TM9SF2 Maintains Golgi Integrity and Regulates Ricin-Induced Cytotoxicity
Abstract
1. Introduction
2. Results
2.1. TM9SF2 Displays Non-Redundant Requirement for Ricin-Induced Cytotoxicity Within the TM9SF Family
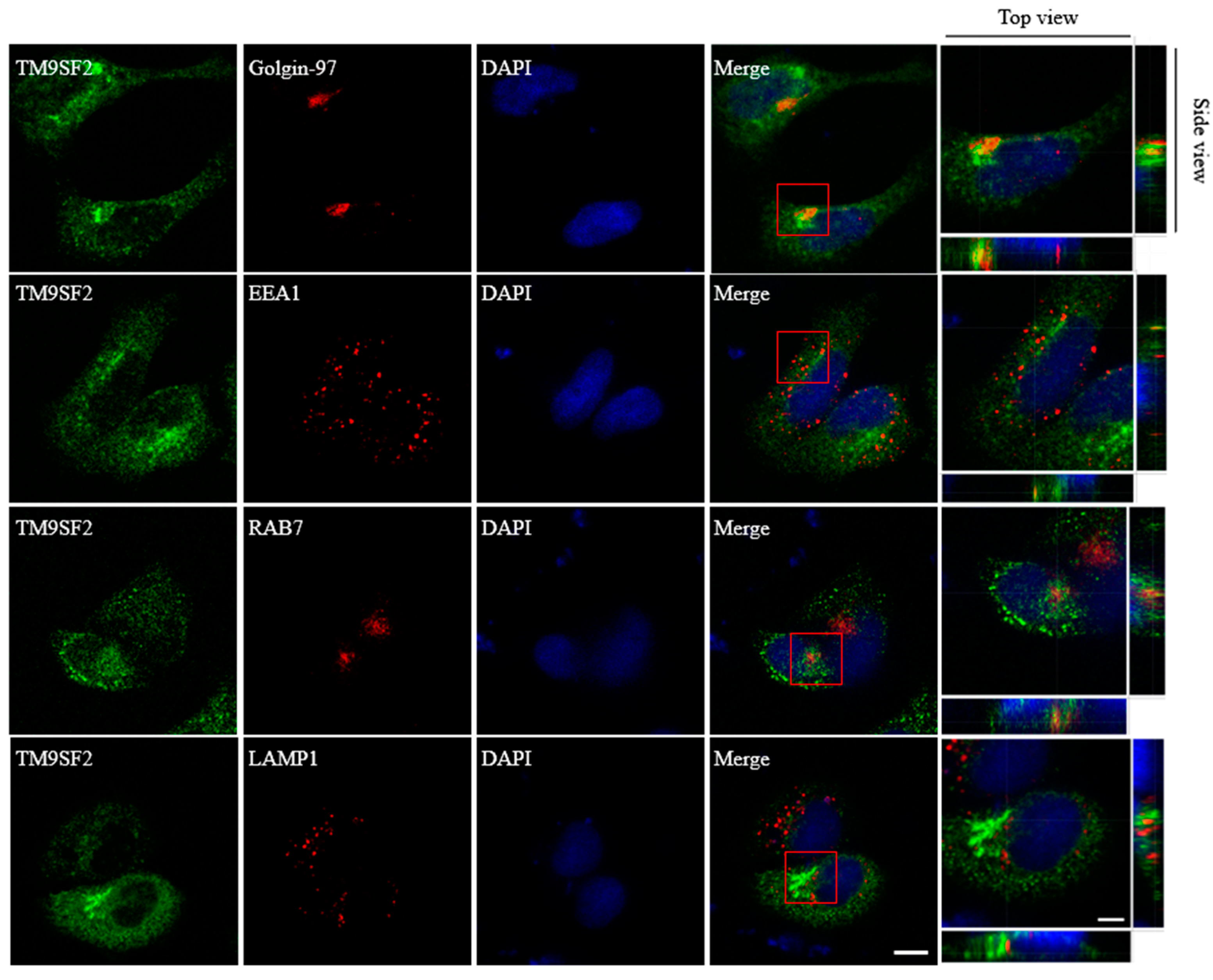
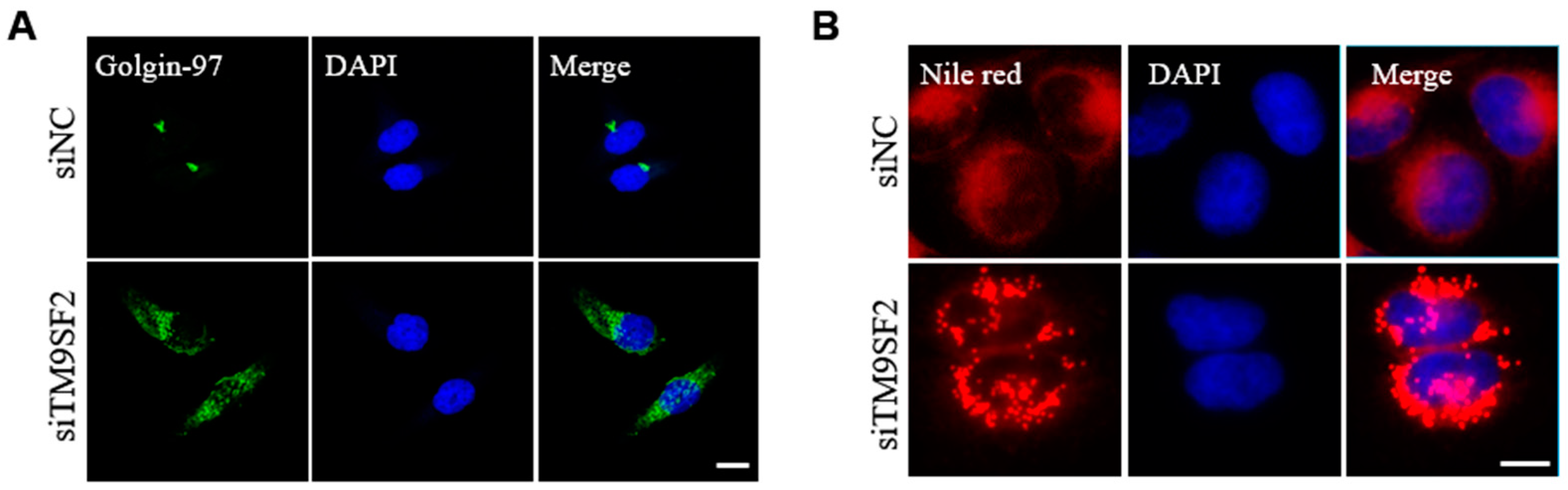
2.2. TM9SF2 Deficiency Impairs Cholesterol Translocation and Causes Fragmentation of Golgi
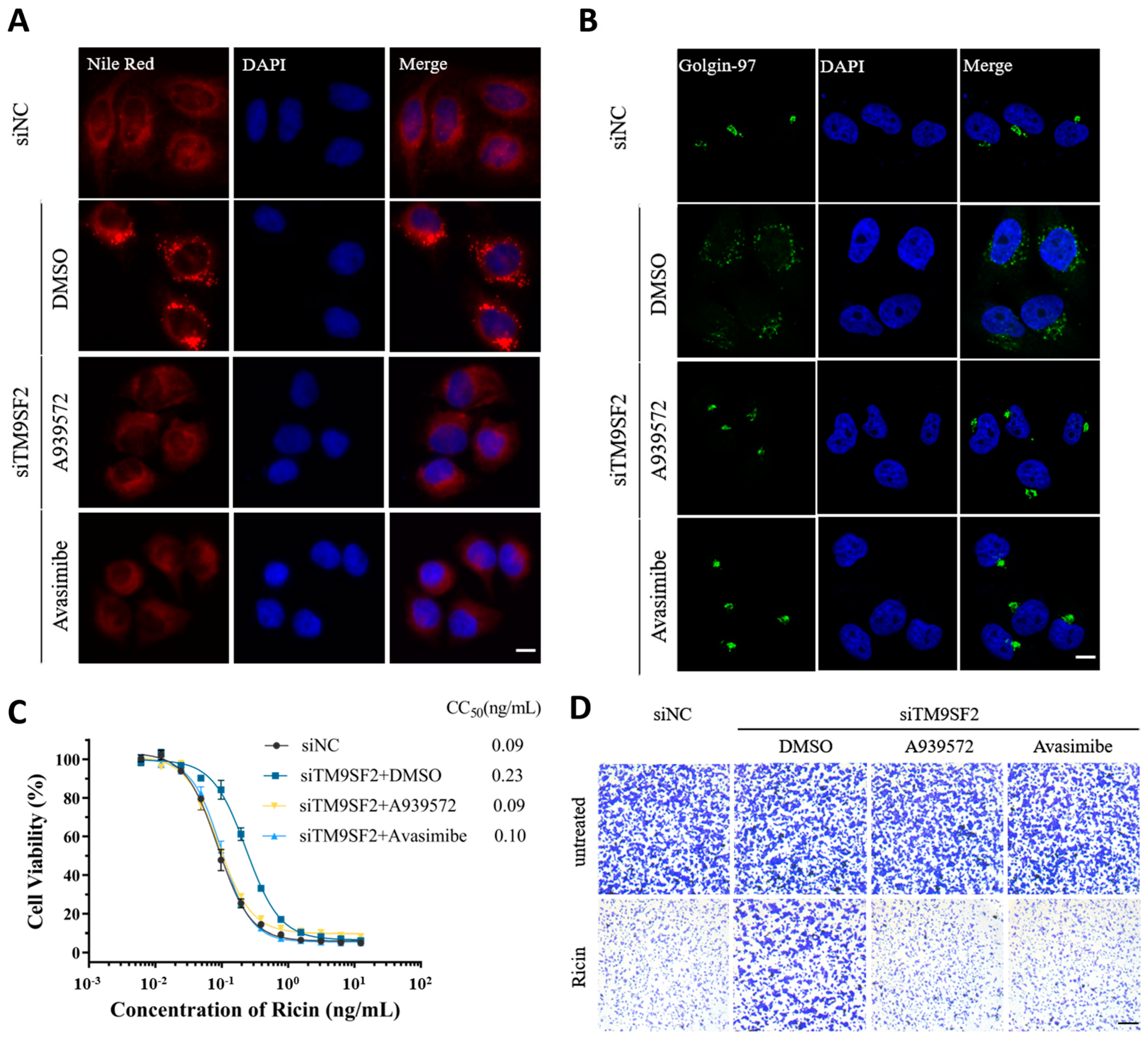
2.3. Pharmacological Intervention of Cholesterol Accumulation Restores Golgi Integrity and Ricin-Induced Cytotoxicity in TM9SF2 Knockdown Settings
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Antibodies, Reagents, and Cell Lines
5.2. Cell Culture Conditions
5.3. cDNA Constructs
5.4. Transient Transfection
5.5. Cell Viability Assay
5.6. Crystal Violet Staining Assay
5.7. qRT-PCR Assay
5.8. Western Blot
5.9. Immunofluorescence
5.10. Nile Red Staining Assay
5.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EEA1 | Early Endosome Antigen 1 |
| RAB7 | RAB7A, Member RAS Oncogene Family |
| LAMP1 | Lysosomal Associated Membrane Protein 1 |
| LDs | Lipid Droplets |
| siRNA | Small Interfering RNA |
| LD50 | Median Lethal Dose |
References
- Grela, P.; Szajwaj, M.; Horbowicz-Drożdżal, P.; Tchórzewski, M. How ricin damages the ribosome. Toxins 2019, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Joshi, K. A Review of Extraction and Detection of Ricin from Castor Plant and the Effect of Ricin on Humans. J. Stud. Res. 2022, 11. [Google Scholar] [CrossRef]
- Audi, J.; Belson, M.; Patel, M.; Schier, J.; Osterloh, J. Ricin poisoning: A comprehensive review. JAMA 2005, 294, 2342–2351. [Google Scholar] [CrossRef] [PubMed]
- Noumi Noumi, L.J.; El-Hanna, S.; Reine Sandrine Mendeuka, N.M.; Van Nuffelen, M. Ricin intoxication by lethal dose of castor seeds ingestion: A case report. J. Med. Case Rep. 2024, 18, 410. [Google Scholar] [CrossRef]
- Abbes, M.; Montana, M.; Curti, C.; Vanelle, P. Ricin poisoning: A review on contamination source, diagnosis, treatment, prevention and reporting of ricin poisoning. Toxicon 2021, 195, 86–92. [Google Scholar] [CrossRef]
- Moshiri, M.; Hamid, F.; Etemad, L. Ricin Toxicity: Clinical and Molecular Aspects. Rep. Biochem. Mol. Biol. 2016, 4, 60–65. [Google Scholar]
- Sowa-Rogozińska, N.; Sominka, H.; Nowakowska-Gołacka, J.; Sandvig, K.; Słomińska-Wojewódzka, M. Intracellular transport and cytotoxicity of the protein toxin ricin. Toxins 2019, 11, 350. [Google Scholar] [CrossRef]
- Tian, S.; Muneeruddin, K.; Choi, M.Y.; Tao, L.; Bhuiyan, R.H.; Ohmi, Y.; Furukawa, K.; Furukawa, K.; Boland, S.; Shaffer, S.A.; et al. Genome-wide CRISPR screens for Shiga toxins and ricin reveal Golgi proteins critical for glycosylation. PLoS Biol. 2018, 16, e2006951. [Google Scholar] [CrossRef]
- Sandvig, K.; Kavaliauskiene, S.; Skotland, T. The protein toxins ricin and Shiga toxin as tools to explore cellular mechanisms of internalization and intracellular transport. Toxins 2021, 13, 377. [Google Scholar] [CrossRef]
- Spooner, R.A.; Lord, J.M. Ricin trafficking in cells. Toxins 2015, 7, 49–65. [Google Scholar] [CrossRef]
- Yamaji, T.; Sekizuka, T.; Tachida, Y.; Sakuma, C.; Morimoto, K.; Kuroda, M.; Hanada, K. A CRISPR Screen Identifies LAPTM4A and TM9SF Proteins as Glycolipid-Regulating Factors. iScience 2019, 11, 409–424. [Google Scholar] [CrossRef]
- Clark, C.R.; Maile, M.; Blaney, P.; Hellweg, S.R.; Strauss, A.; Durose, W.; Priya, S.; Habicht, J.; Burns, M.B.; Blekhman, R. Transposon mutagenesis screen in mice identifies TM9SF2 as a novel colorectal cancer oncogene. Sci. Rep. 2018, 8, 15327. [Google Scholar] [CrossRef]
- Schimmöller, F.; Díaz, E.; Mühlbauer, B.; Pfeffer, S.R. Characterization of a 76 kDa endosomal, multispanning membrane protein that is highly conserved throughout evolution. Gene 1998, 216, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, A.R.; Lazarus, J.E.; Sit, B.; Schmieder, S.; Lencer, W.I.; Blondel, C.J.; Doench, J.G.; Davis, B.M.; Waldor, M.K. CRISPR Screen Reveals that EHEC’s T3SS and Shiga Toxin Rely on Shared Host Factors for Infection. mBio 2018, 9, e01003-18. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Tumkosit, U.; Nakamura, S.; Motooka, D.; Kishishita, N.; Priengprom, T.; Sa-Ngasang, A.; Kinoshita, T.; Takeda, N.; Maeda, Y. Genome-Wide Screening Uncovers the Significance of N-Sulfation of Heparan Sulfate as a Host Cell Factor for Chikungunya Virus Infection. J. Virol. 2017, 91, e00432-17. [Google Scholar] [CrossRef]
- Li, Q.; Lei, C.; Lu, C.; Wang, J.; Gao, M.; Gao, W. LINC01232 exerts oncogenic activities in pancreatic adenocarcinoma via regulation of TM9SF2. Cell Death Dis. 2019, 10, 698. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yang, H.; Song, B.-L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef]
- Anwar, M.U.; Sergeeva, O.A.; Abrami, L.; Mesquita, F.S.; Lukonin, I.; Amen, T.; Chuat, A.; Capolupo, L.; Liberali, P.; D’Angelo, G.; et al. ER-Golgi-localized proteins TMED2 and TMED10 control the formation of plasma membrane lipid nanodomains. Dev. Cell 2022, 57, 2334–2346.e8. [Google Scholar] [CrossRef]
- Sakuma, C.; Sekizuka, T.; Kuroda, M.; Hanada, K.; Yamaji, T. Identification of SYS1 as a host factor required for Shiga toxin-mediated cytotoxicity in Vero cells. Int. J. Mol. Sci. 2021, 22, 4936. [Google Scholar] [CrossRef]
- Tao, L.; Tian, S.; Zhang, J.; Liu, Z.; Robinson-McCarthy, L.; Miyashita, S.-I.; Breault, D.T.; Gerhard, R.; Oottamasathien, S.; Whelan, S.P. Sulfated glycosaminoglycans and low-density lipoprotein receptor contribute to Clostridium difficile toxin A entry into cells. Nat. Microbiol. 2019, 4, 1760–1769. [Google Scholar] [CrossRef]
- Sandvig, K.; Prydz, K.; Hansen, S.H.; van Deurs, B. Ricin transport in brefeldin A-treated cells: Correlation between Golgi structure and toxic effect. J. Cell Biol. 1991, 115, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Simm, R.; Kvalvaag, A.S.; van Deurs, B.; Lindbäck, T.; Sandvig, K. Benzyl alcohol induces a reversible fragmentation of the Golgi apparatus and inhibits membrane trafficking between endosomes and the trans-Golgi network. Exp. Cell Res. 2017, 357, 67–78. [Google Scholar] [CrossRef]
- Grimmer, S.; Iversen, T.G.; van Deurs, B.; Sandvig, K. Endosome to Golgi transport of ricin is regulated by cholesterol. Mol. Biol. Cell 2000, 11, 4205–4216. [Google Scholar] [CrossRef] [PubMed]
- Mesmin, B.; Bigay, J.; Polidori, J.; Jamecna, D.; Lacas-Gervais, S.; Antonny, B. Sterol transfer, PI4P consumption, and control of membrane lipid order by endogenous OSBP. EMBO J. 2017, 36, 3156–3174. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Depta, L.; Rossetti, C.; Caramelle, L.; Cigler, M.; Bryce-Rogers, H.P.; Michon, M.; Rafn Dan, O.; Hoock, J.; Barbier, J. Inhibition of OSBP blocks retrograde trafficking by inducing partial Golgi degradation. Nat. Chem. Biol. 2025, 21, 203–214. [Google Scholar] [CrossRef]
- Sasaki, K.; Adachi, T.; Morishita, F.; Toide, M.; Watanabe, Y.; Sakurai, H.T.; Wakabayashi, S.; Kusumi, S.; Yamaji, T.; Sakurai, K. The cholesterol pathway of the Golgi stress response induces cell death and transcription of Golgi-related genes through metabolic dysregulation of phosphatidylinositol-4-phosphate. bioRxiv 2023. [Google Scholar] [CrossRef]

| Sequences (5′ → 3′) | |
|---|---|
| siRNA-NC | GGAATCTCAYYCGATGATGCATCA |
| siRNA-TM9SF2 #1 | CCTTCACCATATAAGTTTACGTTTA |
| siRNA-TM9SF2 #2 | GTGTTACGATGTTGAAGAT |
| siRNA-TM9SF2 #3 | GCAGTACACTACTTCTTTT |
| Forward Primers (5′-3′) | Reverse Primers (5′-3′) | |
|---|---|---|
| TM9SF2 | ATGGGCGTCTAGATGGGACT | CCTGGGCATCTTCCGTAGAG |
| GAPDH | ATTCCATGGCACCGTCAAGG | TGGACTCCACGACGTACTCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Wan, H.; Wang, X.; Zhang, L.; Xin, R.; Li, L.; Wang, Y.; Xu, C.; Peng, H.; Sun, L.; et al. TM9SF2 Maintains Golgi Integrity and Regulates Ricin-Induced Cytotoxicity. Toxins 2025, 17, 218. https://doi.org/10.3390/toxins17050218
Meng Y, Wan H, Wang X, Zhang L, Xin R, Li L, Wang Y, Xu C, Peng H, Sun L, et al. TM9SF2 Maintains Golgi Integrity and Regulates Ricin-Induced Cytotoxicity. Toxins. 2025; 17(5):218. https://doi.org/10.3390/toxins17050218
Chicago/Turabian StyleMeng, Yue, Hongzhi Wan, Xinyu Wang, Lina Zhang, Ruozheng Xin, Lingyu Li, Yuhui Wang, Chengwang Xu, Hui Peng, Lu Sun, and et al. 2025. "TM9SF2 Maintains Golgi Integrity and Regulates Ricin-Induced Cytotoxicity" Toxins 17, no. 5: 218. https://doi.org/10.3390/toxins17050218
APA StyleMeng, Y., Wan, H., Wang, X., Zhang, L., Xin, R., Li, L., Wang, Y., Xu, C., Peng, H., Sun, L., Wang, B., & Duan, X. (2025). TM9SF2 Maintains Golgi Integrity and Regulates Ricin-Induced Cytotoxicity. Toxins, 17(5), 218. https://doi.org/10.3390/toxins17050218




