Nat. Rev. Dis. Primers 2016, 2, 16020. [Google Scholar] [CrossRef]
- Banaei, N.; Anikst, V.; Schroeder, L.F. Burden of Clostridium difficile Infection in the United States. N. Engl. J. Med. 2015, 372, 2368–2369. [Google Scholar]
- Wang, R.J. Clostridioides difficile Infection: Microbe-Microbe Interactions and Live Biotherapeutics. Front. Microbiol. 2023, 14, 1182612. [Google Scholar] [CrossRef]
- Alam, M.Z.; Markantonis, J.E.; Fallon, J.T. Host Immune Responses to Clostridioides difficile Infection and Potential Novel Therapeutic Approaches. Trop. Med. Infect. Dis. 2023, 8, 506. [Google Scholar] [CrossRef] [PubMed]
- Puerta-Alcalde, P.; Garcia-Vidal, C.; Soriano, A. Prevention and Treatment of C. difficile in Cancer Patients. Curr. Opin. Infect. Dis. 2023, 36, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Kechagias, K.S.; Chorepsima, S.; Triarides, N.A.; Falagas, M.E. Tigecycline for the Treatment of Patients with Clostridium difficile Infection: An Update of the Clinical Evidence. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Koop, A.H.; Travers, P.M.; Khanna, S.; Pardi, D.S.; Farraye, F.A.; Hashash, J.G. Fidaxomicin Treatment for Clostridioides difficile Infection in Patients with Inflammatory Bowel Disease. J. Gastroenterol. Hepatol. 2023, 38, 1910–1916. [Google Scholar] [CrossRef] [PubMed]
- Bratkovic, T.; Zahirovic, A.; Bizjak, M.; Rupnik, M.; Strukelj, B.; Berlec, A. New Treatment Approaches for Clostridioides difficile Infections: Alternatives to Antibiotics and Fecal Microbiota Transplantation. Gut Microbes 2024, 16, 2337312. [Google Scholar] [CrossRef] [PubMed]
- van Prehn, J.; Reigadas, E.; Vogelzang, E.H.; Bouza, E.; Hristea, A.; Guery, B.; Krutova, M.; Norén, T.; Allerberger, F.; Coia, J.E.; et al. European Society of Clinical Microbiology and Infectious Diseases: 2021 Update on the Treatment Guidance Document for Clostridioides difficile Infection in Adults. Clin. Microbiol. Infect. 2021, 27, S1–S21. [Google Scholar] [CrossRef]
- Hou, S.N.; Yu, J.C.; Li, Y.S.; Zhao, D.Y.; Zhang, Z.Y. Advances in Fecal Microbiota Transplantation for Gut Dysbiosis-Related Diseases. Adv. Sci. 2025, 33, 2413197. [Google Scholar] [CrossRef]
- Yadegar, A.; Pakpoor, S.; Ibrahim, F.F.; Nabavi-Rad, A.; Cook, L.; Walter, J.; Seekatz, A.M.; Wong, K.R.; Monaghan, T.M.; Kao, D. Beneficial Effects of Fecal Microbiota Transplantation in Recurrent Clostridioides difficile Infection. Cell Host Microbe 2023, 31, 695–711. [Google Scholar] [CrossRef]
- Bruxelle, J.F.; Péchiné, S.; Collignon, A. Immunization Strategies Against Clostridium difficile. In Updates on Clostridium difficile in Europe: Advances in Microbiology, Infectious Diseases and Public Health; Mastrantonio, P., Rupnik, M., Eds.; Springer International Publishing Ag: Cham, Switzerland, 2018; Volume 8, pp. 197–225. [Google Scholar]
- Pizarro-Guajardo, M.; Chamorro-Veloso, N.; Vidal, R.M.; Paredes-Sabja, D. New Insights for Vaccine Development against Clostridium difficile Infections. Anaerobe 2019, 58, 73–79. [Google Scholar] [CrossRef]
- Chen, S.Y.; Sun, C.L.; Wang, H.Y.; Wang, J.F. The Role of Rho Gtpases in Toxicity of Clostridium difficile Toxins. Toxins 2015, 7, 5254–5267. [Google Scholar] [CrossRef]
- Pruitt, R.N.; Chambers, M.G.; Ng, K.K.S.; Ohi, M.D.; Lacy, D.B. Structural Organization of the Functional Domains of Clostridium difficile Toxins a and B. Proc. Natl. Acad. Sci. USA 2010, 107, 13467–13472. [Google Scholar] [CrossRef] [PubMed]
- Girinathan, B.P.; Monot, M.; Boyle, D.; McAllister, K.N.; Sorg, J.A.; Dupuy, B.; Govind, R. Effect of Tcdr Mutation on Sporulation in the Epidemic Clostridium difficile Strain R20291. Msphere 2017, 2, e00383-16. [Google Scholar] [CrossRef] [PubMed]
- Bouillaut, L.; Dubois, T.; Sonenshein, A.L.; Dupuy, B. Integration of Metabolism and Virulence in Clostridium difficile. Res. Microbiol. 2015, 166, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Kordus, S.L.; Thomas, A.K.; Lacy, D.B. Clostridioides difficile Toxins: Mechanisms of Action and Antitoxin Therapeutics. Nat. Rev. Microbiol. 2022, 20, 285–298. [Google Scholar] [CrossRef]
- Matamouros, S.; England, P.; Dupuy, B. Clostridium difficile Toxin Expression Is Inhibited by the Novel Regulator Tcdc. Mol. Microbiol. 2007, 64, 1274–1288. [Google Scholar] [CrossRef] [PubMed]
- Govind, R.; Dupuy, B. Secretion of Clostridium difficile Toxins A and B Requires the Holin-Like Protein TcdE. PLoS Pathog. 2012, 8, e1002727. [Google Scholar] [CrossRef]
- Mehner-Breiffeld, D.; Rathmann, C.; Riedel, T.; Just, I.; Gerhard, R.; Overmann, J.; Brüser, T. Evidence for an Adaptation of a Phage-Derived Holin/Endolysin System to Toxin Transport in Clostridioides difficile. Front. Microbiol. 2018, 9, 2446. [Google Scholar] [CrossRef]
- Perelle, S.; Gibert, M.; Bourlioux, P.; Corthier, G.; Popoff, M.R. Production of a Complete Binary Toxin (Actin-Specific Adp-Ribosyltransferase) by Clostridium difficile CD196. Infect. Immun. 1997, 65, 1402–1407. [Google Scholar] [CrossRef]
- Metcalf, D.S.; Weese, J.S. Binary Toxin Locus Analysis in Clostridium difficile. J. Med. Microbiol. 2011, 60, 1137–1145. [Google Scholar] [CrossRef]
- Riley, T.V.; Lyras, D.; Douce, G.R. Status of Vaccine Research and Development for Clostridium difficile. Vaccine 2019, 37, 7300–7306. [Google Scholar] [CrossRef]
- Heuler, J.; Chandra, H.; Sun, X.M. Mucosal Vaccination Strategies against Clostridioides difficile Infection. Vaccines 2023, 11, 887. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.H.; Xiong, X.Z.; Zeng, J.; Wang, S.Y.; Tremblay, B.J.M.; Chen, P.; Chen, B.H.; Liu, M.; Chen, P.S.; Sheng, K.W.; et al. Identification of TFPI as a Receptor Reveals Recombination-Driven Receptor Switching in Clostridioides difficile Toxin B Variants. Nat. Commun. 2022, 13, 6786. [Google Scholar] [CrossRef] [PubMed]
- Shen, E.H.; Zhu, K.L.; Li, D.Y.; Pan, Z.R.; Luo, Y.; Bian, Q.; He, L.Q.; Song, X.J.; Zhen, Y.; Jin, D.Z.; et al. Subtyping Analysis Reveals New Variants and Accelerated Evolution of Clostridioides difficile Toxin B. Commun. Biol. 2020, 3, 347. [Google Scholar] [CrossRef] [PubMed]
- Kempher, M.L.; Shadid, T.M.; Larabee, J.L.; Ballard, J.D. A Sequence Invariable Region in Tcdb2 Is Required for Toxin Escape from Clostridioides difficile. J. Bacteriol. 2024, 206, e00096-24. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.R.; Zhang, Y.Y.; Luo, J.H.; Li, D.Y.; Zhou, Y.; He, L.Q.; Yang, Q.; Dong, M.; Tao, L. Functional Analyses of Epidemic Clostridioides difficile Toxin B Variants Reveal Their Divergence in Utilizing Receptors and Inducing Pathology. PLoS Pathog. 2021, 17, e1009197. [Google Scholar] [CrossRef]
- Mansfield, M.J.; Tremblay, B.J.M.; Zeng, J.; Wei, X.; Hodgins, H.; Worley, J.; Bry, L.; Dong, M.; Doxey, A.C. Phylogenomics of 8839 Clostridioides difficile Genomes Reveals Recombination-Driven Evolution and Diversification of Toxin a and B. PLoS Pathog. 2020, 16, e1009181. [Google Scholar] [CrossRef] [PubMed]
- Voneichelstreiber, C.; Laufenbergfeldmann, R.; Sartingen, S.; Schulze, J.; Sauerborn, M. Comparative Sequence-Analysis of the Clostridium difficile Toxin-A and Toxin-B. Mol. Gen. Genet. 1992, 233, 260–268. [Google Scholar] [CrossRef]
- Janezic, S.; Dingle, K.; Alvin, J.; Accetto, T.; Didelot, X.; Crook, D.W.; Lacy, D.B.; Rupnik, M. Comparative Genomics of Clostridioides difficile Toxinotypes Identifies Module-Based Toxin Gene Evolution. Microb. Genomics 2020, 6, e000449. [Google Scholar] [CrossRef]
- Papatheodorou, P.; Barth, H.; Minton, N.; Aktories, K. Cellular Uptake and Mode-of-Action of Clostridium difficile Toxins. In Updates on Clostridium difficile in Europe: Advances in Microbiology, Infectious Diseases and Public Health; Mastrantonio, P., Rupnik, M., Eds.; Springer International Publishing Ag: Cham, Switzerland, 2018; Volume 8, pp. 77–96. [Google Scholar]
- Tao, L.; Tian, S.H.; Zhang, J.; Liu, Z.M.; Robinson-McCarthy, L.; Miyashita, S.I.; Breault, D.T.; Gerhard, R.; Oottamasathien, S.; Whelan, S.P.J.; et al. Sulfated Glycosaminoglycans and Low-Density Lipoprotein Receptor Contribute to Clostridium difficile Toxin A Entry into Cells. Nat. Microbiol. 2019, 4, 1760–1769. [Google Scholar] [CrossRef]
- Schöttelndreier, D.; Langejurgen, A.; Lindner, R.; Genth, H. Low Density Lipoprotein Receptor-Related Protein-1 (Lrp1) Is Involved in the Uptake of Clostridioides difficile Toxin a and Serves as an Internalizing Receptor. Front. Cell. Infect. Microbiol. 2020, 10, 565465. [Google Scholar] [CrossRef]
- Na, X.; Kim, H.; Moyer, M.P.; Pothoulakis, C.; LaMont, J.T. Gp96 Is a Human Colonocyte Plasma Membrane Binding Protein for Clostridium difficile Toxin A. Infect. Immun. 2008, 76, 2862–2871. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zeng, J.; Liu, Z.; Thaker, H.; Wang, S.Y.; Tian, S.H.; Zhang, J.; Tao, L.; Gutierrez, C.B.; Xing, L.; et al. Structural Basis for CSPG4 as a Receptor for TcdB and a Therapeutic Target in Clostridioides difficile Infection. Nat. Commun. 2021, 12, 3748. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.J.; Chen, Y.O.; Liu, J.Z.; Zhang, X.Y.; Liu, Z.H.; Zhou, Z.; Wei, W.S. Low-Density Lipoprotein Receptor-Related Protein 1 Is a Crops-Associated Receptor for Clostridioides difficile Toxin B. Sci. China-Life Sci. 2022, 65, 107–118. [Google Scholar] [CrossRef] [PubMed]
- He, A.N.; Tian, S.H.; Kopper, O.; Horan, D.J.; Chen, P.; Bronson, R.T.; Sheng, R.; Wu, H.; Sui, L.F.; Zhou, K.; et al. Targeted Inhibition of Wnt Signaling with a Clostridioides difficile Toxin B Fragment Suppresses Breast Cancer Tumor Growth. PLoS Biol. 2023, 21, e3002353. [Google Scholar] [CrossRef]
- LaFrance, M.E.; Farrow, M.A.; Chandrasekaran, R.; Sheng, J.S.; Rubin, D.H.; Lacy, D.B. Identification of an Epithelial Cell Receptor Responsible for Clostridium difficile Tcdb-Induced Cytotoxicity. Proc. Natl. Acad. Sci. USA 2015, 112, 7073–7078. [Google Scholar] [CrossRef] [PubMed]
- Qa’Dan, M.; Spyres, L.M.; Ballard, J.D. Ph-Induced Conformational Changes in Clostridium difficile Toxin B. Infect. Immun. 2000, 68, 2470–2474. [Google Scholar] [CrossRef]
- Just, I.; Selzer, J.; Wilm, M.; von Eichel-Streiber, C.; Mann, M.; Aktories, K. Glucosylation of Rho Proteins by Clostridium difficile Toxin B. Nature 1995, 375, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, S.C.; Chen, P.; Tian, S.H.; Zeng, J.; Perry, K.; Dong, M.; Jin, R.S. Structural Basis for Selective Modification of Rho and Ras Gtpases by Clostridioides difficile Toxin B. Sci. Adv. 2021, 7, eabi4582. [Google Scholar] [CrossRef]
- Reineke, J.; Tenzer, S.; Rupnik, M.; Koschinski, A.; Hasselmayer, O.; Schrattenholz, A.; Schild, H.; von Eichel-Streiber, C. Autocatalytic Cleavage of Clostridium difficile Toxin. Nature 2007, 446, 415–419. [Google Scholar] [CrossRef]
- Egerer, M.; Giesemann, T.; Jank, T.; Satchell, K.J.F.; Aktories, K. Auto-Catalytic Cleavage of Clostridium difficile Toxins A and B Depends on Cysteine Protease Activity. J. Biol. Chem. 2007, 282, 25314–25321. [Google Scholar] [CrossRef]
- Xu, H.; Yang, J.L.; Gao, W.Q.; Li, L.; Li, P.; Zhang, L.; Gong, Y.N.; Peng, X.L.; Xi, J.Z.J.; Chen, S.; et al. Innate Immune Sensing of Bacterial Modifications of Rho Gtpases by the Pyrin Inflammasome. Nature 2014, 513, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Paparella, A.S.; Cahill, S.M.; Aboulache, B.L.; Schramm, V.L. Clostridioides difficile Tcdb Toxin Glucosylates Rho Gtpase by an SNi Mechanism and Ion Pair Transition State. ACS Chem. Biol. 2022, 17, 2507–2518. [Google Scholar] [CrossRef] [PubMed]
- Barth, H.; Worek, F.; Steinritz, D.; Papatheodorou, P.; Huber-Lang, M. Trauma-Toxicology: Concepts, Causes, Complications. Naunyn-Schmiedebergs Arch. Pharmacol. 2024, 397, 2935–2948. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic Cancer Vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef] [PubMed]
- Vashishtha, V.M.; Kamath, S. A Brief History of Vaccines against Polio. Indian Pediatr. 2016, 53 (Suppl. 1), S20–S27. [Google Scholar] [PubMed]
- Gupta, S.; Pellett, S. Recent Developments in Vaccine Design: From Live Vaccines to Recombinant Toxin Vaccines. Toxins 2023, 15, 563. [Google Scholar] [CrossRef]
- Melo, A.R.D.; de Macedo, L.S.; Invencao, M.D.V.; de Moura, I.A.; da Gama, M.; de Melo, C.M.L.; Silva, A.J.D.; Batista, M.V.D.; de Freitas, A.C. Third-Generation Vaccines: Features of Nucleic Acid Vaccines and Strategies to Improve Their Efficiency. Genes 2022, 13, 2287. [Google Scholar] [CrossRef]
- Minor, P.D. Live Attenuated Vaccines: Historical Successes and Current Challenges. Virology 2015, 479, 379–392. [Google Scholar] [CrossRef]
- Mascola, J.R.; Fauci, A.S. Novel Vaccine Technologies for the 21st Century. Nat. Rev. Immunol. 2020, 20, 87–88. [Google Scholar] [CrossRef]
- Pace, J.L.; Rossi, H.A.; Esposito, V.M.; Frey, S.M.; Tucker, K.D.; Walker, R.I. Inactivated Whole-Cell Bacterial Vaccines: Current Status and Novel Strategies. Vaccine 1998, 16, 1563–1574. [Google Scholar] [CrossRef]
- Islam, M.S.; Rahman, M.T. A Comprehensive Review on Bacterial Vaccines Combating Antimicrobial Resistance in Poultry. Vaccines 2023, 11, 616. [Google Scholar] [CrossRef] [PubMed]
- Chokephaibulkit, K. Combination Vaccines. J. Med. Assoc. Thail. = Chotmaihet Thangphaet 2002, 85 (Suppl. 2), S694–S699. [Google Scholar]
- Liang, J.L.; Tiwari, T.; Moro, P.; Messonnier, N.E.; Reingold, A.; Sawyer, M.; Clark, T.A. Prevention of Pertussis, Tetanus, and Diphtheria with Vaccines in the United States: Recommendations of the Advisory Committee on Immunization Practices (Acip). MMWR Recomm. Rep. 2018, 67, 1–44. [Google Scholar] [CrossRef]
- Murata, M.; Kovba, A.; Kaneko, A.; Morimoto, M.; Ishigami, A.; Natsume, T.; Washizaki, A.; Miyabe-Nishiwaki, T.; Suzuki, J.; Akari, H. Annual Two-Dose Tetanus Toxoid Vaccination Induces Protective Humoral Immunity to All Age Groups of Rhesus Macaques. Exp. Anim. 2023, 72, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Tsoras, A.N.; Champion, J.A. Protein and Peptide Biomaterials for Engineered Subunit Vaccines and Immunotherapeutic Applications. In Annual Review of Chemical and Biomolecular Engineering; Prausnitz, J.M., Ed.; Annual Reviews: Palo Alto, CA, USA, 2019; Volume 10, pp. 337–359. [Google Scholar]
- Díaz-Dinamarca, D.A.; Salazar, M.L.; Castillo, B.N.; Manubens, A.; Vasquez, A.E.; Salazar, F.; Becker, M.I. Protein-Based Adjuvants for Vaccines as Immunomodulators of the Innate and Adaptive Immune Response: Current Knowledge, Challenges, and Future Opportunities. Pharmaceutics 2022, 14, 1671. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Mahajan, P.; Singh, N.K.; Gupta, A.; Aggarwal, R.; Rappuoli, R.; Johri, A.K. New-Age Vaccine Adjuvants, Their Development, and Future Perspective. Front. Immunol. 2023, 14, 1043139. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging Concepts in the Science of Vaccine Adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.A. A Comparison of Plasmid DNA and Mrna as Vaccine Technologies. Vaccines 2019, 7, 37. [Google Scholar] [CrossRef]
- Liu, T.C.; Liang, Y.J.; Huang, L.P. Development and Delivery Systems of Mrna Vaccines. Front. Bioeng. Biotechnol. 2021, 9, 718753. [Google Scholar] [CrossRef]
- Buschmann, M.D.; Carrasco, M.J.; Alishetty, S.; Paige, M.; Alameh, M.G.; Weissman, D. Nanomaterial Delivery Systems for Mrna Vaccines. Vaccines 2021, 9, 65. [Google Scholar] [CrossRef]
- Muramatsu, H.; Lam, K.; Bajusz, C.; Laczkó, D.; Karikó, K.; Schreiner, P.; Martin, A.; Lutwyche, P.; Heyes, J.; Pardi, N. Lyophilization Provides Long-Term Stability for a Lipid Nanoparticle-Formulated, Nuceoside-Moified Mrna Vaccine. Mol. Ther. 2022, 30, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.Y.; Liu, X.H.; Li, M.; Zhang, Z.L.; Song, L.F.; Zhu, B.Y.; Wu, X.H.; Liu, J.J.; Zhao, D.H.; Li, Y.H. Advances in COVID-19 Mrna Vaccine Development. Signal Transduct. Target. Ther. 2022, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, R.N.; Marbury, T.C.; Foglia, G.; Warny, M. Phase I Dose Finding Studies of an Adjuvanted Clostridium difficile Toxoid Vaccine. Vaccine 2012, 30, 2245–2249. [Google Scholar] [CrossRef] [PubMed]
- de Bruyn, G.; Saleh, J.; Workman, D.; Pollak, R.; Elinoff, V.; Fraser, N.J.; Lefebvre, G.; Martens, M.; Mills, R.E.; Nathan, R.; et al. Defining the Optimal Formulation and Schedule of a Candidate Toxoid Vaccine against Clostridium difficile Infection: A Randomized Phase 2 Clinical Trial. Vaccine 2016, 34, 2170–2178. [Google Scholar] [CrossRef]
- Donald, R.G.K.; Flint, M.; Kalyan, N.; Johnson, E.; Witko, S.E.; Kotash, C.; Zhao, P.; Megati, S.; Yurgelonis, I.; Lee, P.K.; et al. A Novel Approach to Generate a Recombinant Toxoid Vaccine against Clostridium difficile. Microbiology 2013, 159, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, E.; Kitchin, N.; Peng, Y.; Eiden, J.; Gruber, W.; Johnson, E.; Jansen, K.U.; Pride, M.W.; Pedneault, L. A Phase 1, Placebo-Controlled, Randomized Study of the Safety, Tolerability, and Immunogenicity of a Clostridium difficile Vaccine Administered with or without Aluminum Hydroxide in Healthy Adults. Vaccine 2016, 34, 2082–2091. [Google Scholar] [CrossRef]
- Kitchin, N.; Remich, S.A.; Peterson, J.; Peng, Y.H.; Gruber, W.C.; Jansen, K.U.; Pride, M.W.; Anderson, A.S.; Knirsch, C.; Webber, C. A Phase 2 Study Evaluating the Safety, Tolerability, and Immunogenicity of Two 3-Dose Regimens of a Clostridium difficile Vaccine in Healthy Us Adults Aged 65 to 85 Years. Clin. Infect. Dis. 2020, 70, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Donskey, C.J.; Dubberke, E.R.; Klein, N.P.; Liles, E.G.; Szymkowiak, K.; Wilcox, M.H.; Lawrence, J.; Bouguermouh, S.; Zhang, H.Y.; Koury, K.; et al. Clover (Clostridium difficile Vaccine Efficacy Trial) Study: A Phase 3, Randomized Trial Investigating the Efficacy and Safety of a Detoxified Toxin a/B Vaccine in Adults 50 Years and Older at Increased Risk of Clostridioides difficile Infection. Clin. Infect. Dis. 2024, 79, 1503–1511. [Google Scholar] [CrossRef]
- Bézay, N.; Ayad, A.; Dubischar, K.; Firbas, C.; Hochreiter, R.; Kiermayr, S.; Kiss, I.; Pinl, F.; Jilma, B.; Westritschnig, K. Safety, Immunogenicity and Dose Response of Vla84, a New Vaccine Candidate against Clostridium difficile, in Healthy Volunteers. Vaccine 2016, 34, 2585–2592. [Google Scholar] [CrossRef]
- Alameh, M.G.; Semon, A.; Bayard, N.U.; Pan, Y.G.; Dwivedi, G.; Knox, J.; Glover, R.C.; Rangel, P.C.; Tanes, C.; Bittinger, K.; et al. A Multivalent Mrna-Lnp Vaccine Protects against Clostridioides difficile Infection. Science 2024, 386, 69–75. [Google Scholar] [CrossRef]
- Young, V.B. Vaccinating against Clostridioides difficile Infection. N. Engl. J. Med. 2025, 392, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Kirk, J.A.; Banerji, O.; Fagan, R.P. Characteristics of the Clostridium difficile Cell Envelope and Its Importance in Therapeutics. Microb. Biotechnol. 2017, 10, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Deb, D.; Narsaria, U.; Kar, T.; Castiglione, F.; Sanyal, I.; Bade, P.D.; Srivastava, A.P. In Silico Designing of Vaccine Candidate against Clostridium difficile. Sci. Rep. 2021, 11, 14215. [Google Scholar] [CrossRef]
- Fourie, K.R.; Wilson, H.L. Understanding Groel and Dnak Stress Response Proteins as Antigens for Bacterial Diseases. Vaccines 2020, 8, 773. [Google Scholar] [CrossRef]
- Abeyawardhane, D.L.; Godoy-Ruiz, R.; Adipietro, K.A.; Varney, K.M.; Rustandi, R.R.; Pozharski, E.; Weber, D.J. The Importance of Therapeutically Targeting the Binary Toxin from Clostridioides difficile. Int. J. Mol. Sci. 2021, 22, 2926. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Meléndez, A.; Cruz-López, F.; Morfin-Otero, R.; Maldonado-Garza, H.J.; Garza-González, E. An Update on Clostridioides difficile Binary Toxin. Toxins 2022, 14, 305. [Google Scholar] [CrossRef]
- López-Cárdenas, S.; Torres-Martos, E.; Mora-Delgado, J.; Sánchez-Calvo, J.M.; Santos-Peña, M.; López, A.Z.; López-Prieto, M.D.; Pérez-Cortés, S.; Alados, J.C. The Prognostic Value of Toxin B and Binary Toxin in Clostridioides difficile Infection. Gut Microbes 2021, 13, 1884516. [Google Scholar] [CrossRef] [PubMed]
- Secore, S.; Wang, S.; Doughtry, J.; Xie, J.F.; Miezeiewski, M.; Rustandi, R.R.; Horton, M.; Xoconostle, R.; Wang, B.; Lancaster, C.; et al. Development of a Novel Vaccine Containing Binary Toxin for the Prevention of Clostridium difficile Disease with Enhanced Efficacy against Nap1 Strains. PLoS ONE 2017, 12, e0170640. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.H.; Glenn, G.; Flyer, D.; Zhou, B.; Liu, Y.; Sullivan, E.; Wu, H.; Cummings, J.F.; Elllingsworth, L.; Smith, G. Clostridium difficile Chimeric Toxin Receptor Binding Domain Vaccine Induced Protection against Different Strains in Active and Passive Challenge Models. Vaccine 2017, 35, 4079–4087. [Google Scholar] [CrossRef]
- Wright, A.; Wait, R.; Begum, S.; Crossett, B.; Nagy, J.; Brown, K.; Fairweather, N. Proteomic Analysis of Cell Surface Proteins from Clostridium difficile. Proteomics 2005, 5, 2443–2452. [Google Scholar] [CrossRef]
- de la Riva, L.; Willing, S.E.; Tate, E.W.; Fairweather, N.F. Roles of Cysteine Proteases Cwp84 and Cwp13 in Biogenesis of the Cell Wall of Clostridium difficile. J. Bacteriol. 2011, 193, 3276–3285. [Google Scholar] [CrossRef] [PubMed]
- Fagan, R.P.; Albesa-Jové, D.; Qazi, O.; Svergun, D.I.; Brown, K.A.; Fairweather, N.F. Structural Insights into the Molecular Organization of the S-Layer from Clostridium difficile. Mol. Microbiol. 2009, 71, 1308–1322. [Google Scholar] [CrossRef] [PubMed]
- Bruxelle, J.F.; Mizrahi, A.; Hoys, S.; Collignon, A.; Janoir, C.; Péchiné, S. Immunogenic Properties of the Surface Layer Precursor of Clostridium difficile and Vaccination Assays in Animal Models. Anaerobe 2016, 37, 78–84. [Google Scholar] [CrossRef]
- Sidner, B.; Lerma, A.; Biswas, B.; Do, T.V.; Yu, Y.F.; Ronish, L.A.; McCullough, H.; Auchtung, J.M.; Piepenbrink, K.H. Flagellin Is Essential for Initial Attachment to Mucosal Surfaces by Clostridioides difficile. Microbiol. Spectr. 2023, 14, e02120-23. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Takahashi, T. Characteristics and Immunological Roles of Surface Layer Proteins in Clostridium difficile. Ann. Lab. Med. 2018, 38, 189–195. [Google Scholar] [CrossRef]
- Wang, S.H.; Ju, X.H.; Heuler, J.; Zhang, K.S.; Duan, Z.B.; Patabendige, H.; Zhao, S.; Sun, X.M. Recombinant Fusion Protein Vaccine Containing Clostridioides difficile Flic and Flid Protects Mice against C. difficile Infection. Infect. Immun. 2023, 91, e00169-22. [Google Scholar] [CrossRef]
- Razim, A.; Pacyga, K.; Naporowski, P.; Martynowski, D.; Szuba, A.; Gamian, A.; Górska, S. Identification of Linear Epitopes on the Flagellar Proteins of Clostridioides difficile. Sci. Rep. 2021, 11, 9940. [Google Scholar] [CrossRef] [PubMed]
- Ganeshapillai, J.; Vinogradov, E.; Rousseau, J.; Weese, J.S.; Monteiro, M.A. Clostridium difficile Cell-Surface Polysaccharides Composed of Pentaglycosyl and Hexaglycosyl Phosphate Repeating Units. Carbohydr. Res. 2008, 343, 703–710. [Google Scholar] [CrossRef]
- Broecker, F.; Hanske, J.; Martin, C.E.; Baek, J.Y.; Wahlbrink, A.; Wojcik, F.; Hartmann, L.; Rademacher, C.; Anish, C.; Seeberger, P.H. Multivalent Display of Minimal Clostridium difficile Glycan Epitopes Mimics Antigenic Properties of Larger Glycans. Nat. Commun. 2016, 7, 11224. [Google Scholar] [CrossRef]
- Broecker, F.; Wegner, E.; Seco, B.M.S.; Kaplonek, P.; Bräutigam, M.; Ensser, A.; Pfister, F.; Daniel, C.; Martin, C.E.; Mattner, J.; et al. Synthetic Oligosaccharide-Based Vaccines Protect Mice from Clostridioides difficile Infections. ACS Chem. Biol. 2019, 14, 2720–2728. [Google Scholar] [CrossRef]
- Arroyo, L.G.; Hodgins, D.C.; Guest, B.; Costa, M.; Ma, Z.C.; Monteiro, M.A. Serum Igm Antibody Response to Clostridioides difficile Polysaccharide Ps-Ii Vaccination in Pony Foals. Anaerobe 2022, 77, 102635. [Google Scholar] [CrossRef] [PubMed]
- Deakin, L.J.; Clare, S.; Fagan, R.P.; Dawson, L.F.; Pickard, D.J.; West, M.R.; Wren, B.W.; Fairweather, N.F.; Dougan, G.; Lawley, T.D. The Clostridium difficile Spo0a Gene is a Persistence and Transmission Factor. Infect. Immun. 2012, 80, 2704–2711. [Google Scholar] [CrossRef] [PubMed]
- Ghose, C.; Eugenis, I.; Edwards, A.N.; Sun, X.M.; McBride, S.M.; Ho, D.D. Immunogenicity and Protective Efficacy of Clostridium difficile Spore Proteins. Anaerobe 2016, 37, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Díaz-González, F.; Milano, M.; Olguin-Araneda, V.; Pizarro-Cerda, J.; Castro-Córdova, P.; Tzeng, S.C.; Maier, C.S.; Sarker, M.R.; Paredes-Sabja, D. Protein Composition of the Outermost Exosporium-Like Layer of Clostridium difficile 630 Spores. J. Proteom. 2015, 123, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Phetcharaburanin, J.; Hong, H.A.; Colenutt, C.; Bianconi, I.; Sempere, L.; Permpoonpattana, P.; Smith, K.; Dembek, M.; Tan, S.; Brisson, M.C.; et al. The Spore-Associated Protein Bcla1 Affects the Susceptibility of Animals to Colonization and Infection by Clostridium difficile. Mol. Microbiol. 2014, 92, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Maia, A.R.; Reyes-Ramírez, R.; Pizarro-Guajardo, M.; Saggese, A.; Castro-Córdova, P.; Isticato, R.; Ricca, E.; Paredes-Sabja, D.; Baccigalupi, L. Induction of a Specific Humoral Immune Response by Nasal Delivery of Bcla2Ctd of Clostridioides difficile. Int. J. Mol. Sci. 2020, 21, 1277. [Google Scholar] [CrossRef]
- Aubry, A.; Zou, W.; Vinogradov, E.; Williams, D.; Chen, W.X.; Harris, G.; Zhou, H.Y.; Schur, M.J.; Gilbert, M.; Douce, G.R.; et al. In Vitro Production and Immunogenicity of a Clostridium difficile Spore-Specific Bcla3 Glycopeptide Conjugate Vaccine. Vaccines 2020, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Cun, W.Y.; Keller, P.A.; Pyne, S.G. Current and Ongoing Developments in Targeting Clostridioides difficile Infection and Recurrence. Microorganisms 2024, 12, 1206. [Google Scholar] [CrossRef]
- Permpoonpattana, P.; Phetcharaburanin, J.; Mikelsone, A.; Dembek, M.; Tan, S.; Brisson, M.C.; La Ragione, R.; Brisson, A.R.; Fairweather, N.; Hong, H.A.; et al. Functional Characterization of Clostridium difficile Spore Coat Proteins. J. Bacteriol. 2013, 195, 1492–1503. [Google Scholar] [CrossRef]
- Montes-Bravo, N.; Romero-Rodríguez, A.; García-Yunge, J.; Medina, C.; Pizarro-Guajardo, M.; Paredes-Sabja, D. Role of the Spore Coat Proteins Cota and Cotb, and the Spore Surface Protein Cdif630_02480, on the Surface Distribution of Exosporium Proteins in Clostridioides difficile 630 Spores. Microorganisms 2022, 10, 1918. [Google Scholar] [CrossRef]
- Péchiné, S.; Janoir, C.; Boureau, H.; Gleizes, A.; Tsapis, N.; Hoys, S.; Fattal, E.; Collignon, A. Diminished Intestinal Colonization by Clostridium difficile and Immune Response in Mice after Mucosal Immunization with Surface Proteins of Clostridium difficile. Vaccine 2007, 25, 3946–3954. [Google Scholar] [CrossRef] [PubMed]
- Bruxelle, J.F.; Tsapis, N.; Hoys, S.; Collignon, A.; Janoir, C.; Fattal, E.; Péchiné, S. Protection against Clostridium difficile Infection in a Hamster Model by Oral Vaccination Using Flagellin Flic-Loaded Pectin Beads. Vaccine 2018, 36, 6017–6021. [Google Scholar] [CrossRef] [PubMed]
- Bruxelle, J.F.; Mizrahi, A.; Hoys, S.; Collignon, A.; Janoir, C.; Péchiné, S. Clostridium difficile Flagellin Fiic: Evaluation as Adjuvant and Use in a Mucosal Vaccine against Clostridium difficile. PLoS ONE 2017, 12, e0187212. [Google Scholar] [CrossRef] [PubMed]
- Tasteyre, A.; Barc, M.C.; Collignon, A.; Boureau, H.; Karjalainen, T. Role of Flic and Flid Flagellar Proteins of Clostridium difficile in Adherence and Gut Colonization. Infect. Immun. 2001, 69, 7937–7940. [Google Scholar] [CrossRef]
- Razim, A.; Górska, S.; Gamian, A. Non-Toxin-Based Clostridioides difficile Vaccination Approaches. Pathogens 2023, 12, 235. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Ma, Q.; Tian, S. Against Clostridioides difficile Infection: An Update on Vaccine Development. Toxins 2025, 17, 222. https://doi.org/10.3390/toxins17050222
Wang J, Ma Q, Tian S. Against Clostridioides difficile Infection: An Update on Vaccine Development. Toxins. 2025; 17(5):222. https://doi.org/10.3390/toxins17050222
Chicago/Turabian StyleWang, Jingyao, Qianquan Ma, and Songhai Tian. 2025. "Against Clostridioides difficile Infection: An Update on Vaccine Development" Toxins 17, no. 5: 222. https://doi.org/10.3390/toxins17050222
APA StyleWang, J., Ma, Q., & Tian, S. (2025). Against Clostridioides difficile Infection: An Update on Vaccine Development. Toxins, 17(5), 222. https://doi.org/10.3390/toxins17050222






