Snake Venom Compounds: A New Frontier in the Battle Against Antibiotic-Resistant Infections
Abstract
1. Introduction
2. Composition of Snake Venom and Antibacterial Constituents
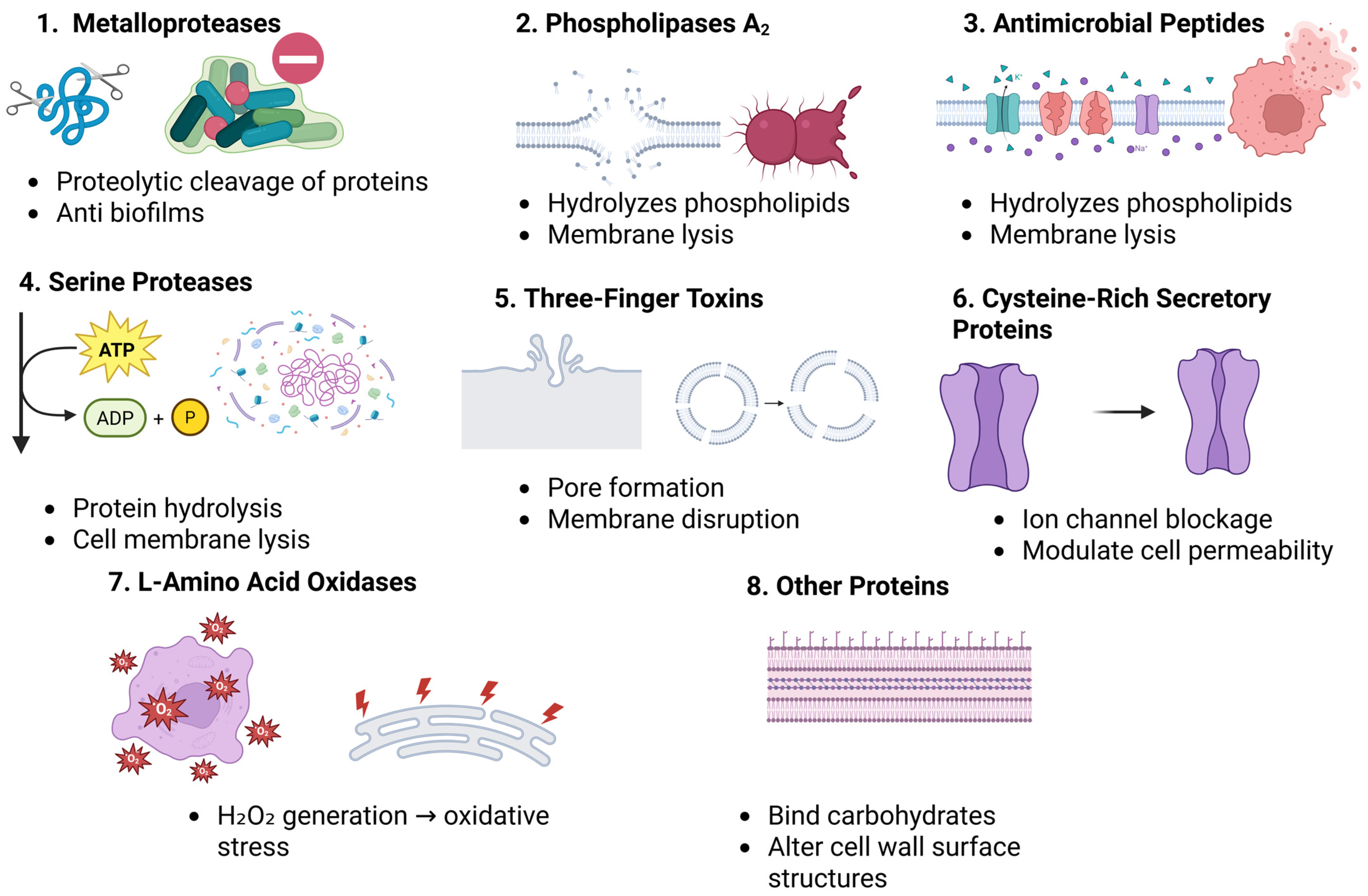
2.1. Metalloproteases (MPs)
2.2. Serine Proteases (SPs)
2.3. Phospholipase A2s (PLA2s)
2.4. Three-Finger Toxins (3FTxs)
2.5. Cysteine-Rich Secretory Proteins (CRISPs)
2.6. L-Amino Acid Oxidases (LAAOs)
2.7. Antimicrobial Peptides (AMPs)
2.8. Other Proteins
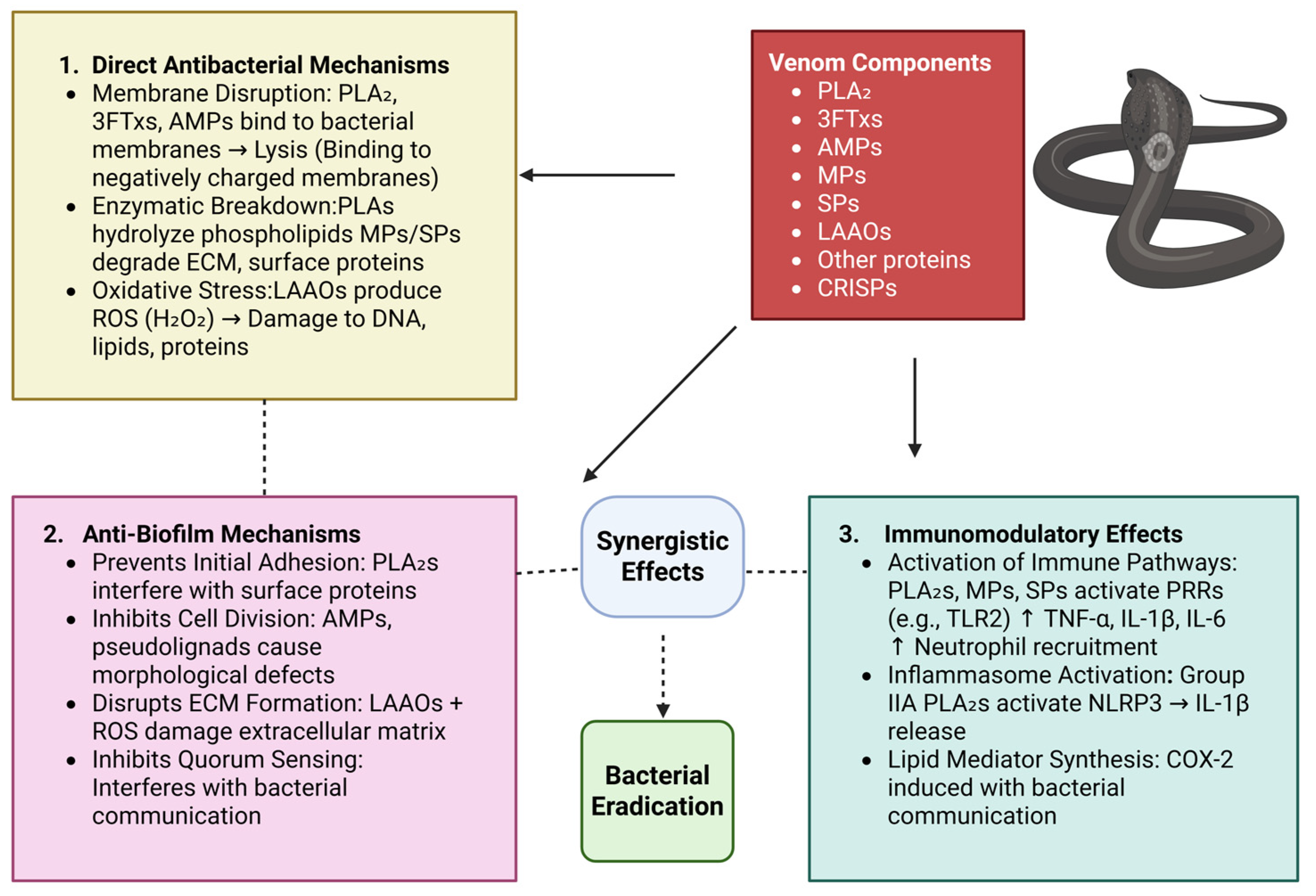
3. Antimicrobial Mechanisms of Snake Venom Components
3.1. Membrane Disruption and Lysis
3.2. Enzymatic Breakdown of Bacterial Components
3.3. Induction of Oxidative Stress
3.4. Inhibition of Biofilm Formation
3.5. Immunomodulatory Effects Against Bacterial Infections
4. Extracellular Vesicles: Types, Composition, and Functional Roles
4.1. The Role of Extracellular Vesicles in the Amplification of Snake Venom-Derived Antibacterial Therapies
4.2. Extracellular Vesicles as Delivery Vessels for Snake Venom-Derived Antibacterial Compounds
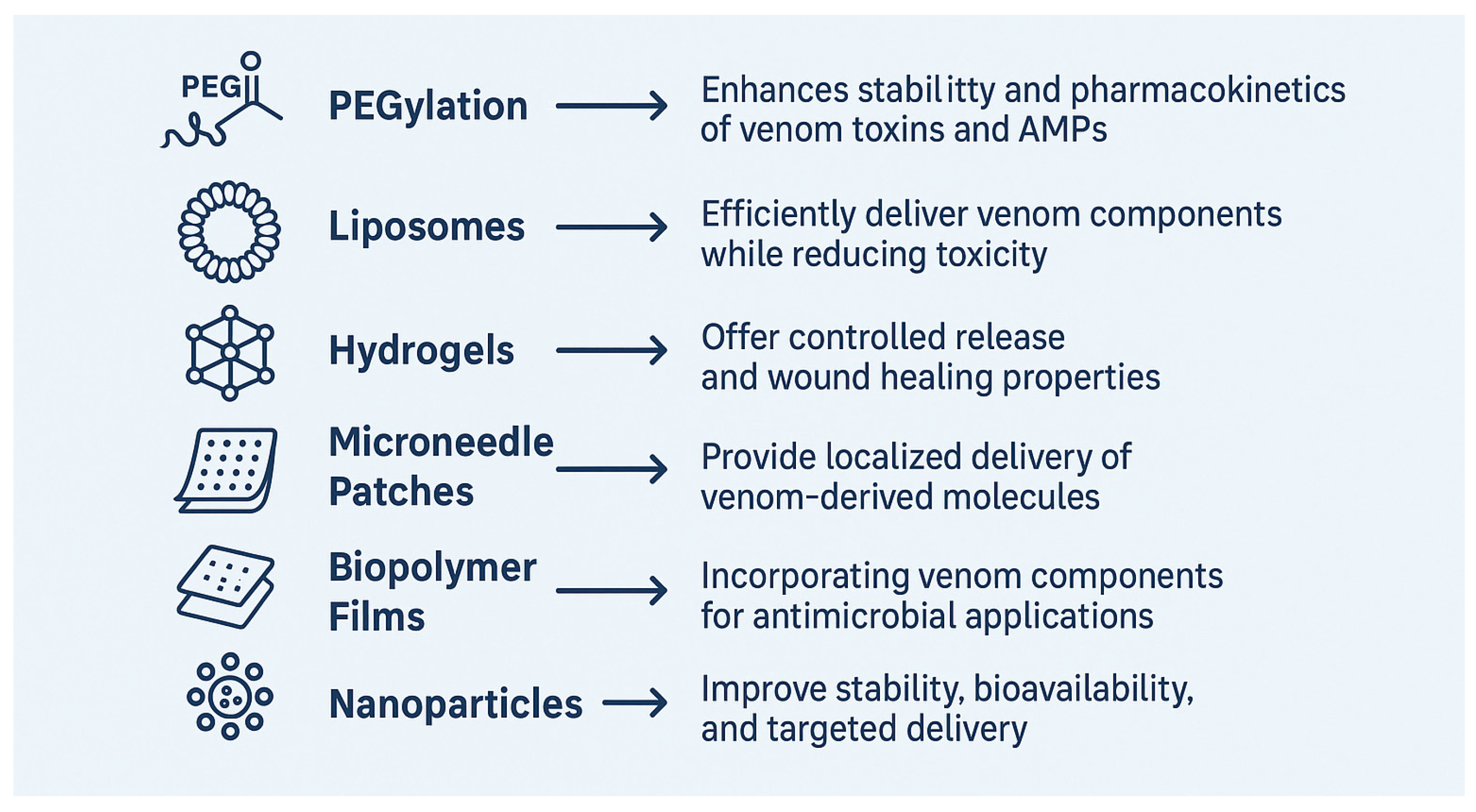
5. Possible Strategies to Improve Antibacterial Activity of Snake Venom
6. Enhancing Antibacterial Activity Through Peptide Modification
7. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.; Klamer, K.; Zimmerman, J.; Kale-Pradhan, P.B.; Bhargava, A. Multidrug Resistant Pseudomonas aeruginosa in Clinical Settings: A Review of Resistance Mechanisms and Treatment Strategies. Pathogens 2024, 13, 975. [Google Scholar] [CrossRef] [PubMed]
- Polianciuc, S.I.; Gurzău, A.E.; Kiss, B.; Ştefan, M.G.; Loghin, F. Antibiotics in the environment: Causes and consequences. Med. Pharm. Rep. 2020, 93, 231–240. [Google Scholar] [CrossRef]
- Subramanian, A. Emerging roles of bacteriophage-based therapeutics in combating antibiotic resistance. Front. Microbiol. 2024, 15, 1384164. [Google Scholar] [CrossRef]
- Mayorga-Ramos, A.; Zúñiga-Miranda, J.; Carrera-Pacheco, S.E.; Barba-Ostria, C.; Guamán, L.P. CRISPR-Cas-Based Antimicrobials: Design, Challenges, and Bacterial Mechanisms of Resistance. ACS Infect. Dis. 2023, 9, 1283–1302. [Google Scholar] [CrossRef]
- Mba, I.E.; Nweze, E.I. Antimicrobial Peptides Therapy: An Emerging Alternative for Treating Drug-Resistant Bacteria. Yale J. Biol. Med. 2022, 95, 445–463. [Google Scholar]
- Oliveira, A.L.; Viegas, M.F.; da Silva, S.L.; Soares, A.M.; Ramos, M.J.; Fernandes, P.A. The chemistry of snake venom and its medicinal potential. Nat. Rev. Chem. 2022, 6, 451–469. [Google Scholar] [CrossRef]
- Bittenbinder, M.A.; van Thiel, J.; Cardoso, F.C.; Casewell, N.R.; Gutiérrez, J.M.; Kool, J.; Vonk, F.J. Tissue damaging toxins in snake venoms: Mechanisms of action, pathophysiology and treatment strategies. Commun. Biol. 2024, 7, 358. [Google Scholar] [CrossRef]
- Casewell, N.R.; Jackson, T.N.W.; Laustsen, A.H.; Sunagar, K. Causes and Consequences of Snake Venom Variation. Trends Pharmacol. Sci. 2020, 41, 570–581. [Google Scholar] [CrossRef]
- Díaz-Gómez, J.L.; Martín-Estal, I.; Rivera-Aboytes, E.; Gaxiola-Muñíz, R.A.; Puente-Garza, C.A.; García-Lara, S.; Castorena-Torres, F. Biomedical applications of synthetic peptides derived from venom of animal origin: A systematic review. Biomed. Pharmacother. 2024, 170, 116015. [Google Scholar] [CrossRef]
- Khan, N.A.; Amorim, F.G.; Dunbar, J.P.; Leonard, D.; Redureau, D.; Quinton, L.; Dugon, M.M.; Boyd, A. Inhibition of bacterial biofilms by the snake venom proteome. Biotechnol. Rep. 2023, 39, e00810. [Google Scholar] [CrossRef] [PubMed]
- Bocian, A.; Hus, K.K. Antibacterial properties of snake venom components. Chem. Pap. 2020, 74, 407–419. [Google Scholar] [CrossRef]
- Almeida, J.R.; Palacios, A.L.V.; Patiño, R.S.P.; Mendes, B.; Teixeira, C.A.S.; Gomes, P.; da Silva, S.L. Harnessing snake venom phospholipases A2 to novel approaches for overcoming antibiotic resistance. Drug Dev. Res. 2019, 80, 68–85. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Junior, N.G.; e Silva Cardoso, M.H.; Franco, O.L. Snake venoms: Attractive antimicrobial proteinaceous compounds for therapeutic purposes. Cell. Mol. Life Sci. 2013, 70, 4645–4658. [Google Scholar] [CrossRef]
- Perumal Samy, R.; Stiles, B.G.; Franco, O.L.; Sethi, G.; Lim, L.H.K. Animal venoms as antimicrobial agents. Biochem. Pharmacol. 2017, 134, 127–138. [Google Scholar] [CrossRef]
- Alam, M.I.; Ojha, R.; Alam, A.; Quasimi, H.; Alam, O. Therapeutic potential of snake venoms as antimicrobial agents. Front. Drug Chem. Clin. Res. 2019, 2, 1–9. [Google Scholar] [CrossRef]
- Adisakwattana, P.; Chanhome, L.; Chaiyabutr, N.; Phuphisut, O.; Onrapak, R.; Thawornkuno, C. Venom-gland transcriptomics of the Malayan pit viper (Calloselasma rhodostoma) for identification, classification, and characterization of venom proteins. Heliyon 2023, 9, e15476. [Google Scholar] [CrossRef]
- Oyama, E.; Takahashi, H. Structures and Functions of Snake Venom Metalloproteinases (SVMP) from Protobothrops venom Collected in Japan. Molecules 2017, 22, 1305. [Google Scholar] [CrossRef]
- Markland, F.S., Jr.; Swenson, S. Snake venom metalloproteinases. Toxicon Off. J. Int. Soc. Toxinol. 2013, 62, 3–18. [Google Scholar] [CrossRef]
- Samy, R.P.; Gopalakrishnakone, P.; Chow, V.T.; Ho, B. Viper metalloproteinase (Agkistrodon halys pallas) with antimicrobial activity against multi-drug resistant human pathogens. J. Cell. Physiol. 2008, 216, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.M.T.; Wang, D.; Shannon, J.D.; Pinto, A.F.M.; Polanowska-Grabowska, R.K.; Fox, J.W. Interaction of the cysteine-rich domain of snake venom metalloproteinases with the A1 domain of von Willebrand factor promotes site-specific proteolysis of von Willebrand factor and inhibition of von Willebrand factor-mediated platelet aggregation. FEBS J. 2007, 274, 3611–3621. [Google Scholar] [CrossRef] [PubMed]
- Lopes Júnior, C.A.; Mendes, M.K.A.; Sousa, M.D.S.; Vieira, E.C.; Andrade, T.A.; de Jesus, J.R. Exploring metalloproteins found in the secretion of venomous species: Biological role and therapeutical applications. Adv. Protein Chem. Struct. Biol. 2024, 141, 539–562. [Google Scholar] [CrossRef] [PubMed]
- Sulca, M.A.; Remuzgo, C.; Cárdenas, J.; Kiyota, S.; Cheng, E.; Bemquerer, M.P.; Machini, M.T. Venom of the Peruvian snake Bothriopsis oligolepis: Detection of antibacterial activity and involvement of proteolytic enzymes and C-type lectins in growth inhibition of Staphylococcus aureus. Toxicon Off. J. Int. Soc. Toxinol. 2017, 134, 30–40. [Google Scholar] [CrossRef]
- Olaoba, O.T.; Karina Dos Santos, P.; Selistre-de-Araujo, H.S.; Ferreira de Souza, D.H. Snake Venom Metalloproteinases (SVMPs): A structure-function update. Toxicon X 2020, 7, 100052. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Latinović, Z.; Leonardi, A.; Koh, C.Y.; Kini, R.M.; Trampuš Bakija, A.; Pungerčar, J.; Križaj, I. The Procoagulant Snake Venom Serine Protease Potentially Having a Dual, Blood Coagulation Factor V and X-Activating Activity. Toxins 2020, 12, 358. [Google Scholar] [CrossRef]
- Serrano, S.M. The long road of research on snake venom serine proteinases. Toxicon Off. J. Int. Soc. Toxinol. 2013, 62, 19–26. [Google Scholar] [CrossRef]
- Amorim, F.G.; Menaldo, D.L.; Carone, S.E.I.; Silva, T.A.; Sartim, M.A.; De Pauw, E.; Quinton, L.; Sampaio, S.V. New Insights on Moojase, a Thrombin-Like Serine Protease from Bothrops moojeni Snake Venom. Toxins 2018, 10, 500. [Google Scholar] [CrossRef]
- Vidal, J.F.D.; Schwartz, M.F.; Garay, A.V.; Valadares, N.F.; Bueno, R.V.; Monteiro, A.C.L.; Freitas, S.M.; Barbosa, J.A.R.G. Exploring the Diversity and Function of Serine Proteases in Toxicofera Reptile Venoms: A Comprehensive Overview. Toxins 2024, 16, 428. [Google Scholar] [CrossRef]
- Oguiura, N.; Sanches, L.; Duarte, P.V.; Sulca-López, M.A.; Machini, M.T. Past, Present, and Future of Naturally Occurring Antimicrobials Related to Snake Venoms. Animals 2023, 13, 744. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Castro-Amorim, J.; Novo de Oliveira, A.; Da Silva, S.L.; Soares, A.M.; Mukherjee, A.K.; Ramos, M.J.; Fernandes, P.A. Catalytically Active Snake Venom PLA2 Enzymes: An Overview of Its Elusive Mechanisms of Reaction. J. Med. Chem. 2023, 66, 5364–5376. [Google Scholar] [CrossRef] [PubMed]
- Sampat, G.H.; Hiremath, K.; Dodakallanavar, J.; Patil, V.S.; Harish, D.R.; Biradar, P.; Mahadevamurthy, R.K.; Barvaliya, M.; Roy, S. Unraveling snake venom phospholipase A2: An overview of its structure, pharmacology, and inhibitors. Pharmacol. Rep. 2023, 75, 1454–1473. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Lomonte, B. Phospholipases A2: Unveiling the secrets of a functionally versatile group of snake venom toxins. Toxicon Off. J. Int. Soc. Toxinol. 2013, 62, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Samy, R.P.; Gopalakrishnakone, P.; Stiles, B.G.; Girish, K.S.; Swamy, S.N.; Hemshekhar, M.; Tan, K.S.; Rowan, E.G.; Sethi, G.; Chow, V.T. Snake venom phospholipases A(2): A novel tool against bacterial diseases. Curr. Med. Chem. 2012, 19, 6150–6162. [Google Scholar] [CrossRef]
- Sudarshan, S.; Dhananjaya, B.L. Antibacterial potential of a basic phospholipase A2 (VRV-PL-V) of Daboia russellii pulchella (Russell’s Viper) venom. Biochemistry. Biokhimiia 2014, 79, 1237–1244. [Google Scholar] [CrossRef]
- Sudarshan, S.; Dhananjaya, B.L. Antibacterial activity of an acidic phospholipase A2 (NN-XIb-PLA2) from the venom of Naja naja (Indian cobra). SpringerPlus 2016, 5, 112. [Google Scholar] [CrossRef]
- Vargas, L.J.; Londoño, M.; Quintana, J.C.; Rua, C.; Segura, C.; Lomonte, B.; Núñez, V. An acidic phospholipase A2 with antibacterial activity from Porthidium nasutum snake venom. Comparative biochemistry and physiology. Part B Biochem. Mol. Biol. 2012, 161, 341–347. [Google Scholar] [CrossRef]
- Accary, C.; Mantash, A.; Mallem, Y.; Fajloun, Z.; Elkak, A. Separation and Biological Activities of Phospholipase A2 (Mb-PLA2) from the Venom of Montivipera bornmuelleri, a Lebanese Viper. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 833–839. [Google Scholar] [CrossRef]
- Nunes, E.; Frihling, B.; Barros, E.; de Oliveira, C.; Verbisck, N.; Flores, T.; de Freitas Júnior, A.; Franco, O.; de Macedo, M.; Migliolo, L.; et al. Antibiofilm Activity of Acidic Phospholipase Isoform Isolated from Bothrops erythromelas Snake Venom. Toxins 2020, 12, 606. [Google Scholar] [CrossRef]
- Diniz-Sousa, R.; Caldeira, C.A.S.; Kayano, A.M.; Paloschi, M.V.; Pimenta, D.C.; Simões-Silva, R.; Ferreira, A.S.; Zanchi, F.B.; Matos, N.B.; Grabner, F.P.; et al. Identification of the Molecular Determinants of the Antibacterial Activity of LmutTX, a Lys49 Phospholipase A2 Homologue Isolated from Lachesis muta muta Snake Venom (Linnaeus, 1766). Basic Clin. Pharmacol. Toxicol. 2018, 122, 413–423. [Google Scholar] [CrossRef]
- Choudhury, M.; McCleary, R.J.R.; Kini, R.M.; Velmurugan, D. Orphan Three-Finger Toxins Bind at Tissue Factor-Factor VIIa Interface to Inhibit Factor X Activation: Identification of Functional Site by Docking. TH Open Companion J. Thromb. Haemost. 2018, 2, e303–e314. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dubovskii, P.V.; Ignatova, A.A.; Feofanov, A.V.; Utkin, Y.N.; Efremov, R.G. Antibacterial activity of cardiotoxin-like basic polypeptide from cobra venom. Bioorg. Med. Chem. Lett. 2020, 30, 126890. [Google Scholar] [CrossRef] [PubMed]
- Okumu, M.O.; Eyaan, K.L.; Bett, L.K.; Gitahi, N. Antibacterial Activity of Venom from the Puff Adder (Bitis arietans), Egyptian Cobra (Naja haje), and Red Spitting Cobra (Naja pallida). Int. J. Microbiol. 2023, 2023, 7924853. [Google Scholar] [CrossRef]
- Gajic, I.; Kekic, D.; Jankovic, M.; Tomic, N.; Skoric, M.; Petrovic, M.; Mitic Culafic, D.; Opavski, N.; Ristivojevic, P.; Krstic Ristivojevic, M.; et al. Nature’s Arsenal: Uncovering Antibacterial Agents Against Antimicrobial Resistance. Antibiotics 2025, 14, 253. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M.; Doley, R. Structure, function and evolution of three-finger toxins: Mini proteins with multiple targets. Toxicon Off. J. Int. Soc. Toxinol. 2010, 56, 855–867. [Google Scholar] [CrossRef]
- Tadokoro, T.; Modahl, C.M.; Maenaka, K.; Aoki-Shioi, N. Cysteine-Rich Secretory Proteins (CRISPs) from Venomous Snakes: An Overview of the Functional Diversity in a Large and Underappreciated Superfamily. Toxins 2020, 12, 175. [Google Scholar] [CrossRef]
- Salazar, E.; Cirilo, A.; Reyes, A.; Barrientos, M.; Galan, J.; Sánchez, E.E.; Suntravat, M. Snake venom cysteine-rich secretory protein from Mojave rattlesnake venom (Css-CRiSP) induces acute inflammatory responses on different experimental models. Toxicon X 2023, 21, 100180. [Google Scholar] [CrossRef]
- Messadi, E. Snake venom components as therapeutic drugs in ischemic heart disease. Biomolecules 2023, 13, 1539. [Google Scholar] [CrossRef]
- Adade, C.M.; Carvalho, A.L.; Tomaz, M.A.; Costa, T.F.; Godinho, J.L.; Melo, P.A.; Lima, A.P.; Rodrigues, J.C.; Zingali, R.B.; Souto-Padrón, T. Crovirin, a snake venom cysteine-rich secretory protein (CRISP) with promising activity against Trypanosomes and Leishmania. PLoS Neglected Trop. Dis. 2014, 8, e3252. [Google Scholar] [CrossRef]
- Wang, Y.L.; Kuo, J.H.; Lee, S.C.; Liu, J.S.; Hsieh, Y.C.; Shih, Y.T.; Chen, C.J.; Chiu, J.J.; Wu, W.G. Cobra CRISP functions as an inflammatory modulator via a novel Zn2+-and heparan sulfate-dependent transcriptional regulation of endothelial cell adhesion molecules. J. Biol. Chem. 2010, 285, 37872–37883. [Google Scholar] [CrossRef]
- Badari, J.C.; Díaz-Roa, A.; Teixeira Rocha, M.M.; Mendonça, R.Z.; da Silva Junior, P.I. Patagonin-CRISP: Antimicrobial Activity and Source of Antimicrobial Molecules in Duvernoy’s Gland Secretion (Philodryas patagoniensis Snake). Front. Pharmacol. 2021, 11, 586705. [Google Scholar] [CrossRef] [PubMed]
- Izidoro, L.F.M.; Sobrinho, J.C.; Mendes, M.M.; Costa, T.R.; Grabner, A.N.; Rodrigues, V.M.; da Silva, S.L.; Zanchi, F.B.; Zuliani, J.P.; Fernandes, C.F.C.; et al. Snake venom L-amino acid oxidases: Trends in pharmacology and biochemistry. BioMed Res. Int. 2014, 2014, 196754. [Google Scholar] [CrossRef]
- Pollegioni, L.; Motta, P.; Molla, G. L-amino acid oxidase as biocatalyst: A dream too far? Appl. Microbiol. Biotechnol. 2013, 97, 9323–9341. [Google Scholar] [CrossRef] [PubMed]
- Abdelkafi-Koubaa, Z.; Morjen, M.; Srairi-Abid, N.; El Ayeb, M.; Marrakchi, N. Snake venom L-amino acid oxidases potential biomedical applications. Arch. Inst. Pasteur Tunis. 2014, 91, 15–32. [Google Scholar] [PubMed]
- Phua, C.S.; Vejayan, J.; Ambu, S.; Gorajana, A.; Ibrahim, H. Purification and antibacterial activities of an L-amino acid oxidase from king cobra (Ophiophagus hannah) venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2012, 18, 198–207. [Google Scholar] [CrossRef]
- Lu, Q.M.; Wei, Q.; Jin, Y.; Wei, J.F.; Wang, W.Y.; Xiong, Y.L. L-amino acid oxidase from Trimeresurus jerdonii snake venom: Purification, characterization, platelet aggregation-inducing and antibacterial effects. J. Nat. Toxins 2002, 11, 345–352. [Google Scholar]
- Zhong, S.R.; Jin, Y.; Wu, J.B.; Jia, Y.H.; Xu, G.L.; Wang, G.C.; Xiong, Y.L.; Lu, Q.M. Purification and characterization of a new L-amino acid oxidase from Daboia russellii siamensis venom. Toxicon Off. J. Int. Soc. Toxinol. 2009, 54, 763–771. [Google Scholar] [CrossRef]
- Hanane-Fadila, Z.M.; Fatima, L.D. Purification, characterization and antibacterial activity of L-amino acid oxidase from Cerastes cerastes. J. Biochem. Mol. Toxicol. 2014, 28, 347–354. [Google Scholar] [CrossRef]
- Vargas, L.J.; Quintana, J.C.; Pereañez, J.A.; Núñez, V.; Sanz, L.; Calvete, J. Cloning and characterization of an antibacterial L-amino acid oxidase from Crotalus durissus cumanensis venom. Toxicon Off. J. Int. Soc. Toxinol. 2013, 64, 1–11. [Google Scholar] [CrossRef]
- Samel, M.; Tõnismägi, K.; Rönnholm, G.; Vija, H.; Siigur, J.; Kalkkinen, N.; Siigur, E. L-Amino acid oxidase from Naja naja oxiana venom. Comparative biochemistry and physiology. Part B Biochem. Mol. Biol. 2008, 149, 572–580. [Google Scholar] [CrossRef]
- Fernandes, T.A.d.M.; Costa, T.R.; Menezes, R.d.P.; de Souza, M.A.; Martins, C.H.G.; Junior, N.N.; Amorim, F.G.; Quinton, L.; Polloni, L.; Teixeira, S.C.; et al. Bothrops snake venom L-amino acid oxidases impair biofilm formation of clinically relevant bacteria. Toxicon Off. J. Int. Soc. Toxinol. 2024, 238, 107569. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, T.B.; Clark, R.J. Advances in venom peptide drug discovery: Where are we at and where are we heading? Expert Opin. Drug Discov. 2021, 16, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, M.; Chang, W. Antimicrobial peptides and proteins against drug-resistant pathogens. Cell Surf. 2024, 12, 100135. [Google Scholar] [CrossRef] [PubMed]
- Dean, S.N.; Bishop, B.M.; van Hoek, M.L. Natural and synthetic cathelicidin peptides with anti-microbial and anti-biofilm activity against Staphylococcus aureus. BMC Microbiol. 2011, 11, 114. [Google Scholar] [CrossRef]
- Blower, R.J.; Barksdale, S.M.; van Hoek, M.L. Snake Cathelicidin NA-CATH and Smaller Helical Antimicrobial Peptides Are Effective against Burkholderia thailandensis. PLoS Neglected Trop. Dis. 2015, 9, e0003862. [Google Scholar] [CrossRef]
- Kościuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzałkowska, N.; Jóźwik, A.; Horbańczuk, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of antimicrobial peptides. A review. Mol. Biol. Rep. 2012, 39, 10957–10970. [Google Scholar] [CrossRef]
- Gan, B.H.; Gaynord, J.; Rowe, S.M.; Deingruber, T.; Spring, D.R. The multifaceted nature of antimicrobial peptides: Current synthetic chemistry approaches and future directions. Chem. Soc. Rev. 2021, 50, 7820–7880. [Google Scholar] [CrossRef]
- Li, S.A.; Lee, W.H.; Zhang, Y. Efficacy of OH-CATH30 and its analogs against drug-resistant bacteria in vitro and in mouse models. Antimicrob. Agents Chemother. 2012, 56, 3309–3317. [Google Scholar] [CrossRef]
- Rizzetto, G.; Gambini, D.; Maurizi, A.; Molinelli, E.; De Simoni, E.; Pallotta, F.; Brescini, L.; Cirioni, O.; Offidani, A.; Simonetti, O.; et al. The sources of antimicrobial peptides against Gram-positives and Gramnegatives: Our research experience. Le Infez. Med. 2023, 31, 306–322. [Google Scholar]
- Pérez-Peinado, C.; Defaus, S.; Andreu, D. Hitchhiking with Nature: Snake Venom Peptides to Fight Cancer and Superbugs. Toxins 2020, 12, 255. [Google Scholar] [CrossRef]
- Ballén, V.; Cepas, V.; Ratia, C.; Gabasa, Y.; Soto, S.M. Clinical Escherichia coli: From Biofilm Formation to New Antibiofilm Strategies. Microorganisms 2022, 10, 1103. [Google Scholar] [CrossRef] [PubMed]
- Sachidananda, M.K.; Murari, S.K.; Gowda, C.D. Characterization of an antibacterial peptide from Indian cobra (Naja naja) venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2007, 13, 446–461. [Google Scholar] [CrossRef]
- Sen, S.; Samat, R.; Jash, M.; Ghosh, S.; Roy, R.; Mukherjee, N.; Ghosh, S.; Sarkar, J.; Ghosh, S. Potential Broad-Spectrum Antimicrobial, Wound Healing, and Disinfectant Cationic Peptide Crafted from Snake Venom. J. Med. Chem. 2023, 66, 11555–11572. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.; Lancellotti, M.; Soares, A.M.; Calderon, L.A.; Ramírez, D.; González, W.; Marangoni, S.; Da Silva, S.L. CoaTx-II, a new dimeric Lys49 phospholipase A2 from Crotalus oreganus abyssus snake venom with bactericidal potential: Insights into its structure and biological roles. Toxicon Off. J. Int. Soc. Toxinol. 2016, 120, 147–158. [Google Scholar] [CrossRef]
- Samy, R.P.; Mackessy, S.P.; Jeyasankar, A.; Ponraj, M.R.; Franco, O.L.; Cooper, M.A.; Kandasamy, M.; Mohanta, T.K.; Bhagavathsingh, J.; Vaiyapuri, S. Purification of PaTx-II from the Venom of the Australian King Brown Snake and Characterization of Its Antimicrobial and Wound Healing Activities. Int. J. Mol. Sci. 2023, 24, 4359. [Google Scholar] [CrossRef]
- Chen, L.W.; Kao, P.H.; Fu, Y.S.; Lin, S.R.; Chang, L.S. Membrane-damaging activity of Taiwan cobra cardiotoxin 3 is responsible for its bactericidal activity. Toxicon Off. J. Int. Soc. Toxinol. 2011, 58, 46–53. [Google Scholar] [CrossRef]
- Sala, A.; Cabassi, C.S.; Santospirito, D.; Polverini, E.; Flisi, S.; Cavirani, S.; Taddei, S. Novel Naja atra cardiotoxin 1 (CTX-1) derived antimicrobial peptides with broad spectrum activity. PLoS ONE 2018, 13, e0190778. [Google Scholar] [CrossRef]
- Kao, P.H.; Lin, S.R.; Hu, W.P.; Chang, L.S. Naja naja atra and Naja nigricollis cardiotoxins induce fusion of Escherichia coli and Staphylococcus aureus membrane-mimicking liposomes. Toxicon Off. J. Int. Soc. Toxinol. 2012, 60, 367–377. [Google Scholar] [CrossRef]
- Yacoub, T.; Rima, M.; Karam, M.; Sabatier, J.-M.; Fajloun, Z. Antimicrobials from Venomous Animals: An Overview. Molecules 2020, 25, 2402. [Google Scholar] [CrossRef]
- de Oliveira, A.L.N.; Lacerda, M.T.; Ramos, M.J.; Fernandes, P.A. Viper Venom Phospholipase A2 Database: The Structural and Functional Anatomy of a Primary Toxin in Envenomation. Toxins 2024, 16, 71. [Google Scholar] [CrossRef]
- Srinivasan, K.; Nampoothiri, M.; Khandibharad, S.; Singh, S.; Nayak, A.G.; Hariharapura, R.C. Proteomic diversity of Russell’s viper venom: Exploring PLA2 isoforms, pharmacological effects, and inhibitory approaches. Arch. Toxicol. 2024, 98, 3569–3584. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Ilies, M.A. The Phospholipase A2 Superfamily: Structure, Isozymes, Catalysis, Physiologic and Pathologic Roles. Int. J. Mol. Sci. 2023, 24, 1353. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Park, Y.; Hahm, K.S. The role of antimicrobial peptides in preventing multidrug-resistant bacterial infections and biofilm formation. Int. J. Mol. Sci. 2011, 12, 5971–5992. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Waseem, R.; Zehra, Z.; Aiman, A.; Bhardwaj, P.; Ansari, J.; Hassan, M.I.; Islam, A. Mitochondrial Dysfunction: Pathophysiology and Mitochondria-Targeted Drug Delivery Approaches. Pharmaceutics 2022, 14, 2657. [Google Scholar] [CrossRef]
- Wong, F.; Amir, A. Mechanics and Dynamics of Bacterial Cell Lysis. Biophys. J. 2019, 116, 2378–2389. [Google Scholar] [CrossRef]
- Samy, R.P.; Manikandan, J.; Sethi, G.; Franco, O.L.; Okonkwo, J.C.; Stiles, B.G.; Chow, V.T.; Gopalakrishnakone, P.; Al Qahtani, M. Snake venom proteins: Development into antimicrobial and wound healing agents. Mini-Rev. Org. Chem. 2014, 11, 15–27. [Google Scholar] [CrossRef]
- Hahn, K.; Neumeister, K.; Mix, A.; Kottke, T.; Gröger, H.; Fischer von Mollard, G. Recombinant expression and characterization of a L-amino acid oxidase from the fungus Rhizoctonia solani. Appl. Microbiol. Biotechnol. 2017, 101, 2853–2864. [Google Scholar] [CrossRef]
- Gasanov, S.E.; Dagda, R.K.; Rael, E.D. Snake Venom Cytotoxins, Phospholipase A2s, and Zn2+-dependent Metalloproteinases: Mechanisms of Action and Pharmacological Relevance. J. Clin. Toxicol. 2014, 4, 1000181. [Google Scholar] [CrossRef]
- Yunus, J.; Wan Dagang, W.R.Z.; Jamaluddin, H.; Jemon, K.; Mohamad, S.E.; Jonet, M.A. Bacterial biofilm growth and perturbation by serine protease from Bacillus sp. Arch. Microbiol. 2024, 206, 138. [Google Scholar] [CrossRef]
- Hiu, J.J.; Yap, M.K.K. Cytotoxicity of snake venom enzymatic toxins: Phospholipase A2 and l-amino acid oxidase. Biochem. Soc. Trans. 2020, 48, 719–731. [Google Scholar] [CrossRef]
- Resiere, D.; Mehdaoui, H.; Neviere, R. Inflammation and Oxidative Stress in Snakebite Envenomation: A Brief Descriptive Review and Clinical Implications. Toxins 2022, 14, 802. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.R.; Burin, S.M.; Menaldo, D.L.; de Castro, F.A.; Sampaio, S.V. Snake venom L-amino acid oxidases: An overview on their antitumor effects. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Cao, J.; Hsu, Y.H.; Magrioti, V.; Kokotos, G. Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011, 111, 6130–6185. [Google Scholar] [CrossRef] [PubMed]
- Ouertani, A.; Mollet, C.; Boughanmi, Y.; de Pomyers, H.; Mosbah, A.; Ouzari, H.I.; Cherif, A.; Gigmes, D.; Maresca, M.; Mabrouk, K. Screening of antimicrobial activity in venom: Exploring key parameters. Toxicon Off. J. Int. Soc. Toxinol. 2024, 251, 108135. [Google Scholar] [CrossRef]
- Raza, S.; Matuła, K.; Karoń, S.; Paczesny, J. Resistance and Adaptation of Bacteria to Non-Antibiotic Antibacterial Agents: Physical Stressors, Nanoparticles, and Bacteriophages. Antibiotics 2021, 10, 435. [Google Scholar] [CrossRef]
- Mourenza, Á.; Gil, J.A.; Mateos, L.M.; Letek, M. Oxidative Stress-Generating Antimicrobials, a Novel Strategy to Overcome Antibacterial Resistance. Antioxidants 2020, 9, 361. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control. 2019, 8, 76. [Google Scholar] [CrossRef]
- Schneider, R.; Primon-Barros, M.; Von Borowski, R.G.; Chat, S.; Nonin-Lecomte, S.; Gillet, R.; Macedo, A.J. Pseudonajide peptide derived from snake venom alters cell envelope integrity interfering on biofilm formation in Staphylococcus epidermidis. BMC Microbiol. 2020, 20, 237. [Google Scholar] [CrossRef]
- Truong, N.V.; Phan, T.T.T.; Hsu, T.S.; Phu Duc, P.; Lin, L.Y.; Wu, W.G. Action mechanism of snake venom l-amino acid oxidase and its double-edged sword effect on cancer treatment: Role of pannexin 1-mediated interleukin-6 expression. Redox Biol. 2023, 64, 102791. [Google Scholar] [CrossRef]
- Di Somma, A.; Moretta, A.; Canè, C.; Cirillo, A.; Duilio, A. Antimicrobial and Antibiofilm Peptides. Biomolecules 2020, 10, 652. [Google Scholar] [CrossRef]
- Fontanot, A.; Ellinger, I.; Unger, W.W.J.; Hays, J.P. A Comprehensive Review of Recent Research into the Effects of Antimicrobial Peptides on Biofilms—January 2020 to September 2023. Antibiotics 2024, 13, 343. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Gao, J.; Zhang, S.; Wu, S.; Xie, Z.; Ling, G.; Kuang, Y.Q.; Yang, Y.; Yu, H.; Wang, Y. Identification and Characterization of the First Cathelicidin from Sea Snakes with Potent Antimicrobial and Anti-inflammatory Activity and Special Mechanism. J. Biol. Chem. 2015, 290, 16633–16652. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.F.; Landucci, E.C.; Antunes, E.; Chacur, M.; Cury, Y. Inflammatory effects of snake venom myotoxic phospholipases A2. Toxicon Off. J. Int. Soc. Toxinol. 2003, 42, 947–962. [Google Scholar] [CrossRef] [PubMed]
- Zoccal, K.F.; da Silva Bitencourt, C.; Paula-Silva, F.W.G.; Sorgi, C.A.; de Castro Figueiredo Bordon, K.; Arantes, E.C.; Faccioli, L.H. TLR2, TLR4 and CD14 recognize venom-associated molecular patterns from Tityus serrulatus to induce macrophage-derived inflammatory mediators. PLoS ONE 2014, 9, e88174. [Google Scholar] [CrossRef]
- Stone, S.F.; Isbister, G.K.; Shahmy, S.; Mohamed, F.; Abeysinghe, C.; Karunathilake, H.; Ariaratnam, A.; Jacoby-Alner, T.E.; Cotterell, C.L.; Brown, S.G. Immune response to snake envenoming and treatment with antivenom; complement activation, cytokine production and mast cell degranulation. PLoS Neglected Trop. Dis. 2013, 7, e2326. [Google Scholar] [CrossRef]
- de Barros, E.; Gonçalves, R.M.; Cardoso, M.H.; Santos, N.C.; Franco, O.L.; Cândido, E.S. Snake Venom Cathelicidins as Natural Antimicrobial Peptides. Front. Pharmacol. 2019, 10, 1415. [Google Scholar] [CrossRef]
- Samy, R.P.; Kandasamy, M.; Gopalakrishnakone, P.; Stiles, B.G.; Rowan, E.G.; Becker, D.; Shanmugam, M.K.; Sethi, G.; Chow, V.T.K. Wound healing activity and mechanisms of action of an antibacterial protein from the venom of the eastern diamondback rattlesnake (Crotalus adamanteus). PLoS ONE 2014, 9, e80199. [Google Scholar] [CrossRef]
- Giannotti, K.C.; Leiguez, E.; Moreira, V.; Nascimento, N.G.; Lomonte, B.; Gutiérrez, J.M.; Lopes de Melo, R.; Teixeira, C. A Lys49 phospholipase A2, isolated from Bothrops asper snake venom, induces lipid droplet formation in macrophages which depends on distinct signaling pathways and the C-terminal region. BioMed Res. Int. 2013, 2013, 807982. [Google Scholar] [CrossRef]
- Moreira, V.; Lomonte, B.; Vinolo, M.A.; Curi, R.; Gutiérrez, J.M.; Teixeira, C. An Asp49 phospholipase A2 from snake venom induces cyclooxygenase-2 expression and prostaglandin E2 production via activation of NF-κB, p38MAPK, and PKC in macrophages. Mediat. Inflamm. 2014, 2014, 105879. [Google Scholar] [CrossRef]
- Avalo, Z.; Barrera, M.C.; Agudelo-Delgado, M.; Tobón, G.J.; Cañas, C.A. Biological Effects of Animal Venoms on the Human Immune System. Toxins 2022, 14, 344. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular vesicles and nanoparticles: Emerging complexities. Trends Cell biology 2023, 33, 667–681. [Google Scholar] [CrossRef]
- Yokoi, A.; Ochiya, T. Exosomes and extracellular vesicles: Rethinking the essential values in cancer biology. Semin. Cancer Biol. 2021, 74, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, D.; Bakkannavar, S.; Bhat, V.R.; Sharan, K. Extracellular vesicles as novel drug delivery systems to target cancer and other diseases: Recent advancements and future perspectives. F1000Research 2023, 12, 329. [Google Scholar] [CrossRef] [PubMed]
- Muttiah, B.; Law, J.X. Milk-derived extracellular vesicles and gut health. NPJ Sci. Food 2025, 9, 12. [Google Scholar] [CrossRef]
- Ciferri, M.C.; Quarto, R.; Tasso, R. Extracellular Vesicles as Biomarkers and Therapeutic Tools: From Pre-Clinical to Clinical Applications. Biology 2021, 10, 359. [Google Scholar] [CrossRef]
- Islam Khan, M.Z.; Tam, S.Y.; Law, H.K.W. Advances in High Throughput Proteomics Profiling in Establishing Potential Biomarkers for Gastrointestinal Cancer. Cells 2022, 11, 973. [Google Scholar] [CrossRef]
- Mosquera-Heredia, M.I.; Morales, L.C.; Vidal, O.M.; Barceló, E.; Silvera-Redondo, C.; Vélez, J.I.; Garavito-Galofre, P. Exosomes: Potential Disease Biomarkers and New Therapeutic Targets. Biomedicines 2021, 9, 1061. [Google Scholar] [CrossRef]
- Iqbal, Z.; Rehman, K.; Mahmood, A.; Shabbir, M.; Liang, Y.; Duan, L.; Zeng, H. Exosome for mRNA delivery: Strategies and therapeutic applications. J. Nanobiotechnol. 2024, 22, 395. [Google Scholar] [CrossRef]
- De Sousa, K.P.; Rossi, I.; Abdullahi, M.; Ramirez, M.I.; Stratton, D.; Inal, J.M. Isolation and characterization of extracellular vesicles and future directions in diagnosis and therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1835. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, B.; Wang, Y.; Du, H.; Fang, L. Extracellular Vesicles as Mediators and Potential Targets in Combating Cancer Drug Resistance. Molecules 2025, 30, 498. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Yadav, A.; Nandy, A.; Ghatak, S. Insight into the Functional Dynamics and Challenges of Exosomes in Pharmaceutical Innovation and Precision Medicine. Pharmaceutics 2024, 16, 709. [Google Scholar] [CrossRef] [PubMed]
- Bala, A.A.; Oukkache, N.; Sanchez, E.E.; Suntravat, M.; Galan, J.A. Venoms and Extracellular Vesicles: A New Frontier in Venom Biology. Toxins 2025, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Xun, C.; Wang, L.; Yang, H.; Xiao, Z.; Deng, M.; Xu, R.; Zhou, X.; Chen, P.; Liu, Z. Origin and Characterization of Extracellular Vesicles Present in the Spider Venom of Ornithoctonus hainana. Toxins 2021, 13, 579. [Google Scholar] [CrossRef]
- K, N.; Bakkannavar, S.M.; Bhat, V.R.; Sirur, F.M. A review on snake venom extracellular vesicles: Past to present. Toxicon Off. J. Int. Soc. Toxinol. 2024, 244, 107772. [Google Scholar] [CrossRef]
- Dang, X.T.T.; Kavishka, J.M.; Zhang, D.X.; Pirisinu, M.; Le, M.T.N. Extracellular Vesicles as an Efficient and Versatile System for Drug Delivery. Cells 2020, 9, 2191. [Google Scholar] [CrossRef]
- Cavallo, I.; Sivori, F.; Mastrofrancesco, A.; Abril, E.; Pontone, M.; Di Domenico, E.G.; Pimpinelli, F. Bacterial Biofilm in Chronic Wounds and Possible Therapeutic Approaches. Biology 2024, 13, 109. [Google Scholar] [CrossRef]
- Espejo, C.; Wilson, R.; Pye, R.J.; Ratcliffe, J.C.; Ruiz-Aravena, M.; Willms, E.; Wolfe, B.W.; Hamede, R.; Hill, A.F.; Jones, M.E.; et al. Cathelicidin-3 Associated With Serum Extracellular Vesicles Enables Early Diagnosis of a Transmissible Cancer. Front. Immunol. 2022, 13, 858423. [Google Scholar] [CrossRef]
- Zeng, Y.; Qiu, Y.; Jiang, W.; Shen, J.; Yao, X.; He, X.; Li, L.; Fu, B.; Liu, X. Biological Features of Extracellular Vesicles and Challenges. Front. Cell Dev. Biol. 2022, 10, 816698. [Google Scholar] [CrossRef]
- AlQurashi, D.M.; AlQurashi, T.F.; Alam, R.I.; Shaikh, S.; Tarkistani, M.A.M. Advanced Nanoparticles in Combating Antibiotic Resistance: Current Innovations and Future Directions. J. Nanotheranostics 2025, 6, 9. [Google Scholar] [CrossRef]
- Barathan, M.; Ng, S.L.; Lokanathan, Y.; Ng, M.H.; Law, J.X. Unseen Weapons: Bacterial Extracellular Vesicles and the Spread of Antibiotic Resistance in Aquatic Environments. Int. J. Mol. Sci. 2024, 25, 3080. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.; Moreira, J.N.; Rodrigues, L.R. New advances in exosome-based targeted drug delivery systems. Crit. Rev. Oncol./Hematol. 2022, 172, 103628. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, X.; Chandra, S.; Lyon, C.; Ning, B.; Jiang, L.; Fan, J.; Hu, T.Y. Extracellular vesicles: Emerging tools as therapeutic agent carriers. Acta Pharm. Sin. B 2022, 12, 3822–3842. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A. Structure-function studies and mechanism of action of snake venom L-amino acid oxidases. Front. Pharmacol. 2020, 11, 110. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Huang, T.H.; Yang, S.C.; Chen, C.C.; Fang, J.Y. Nano-Based Drug Delivery or Targeting to Eradicate Bacteria for Infection Mitigation: A Review of Recent Advances. Front. Chem. 2020, 8, 286. [Google Scholar] [CrossRef]
- Johnson, V.; Vasu, S.; Kumar, U.S.; Kumar, M. Surface-Engineered Extracellular Vesicles in Cancer Immunotherapy. Cancers 2023, 15, 2838. [Google Scholar] [CrossRef]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- da-Silva-Freitas, D.; Boldrini-França, J.; Arantes, E.C. PEGylation: A successful approach to improve the biopharmaceutical potential of snake venom thrombin-like serine protease. Protein Pept. Lett. 2015, 22, 1133–1139. [Google Scholar] [CrossRef]
- Makharadze, D.; del Valle, L.J.; Katsarava, R.; Puiggalí, J. The Art of PEGylation: From Simple Polymer to Sophisticated Drug Delivery System. Int. J. Mol. Sci. 2025, 26, 3102. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99 Pt A, 28–51. [Google Scholar] [CrossRef]
- Benincasa, M.; Zahariev, S.; Pelillo, C.; Milan, A.; Gennaro, R.; Scocchi, M. PEGylation of the peptide Bac7(1-35) reduces renal clearance while retaining antibacterial activity and bacterial cell penetration capacity. Eur. J. Med. Chem. 2015, 95, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Alves, Á.E.F.; Barros, A.B.C.; Silva, L.C.F.; Carvalho, L.M.M.; Pereira, G.M.A.; Uchôa, A.F.C.; Barbosa-Filho, J.M.; Silva, M.S.; Luna, K.P.O.; Soares, K.S.R.; et al. Emerging Trends in Snake Venom-Loaded Nanobiosystems for Advanced Medical Applications: A Comprehensive Overview. Pharmaceutics 2025, 17, 204. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Méndez, E.; Fuglsang-Madsen, A.; Føns, S.; Lomonte, B.; Gutiérrez, J.M.; Laustsen, A.H. Innovative Immunization Strategies for Antivenom Development. Toxins 2018, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Thompson, D.H. Stimuli-responsive liposomes for drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1450. [Google Scholar] [CrossRef]
- Ashfaq, R.; Rasul, A.; Asghar, S.; Kovács, A.; Berkó, S.; Budai-Szűcs, M. Lipid Nanoparticles: An Effective Tool to Improve the Bioavailability of Nutraceuticals. Int. J. Mol. Sci. 2023, 24, 15764. [Google Scholar] [CrossRef]
- Izumi, K.; Saito, C.; Kawano, R. Liposome Deformation Induced by Membrane-Binding Peptides. Micromachines 2023, 14, 373. [Google Scholar] [CrossRef]
- Badiee, A.; Khamesipour, A.; Samiei, A.; Soroush, D.; Shargh, V.H.; Kheiri, M.T.; Barkhordari, F.; Robert Mc Master, W.; Mahboudi, F.; Jaafari, M.R. The role of liposome size on the type of immune response induced in BALB/c mice against leishmaniasis: Rgp63 as a model antigen. Exp. Parasitol. 2012, 132, 403–409. [Google Scholar] [CrossRef]
- Schwendener, R.A. Liposomes as vaccine delivery systems: A review of the recent advances. Ther. Adv. Vaccines 2014, 2, 159–182. [Google Scholar] [CrossRef]
- Ferreira, M.; Ogren, M.; Dias, J.N.R.; Silva, M.; Gil, S.; Tavares, L.; Aires-da-Silva, F.; Gaspar, M.M.; Aguiar, S.I. Liposomes as Antibiotic Delivery Systems: A Promising Nanotechnological Strategy against Antimicrobial Resistance. Molecules 2021, 26, 2047. [Google Scholar] [CrossRef]
- Liu, M.; Jin, J.; Zhong, X.; Liu, L.; Tang, C.; Cai, L. Polysaccharide hydrogels for skin wound healing. Heliyon 2024, 10, e35014. [Google Scholar] [CrossRef]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: Current achievements and future directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Gounden, V.; Singh, M. Hydrogels and Wound Healing: Current and Future Prospects. Gels 2024, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Samat, R.; Sen, S.; Jash, M.; Ghosh, S.; Garg, S.; Sarkar, J.; Ghosh, S. Venom: A Promising Avenue for Antimicrobial Therapeutics. ACS Infect. Dis. 2024, 10, 3098–3125. [Google Scholar] [CrossRef]
- Yegappan, R.; Lauko, J.; Wang, Z.; Lavin, M.F.; Kijas, A.W.; Rowan, A.E. Snake Venom Hydrogels as a Rapid Hemostatic Agent for Uncontrolled Bleeding. Adv. Healthc. Mater. 2022, 11, e2200574. [Google Scholar] [CrossRef]
- Nazary Abrbekoh, F.; Salimi, L.; Saghati, S.; Amini, H.; Fathi Karkan, S.; Moharamzadeh, K.; Sokullu, E.; Rahbarghazi, R. Application of microneedle patches for drug delivery; doorstep to novel therapies. J. Tissue Eng. 2022, 13, 20417314221085390. [Google Scholar] [CrossRef]
- Bae, W.-G.; Ko, H.; So, J.-Y.; Yi, H.; Lee, C.-H.; Lee, D.-H.; Ahn, Y.; Lee, S.-H.; Lee, K.; Jun, J.; et al. Snake fang-inspired stamping patch for transdermal delivery of liquid formulations. Sci. Transl. Med. 2019, 11, eaaw3329. [Google Scholar] [CrossRef]
- Luo, R.; Xu, H.; Lin, Q.; Chi, J.; Liu, T.; Jin, B.; Ou, J.; Xu, Z.; Peng, T.; Quan, G.; et al. Emerging Trends in Dissolving-Microneedle Technology for Antimicrobial Skin-Infection Therapies. Pharmaceutics 2024, 16, 1188. [Google Scholar] [CrossRef]
- Zona Rubio, D.C.; Aragón, D.M.; Almeida Alves, I. Innovations in Snake Venom-Derived Therapeutics: A Systematic Review of Global Patents and Their Pharmacological Applications. Toxins 2025, 17, 136. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, J.; Fan, D. A dissolving microneedle patch for antibiotic/enzymolysis/photothermal triple therapy against bacteria and their biofilms. Chem. Eng. J. 2022, 437, 135475. [Google Scholar] [CrossRef]
- Reis, C.A.; Gomes, A.; do Amaral Sobral, P.J. Films Based on Biopolymers Incorporated with Active Compounds Encapsulated in Emulsions: Properties and Potential Applications-A Review. Foods 2023, 12, 3602. [Google Scholar] [CrossRef] [PubMed]
- Sáenz-Santos, C.M.; Opemipo Oyedara, O.; García-Tejeda, Y.V.; Romero-Bastida, C.A.; García-Oropesa, E.M.; Villalobo, E.; Rodríguez-Pérez, M.A. Active Biopolymeric Films Inoculated with Bdellovibrio bacteriovorus, a Predatory Bacterium. Coatings 2021, 11, 605. [Google Scholar] [CrossRef]
- Caseli, L.; Tiburcio, V.L.B.; Vargas, F.F.R.; Marangoni, S.; Siqueira, J.R., Jr. Enhanced architecture of lipid-carbon nanotubes as langmuir-blodgett films to investigate the enzyme activity of phospholipases from snake venom. J. Phys. Chem. B 2012, 116, 13424–13429. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.; Gomes, D.; da Costa, A.; Dias, S.C.; Casal, M.; Machado, R. Protein-Engineered Polymers Functionalized with Antimicrobial Peptides for the Development of Active Surfaces. Appl. Sci. 2021, 11, 5352. [Google Scholar] [CrossRef]
- Roque-Borda, C.A.; Gualque, M.W.L.; da Fonseca, F.H.; Pavan, F.R.; Santos-Filho, N.A. Nanobiotechnology with Therapeutically Relevant Macromolecules from Animal Venoms: Venoms, Toxins, and Antimicrobial Peptides. Pharmaceutics 2022, 14, 891. [Google Scholar] [CrossRef]
- Joglekar, A.V.; Dehari, D.; Anjum, M.; Dulla, N.; Chaudhuri, A.; Singh, S.; Agrawal, A.K. Therapeutic potential of venom peptides: Insights in the nanoparticle-mediated venom formulations. Futur. J. Pharm. Sci. 2022, 8, 34. [Google Scholar] [CrossRef]
- Biswas, A.; Gomes, A.; Sengupta, J.; Datta, P.; Singha, S.; Dasgupta, A.K.; Gomes, A. Nanoparticle-conjugated animal venom-toxins and their possible therapeutic potential. J. Venom Res. 2012, 3, 15–21. [Google Scholar]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef]
- Akdaşçi, E.; Duman, H.; Eker, F.; Bechelany, M.; Karav, S. Chitosan and Its Nanoparticles: A Multifaceted Approach to Antibacterial Applications. Nanomaterials 2025, 15, 126. [Google Scholar] [CrossRef]
- Sánchez, A.; Mejía, S.P.; Orozco, J. Recent Advances in Polymeric Nanoparticle-Encapsulated Drugs against Intracellular Infections. Molecules 2020, 25, 3760. [Google Scholar] [CrossRef]
- Oguiura, N.; Corrêa, P.G.; Rosmino, I.L.; de Souza, A.O.; Pasqualoto, K.F.M. Antimicrobial Activity of Snake β-Defensins and Derived Peptides. Toxins 2022, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Gagat, P.; Ostrówka, M.; Duda-Madej, A.; Mackiewicz, P. Enhancing Antimicrobial Peptide Activity through Modifications of Charge, Hydrophobicity, and Structure. Int. J. Mol. Sci. 2024, 25, 10821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Cheng, X.R.; Liu, J.; Kang, J.; Fu, C.Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.D.; Kim, E.Y.; Radhakrishnan, N.K.; Bang, J.K.; Yang, S.; Shin, S.Y. Enhanced Antibacterial, Anti-Inflammatory, and Antibiofilm Activities of Tryptophan-Substituted Peptides Derived from Cecropin A-Melittin Hybrid Peptide BP100. Molecules 2024, 29, 5231. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar]
- Cañas, C.A.; Castaño-Valencia, S.; Castro-Herrera, F.; Cañas, F.; Tobón, G.J. Biomedical applications of snake venom: From basic science to autoimmunity and rheumatology. J. Transl. Autoimmun. 2020, 4, 100076. [Google Scholar] [CrossRef]
- Sciani, J.M.; Pimenta, D.C. The modular nature of bradykinin-potentiating peptides isolated from snake venoms. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 45. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Albulescu, L.O.; Clare, R.H.; Casewell, N.R.; Abd El-Aziz, T.M.; Escalante, T.; Rucavado, A. The Search for Natural and Synthetic Inhibitors That Would Complement Antivenoms as Therapeutics for Snakebite Envenoming. Toxins 2021, 13, 451. [Google Scholar] [CrossRef]
- Uko, S.O.; Malami, I.; Ibrahim, K.G.; Lawal, N.; Bello, M.B.; Abubakar, M.B.; Imam, M.U. Revolutionizing snakebite care with novel antivenoms: Breakthroughs and barriers. Heliyon 2024, 10, e25531. [Google Scholar] [CrossRef]
- Singh, R.; Chandley, P.; Rohatgi, S. Recent Advances in the Development of Monoclonal Antibodies and Next-Generation Antibodies. ImmunoHorizons 2023, 7, 886–897. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
| Snake Venom Component | Type/Structure | Bacterial Targets | Mechanisms of Action | Example(s) | MIC (µg/mL) |
|---|---|---|---|---|---|
| Metalloproteases (MPs) | Protease enzymes (P-I, P-II, P-III) | Gram-positive and Gram-negative bacteria | Disrupt bacterial membranes, inhibit ion channels, hydrolyze structural components | Agkistrodon halys metalloproteinase (AHM), Bothriopsis oligolepis PIII-MP | 1.875–60 (AHM), MIC for S. aureus: 80 |
| Serine Proteases (SPs) | Single-chain enzymes (26–33 kDa) | Gram-positive bacteria | Membrane disruption, proteolytic degradation of proteins | B. oligolepis SP | 80 (S. aureus) |
| Phospholipase A2s (PLA2s) | Enzyme hydrolyzing phospholipids | Gram-positive and Gram-negative bacteria | Membrane disruption via enzymatic hydrolysis | Daboia russelii PLA2, Naja naja PLA2 | ~11.1–20 (S. aureus), 10–20 (Bacillus subtilis) |
| Three-Finger Toxins (3FTxs) | Non-enzymatic proteins | Gram-positive bacteria | Disrupt bacterial membranes | A5 from Naja naja | No activity against Gram-negative bacteria |
| Cysteine-Rich Secretory Proteins (CRISPs) | Single-chain proteins (20–30 kDa) | Gram-negative bacteria, fungi | Modulate ion channels, disrupt bacterial membrane | Patagonin-CRISP (Philodryas patagoniensis) | 15–7.5 (P. aeruginosa) |
| L-Amino Acid Oxidases (LAAOs) | Flavoproteins, catalyze oxidative deamination | Gram-positive and Gram-negative bacteria | Produce H2O2, leading to oxidative stress, biofilm inhibition | Ophiophagus hannah LAAO, Trimeresurus jerdonii LAAO | 4.5–36 (S. aureus), 9–288 (varied bacteria) |
| Antimicrobial Peptides (AMPs) | Small cationic peptides | Gram-positive and Gram-negative bacteria | Disrupt bacterial membranes, inhibit cell wall synthesis | Cathelicidins, defensins, aprotinin | 10–100 (S. aureus, E. coli) |
| Nucleotidases | Enzyme hydrolyzing nucleotides | Gram-negative bacteria | Degrade bacterial DNA and RNA, disrupt bacterial integrity | Nucleotidase from Bothrops asper | 20–80 (P. aeruginosa) |
| Snake Venom Phosphatases | Enzyme (phosphatase activity) | Gram-negative bacteria | Disrupt bacterial surface structures, interfere with signal transduction | Phosphatase from Naja naja | 50 (E. coli) |
| Aspect | Details |
|---|---|
| Source and Nature of SVEVs | Naturally secreted by snake venom gland epithelial cells. Nano-sized, lipid bilayer-enclosed vesicles. |
| Bioactive Cargo | AMPs (cathelicidins, defensins), enzymes (PLA2s, LAAOs, 5′-nucleotidases), toxins, lipids. |
| Isolation Techniques | Ultracentrifugation, SEC, EVTRAP, polymer precipitation, antibody-based capture. |
| Mechanisms of Antibacterial Action | Membrane damage, oxidative stress induction, enzymatic degradation of bacterial components. |
| Advantages over Traditional Antibiotics | Reduced resistance development, enhanced stability, bioavailability, and synergistic potency. |
| Therapeutic Applications | Wound healing, sepsis treatment, adjuvant to antibiotics, and biofilm penetration. |
| Engineering for Drug Delivery | Functionalization with targeting ligands/antibodies, encapsulation of antibacterial drugs like cathelicidins and defensins. |
| Benefits as Delivery Vehicles | Protection of cargo, targeted delivery, low immunogenicity, biocompatibility, and sustained release. |
| Challenges to Clinical Translation | Standardization of isolation, cytotoxicity removal, in vivo validation, and clinical testing. |
| Notable Example | Bothrops jararaca SVEVs delivering antimicrobial enzymes and proteins. |
| Complementary Delivery Approaches | Exosome-based delivery, PEGylation, liposomes, hydrogels, microneedle patches, and biopolymer films. |
| Delivery System | Advantages | Applications | References |
|---|---|---|---|
| Polymeric Nanoparticles (e.g., Chitosan) | Biocompatible, mucoadhesive, intrinsic antimicrobial activity, enhances venom stability, controlled release | Targeted antibacterial therapy, treatment of infections, reduced inflammation | [165,169,170,171] |
| PEGylation of Snake Venom Toxins | Improved pharmacokinetics, reduced immunogenicity, increased enzymatic activity, stability, reduced renal clearance | Development of new antimicrobial drugs, enhanced bioavailability, reduced toxicity | [138,139,140,141,142] |
| Liposomes | Enhanced immunogenicity, minimized toxicity, high encapsulation efficiency, targeted delivery | Snake venom-based immunization, antimicrobial therapy, drug resistance treatment | [143,144,145,146,147,148,149] |
| Hydrogels | Sustained release, local delivery, multifunctional (antimicrobial, hemostatic, regenerative properties) | Wound healing, infection control, tissue regeneration | [150,151,152,153,154,155] |
| Microneedles | Minimally invasive, controlled local delivery, reduced systemic toxicity, high therapeutic efficacy | Transdermal drug delivery, biofilm-associated infection treatment | [156,157,158,159,160,161] |
| Biopolymer Films | Stable antimicrobial action, biodegradable, functionalized for broad-spectrum activity | Infection-resistant wound dressings, antimicrobial medical devices, food packaging | [161,162,163,164,165] |
| Category | Strategy | Description/Example |
|---|---|---|
| 1. Enhancing Antibacterial Activity | Amino Acid Substitution | Alters hydrophobicity, amphipathicity, and charge to enhance membrane interaction and disruption. |
| Cysteine to Alanine Substitution | Prevents disulfide bond formation while retaining activity (e.g., crotamine, bothropstins). | |
| Tryptophan Incorporation | Enhances membrane penetration and destabilization (e.g., melittin, vipericidins). | |
| Positive Charge Addition | Boosts electrostatic interaction with bacterial membranes, especially effective against Gram-positive strains. | |
| Insertion/Deletion Mutations | Alters α-helix or β-sheet formation, affecting flexibility and membrane penetration. | |
| 2. Synthetic Approaches and Analog Design | Shortened Derivatives | Examples: LZ1, ZY13—retain potency, with improved pharmacokinetics. |
| Directed Evolution | Introduces random mutations to improve affinity, activity, and proteolytic resistance. | |
| Synthetic Analogs | Engineered for better selectivity, in vivo stability, and broader activity profiles. | |
| 3. Reducing Venom Toxicity | Component Isolation and Modification | Focus on non-toxic components like BPPs; enabled drugs like Captopril. |
| Genetic/Chemical Modification | Modify toxic molecules to preserve function but reduce systemic toxicity. | |
| Natural Inhibitors (e.g., sera, plant extracts, EDTA) | Neutralize enzymatic activity of venom components like PLA2, metalloproteinases. | |
| Monoclonal Antibodies (mAbs) | Target specific venom toxins with high specificity and low immunogenicity. | |
| Nanoparticle Delivery Systems | Enable targeted delivery and reduced off-target toxicity, potential for theranostic use. | |
| Combination Therapies | Use of mAbs, nanoparticles, or natural inhibitors alongside antivenoms. | |
| Immunization and Vaccine Development | Inactivated venom components used to stimulate protective immunity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muttiah, B.; Hanafiah, A. Snake Venom Compounds: A New Frontier in the Battle Against Antibiotic-Resistant Infections. Toxins 2025, 17, 221. https://doi.org/10.3390/toxins17050221
Muttiah B, Hanafiah A. Snake Venom Compounds: A New Frontier in the Battle Against Antibiotic-Resistant Infections. Toxins. 2025; 17(5):221. https://doi.org/10.3390/toxins17050221
Chicago/Turabian StyleMuttiah, Barathan, and Alfizah Hanafiah. 2025. "Snake Venom Compounds: A New Frontier in the Battle Against Antibiotic-Resistant Infections" Toxins 17, no. 5: 221. https://doi.org/10.3390/toxins17050221
APA StyleMuttiah, B., & Hanafiah, A. (2025). Snake Venom Compounds: A New Frontier in the Battle Against Antibiotic-Resistant Infections. Toxins, 17(5), 221. https://doi.org/10.3390/toxins17050221






