Electrical Cell Impedance Sensing (ECIS): Feasibility of a Novel In Vitro Approach to Studying Venom Toxicity and Potential Therapeutics
Abstract
1. Introduction
2. Results
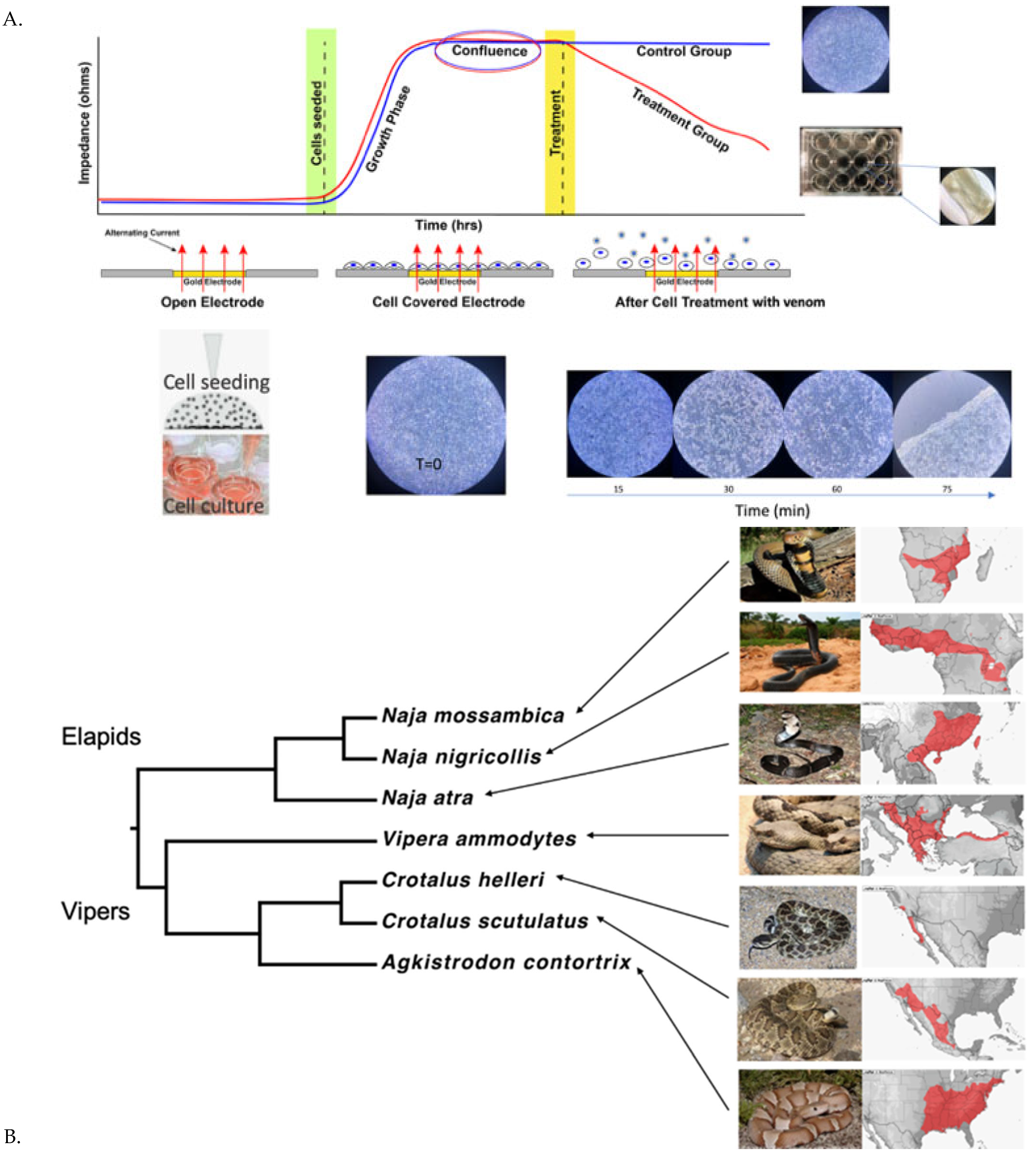
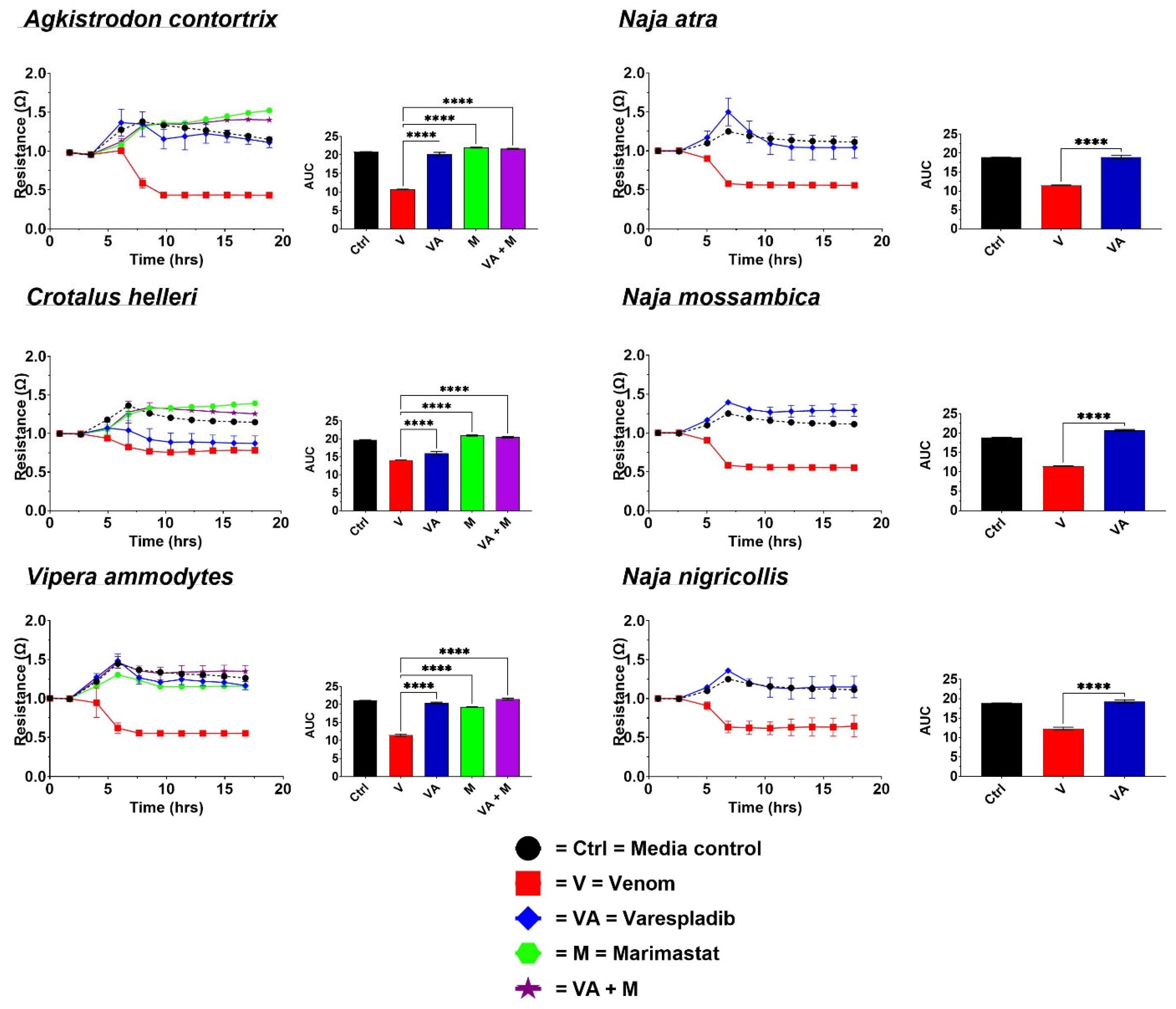
2.1. Cell Detachment Analysis—ECIS
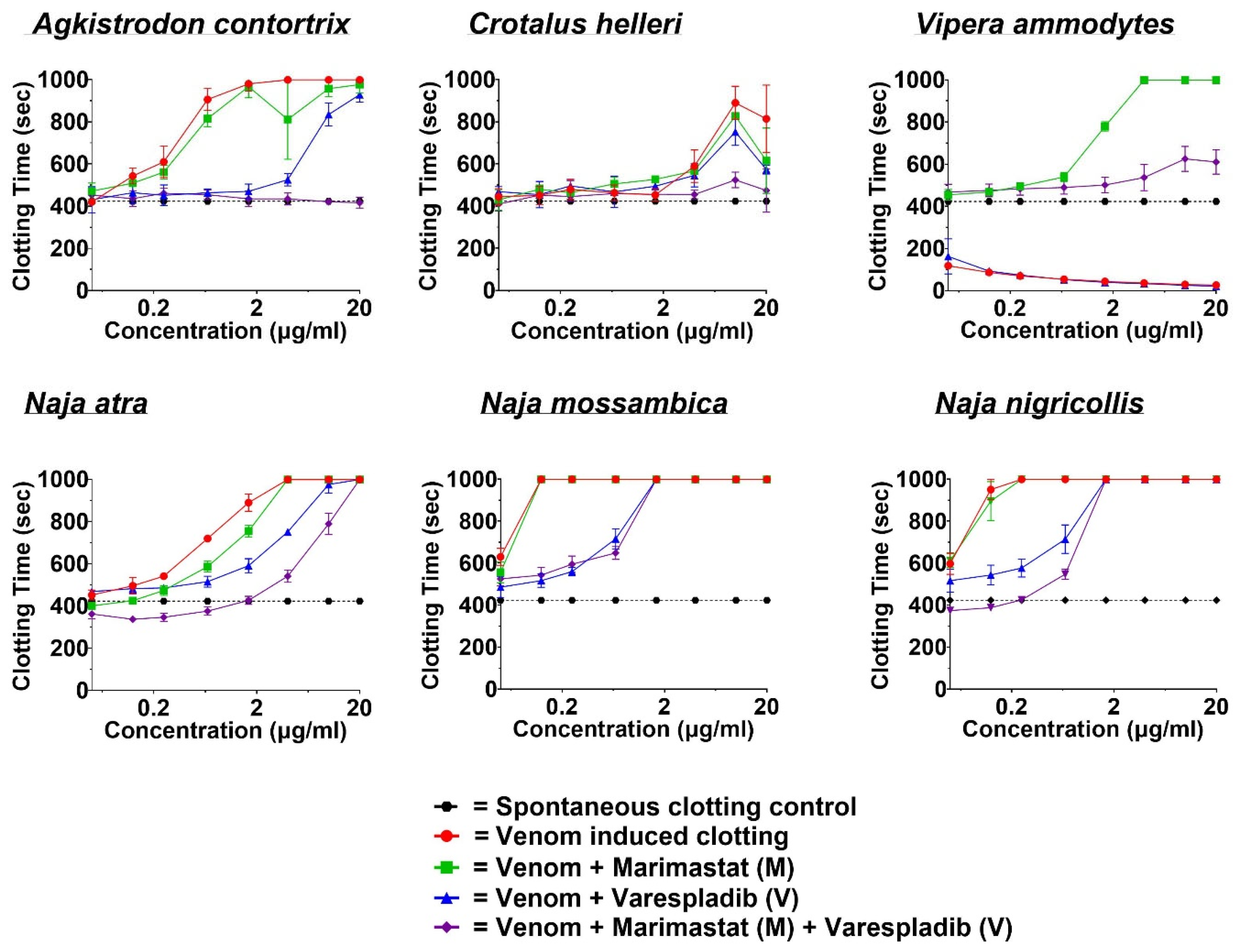
2.2. Coagulotoxicity Analysis—StarMax
3. Discussion
4. Materials and Methods
4.1. Approvals
4.2. Stock Preparation
4.2.1. Venoms
4.2.2. Culturing Protocol for H1975 Cells
4.2.3. Plasma
4.2.4. Direct Toxin Inhibitors (DTIs)
4.3. Assay Conditions
4.3.1. Cellular Detachment Effects Evaluation by Electric Cell-Substrate Impedance Sensing (ECIS)
4.3.2. Coagulotoxic Effect Evaluation by STA-R Max®
4.4. Data Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Minghui, R.; Malecela, M.N.; Cooke, E.; Abela-Ridder, B. Snakebite-Emerging from the Shadows of Neglect. Lancet 2019, 7, e837–e838. [Google Scholar]
- WHO Snakebite Envenoming. Available online: https://www.who.int/news-room/fact-sheets/detail/snakebite-envenoming (accessed on 26 August 2024).
- Chippaux, J.P. Snake-Bites: Appraisal of the Global Situation. Bull. World Health Organ. 1998, 76, 515–524. [Google Scholar] [PubMed]
- Roberts, N.L.S.; Johnson, E.K.; Zeng, S.M.; Hamilton, E.B.; Abdoli, A.; Alahdab, F.; Alipour, V.; Ancuceanu, R.; Andrei, C.L.; Anvari, D.; et al. Global Mortality of Snakebite Envenoming between 1990 and 2019. Nat. Commun. 2022, 13, 6160. [Google Scholar] [CrossRef]
- Zdenek, C.N.; Llinas, J.; Dobson, J.; Allen, L.; Dunstan, N.; Sousa, L.F.; Moura da Silva, A.M.; Fry, B.G. Pets in Peril: The Relative Susceptibility of Cats and Dogs to Procoagulant Snake Venoms. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 236, 108769. [Google Scholar] [CrossRef]
- Monsó, S.; Benz-Schwarzburg, J.; Bremhorst, A. Animal Morality: What It Means and Why It Matters. J. Ethics 2018, 22, 283. [Google Scholar] [CrossRef]
- Kiani, A.K.; Pheby, D.; Henehan, G.; Brown, R.; Sieving, P.; Sykora, P.; Marks, R.; Falsini, B.; Capodicasa, N.; Miertus, S.; et al. Ethical Considerations Regarding Animal Experimentation. J. Prev. Med. Hyg. 2022, 63, E255. [Google Scholar] [CrossRef]
- Humane Society International Costs of Animal and Non-Animal Testing—Humane Society International. Available online: https://www.hsi.org/news-resources/time_and_cost/ (accessed on 26 August 2024).
- Haas, J.; Manro, J.; Shannon, H.; Anderson, W.; Brozinick, J.; Chakravartty, A.; Chambers, M.; Du, J.; Eastwood, B.; Heuer, J.; et al. In Vivo Assay Guidelines. Assay Guid. Man. 2012, 1. [Google Scholar]
- Oeyen, M.; Meyen, E.; Doijen, J.; Schols, D. In-Depth Characterization of Zika Virus Inhibitors Using Cell-Based Electrical Impedance. Microbiol. Spectr. 2022, 10, e0049122. [Google Scholar] [CrossRef]
- Fallarero, A.; Batista-González, A.E.; Hiltunen, A.K.; Liimatainen, J.; Karonen, M.; Vuorela, P.M. Online Measurement of Real-Time Cytotoxic Responses Induced by Multi-Component Matrices, Such as Natural Products, through Electric Cell-Substrate Impedance Sensing (ECIS). Int. J. Mol. Sci. 2015, 16, 27044–27057. [Google Scholar] [CrossRef]
- Tolardo, V.; Magrì, D.; Fumagalli, F.; Cassano, D.; Athanassiou, A.; Fragouli, D.; Gioria, S. In Vitro High-Throughput Toxicological Assessment of Nanoplastics. Nanomaterials 2022, 12, 1947. [Google Scholar] [CrossRef]
- Huang, C.C.; Chiu, S.C.; Chao, S.C.; Liao, H.Y.; Lee, S.P.; Huang, C.C.; Cho, D.Y. Real-Time Monitoring of the Cytotoxic and Antimetastatic Properties of Cannabidiol in Human Oral Squamous Cell Carcinoma Cells Using Electric Cell-Substrate Impedance Sensing. Int. J. Mol. Sci. 2022, 23, 15842. [Google Scholar] [CrossRef] [PubMed]
- Cytotoxicity with ECIS—Applied BioPhysics. Available online: https://www.biophysics.com/cytotoxicity.php (accessed on 22 August 2024).
- Chowdhury, A.; Lewin, M.R.; Carter, R.W.; Casewell, N.R.; Fry, B.G. Keel Venom: Rhabdophis Subminiatus (Red-Necked Keelback) Venom Pathophysiologically Affects Diverse Blood Clotting Pathways. Toxicon 2022, 218, 19–24. [Google Scholar] [CrossRef]
- Chowdhury, A.; Youngman, N.J.; Liu, J.; Lewin, M.R.; Carter, R.W.; Fry, B.G. The Relative Efficacy of Chemically Diverse Small-Molecule Enzyme-Inhibitors against Anticoagulant Activities of Black Snake (Pseudechis Spp.) Venoms. Toxicol. Lett. 2022, 366, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Lewin, M.R.; Zdenek, C.N.; Carter, R.W.; Fry, B.G.; Youngman, N.J.; Liu, J.; Lewin, M.R.; Carter, R.W.; Fry, B.G. The Relative Efficacy of Chemically Diverse Small-Molecule Enzyme-Inhibitors Against Anticoagulant Activities of African Spitting Cobra (Naja Species) Venoms. Front. Immunol. 2021, 12, 752442. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Zdenek, C.N.; Dobson, J.S.; Bourke, L.A.; Soria, R.; Fry, B.G. Clinical Implications of Differential Procoagulant Toxicity of the Palearctic Viperid Genus Macrovipera, and the Relative Neutralization Efficacy of Antivenoms and Enzyme Inhibitors. Toxicol. Lett. 2021, 340, 77–88. [Google Scholar] [CrossRef]
- Chowdhury, A.; Zdenek, C.N.; Lewin, M.R.; Carter, R.; Jagar, T.; Ostanek, E.; Harjen, H.; Aldridge, M.; Soria, R.; Haw, G.; et al. Venom-Induced Blood Disturbances by Palearctic Viperid Snakes, and Their Relative Neutralization by Antivenoms and Enzyme-Inhibitors. Front. Immunol. 2021, 12, 688802. [Google Scholar] [CrossRef]
- Youngman, N.J.; Lewin, M.R.; Carter, R.; Naude, A.; Fry, B.G. Efficacy and Limitations of Chemically Diverse Small-Molecule Enzyme-Inhibitors against the Synergistic Coagulotoxic Activities of Bitis Viper Venoms. Molecules 2022, 27, 1733. [Google Scholar] [CrossRef]
- Youngman, N.J.; Walker, A.; Naude, A.; Coster, K.; Sundman, E.; Fry, B.G. Varespladib (LY315920) Neutralises Phospholipase A 2 Mediated Prothrombinase-Inhibition Induced by Bitis Snake Venoms. Comp. Biochem. Physiol. Part C 2020, 236, 108818. [Google Scholar] [CrossRef]
- Wasserberger, J.; Ordog, G.; Merkin, T.E. Southern Pacific Rattlesnake Bite: A Unique Clinical Challenge. J. Emerg. Med. 2006, 31, 263–266. [Google Scholar] [CrossRef]
- Parrish, H.M.; Carr, C.A. Bites by Copperheads (Ancistrodon Contortrix) in the United States. J. Am. Med. Assoc. 1967, 201, 927–932. [Google Scholar] [CrossRef]
- Warrell, D.A.; Ormerod, L.D. Snake Venom Ophthalmia and Blindness Caused by the Spitting Cobra (Naja Nigricollis) in Nigeria. Am. J. Trop. Med. Hyg. 1976, 25, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J. Venom Ophthalmia from Naja Mossambica in KwaZulu Natal, South Africa: A Reminder to All That for Ocular Chemical Injury, Dilution Is the Solution. Trop. Doct. 2015, 45, 250–251. [Google Scholar] [CrossRef] [PubMed]
- Paolino, G.; Di Nicola, M.R.; Pontara, A.; Didona, D.; Moliterni, E.; Mercuri, S.R.; Grano, M.; Borgianni, N.; Kumar, R.; Pampena, R. Vipera Snakebite in Europe: A Systematic Review of a Neglected Disease. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2247–2260. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Sung, W.C.; Mu, H.W.; Hung, D.Z. Local Cytotoxic Effects in Cobra Envenoming: A Pilot Study. Toxins 2022, 14, 122. [Google Scholar] [CrossRef]
- Warrell, D.A.; Greenwood, B.M.; Davidson, N.M.; Ormerod, L.D.; Prentice, C.R.M. Necrosis, Haemorrhage and Complement Depletion Following Bites by the Spitting Cobra. QJM 1976, 45, 2–22. [Google Scholar] [CrossRef]
- Casewell, N.R.; Sunagar, K.; Takacs, Z.; Calvete, J.J.; Jackson, T.N.; Fry, B.G. Snake Venom Metalloprotease Enzymes. In Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery; Fry, B.G., Ed.; Oxford University Press: New York, NY, USA, 2015; pp. 347–363. ISBN 9780199309399. [Google Scholar]
- Sunagar, K.; Jackson, T.; Reeks, T.; Fry, B.G. Group I Phospholipase A2 Enzymes. In Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery; Oxford University Press: New York, NY, USA, 2015; pp. 327–334. [Google Scholar]
- Ferraz, C.R.; Arrahman, A.; Xie, C.; Casewell, N.R.; Lewis, R.J.; Kool, J.; Cardoso, F.C. Multifunctional Toxins in Snake Venoms and Therapeutic Implications: From Pain to Hemorrhage and Necrosis. Front. Ecol. Evol. 2019, 7, 218. [Google Scholar] [CrossRef]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.A.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S.; et al. The Toxicogenomic Multiverse: Convergent Recruitment of Proteins into Animal Venoms. Annu. Rev. Genomics Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef]
- Sunagar, K.; Tsai, I.; Lomonte, B.; Jackson, T.N.; Reeks, T.; Fry, B.G. Group II Phospholipase A2 Enzymes. In Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery; Oxford University Press: New York, NY, USA, 2015; pp. 335–346. [Google Scholar]
- Murakami, T.; Sinha, M.; Kumar, R.P.; Kaur, P.; Kumar, S.; Dey, S.; Sharma, S.; Vrielink, A.; Betzel, C.; Takeda, S.; et al. Enzymatic Toxins from Snake Venom: Structural Characterization and Mechanism of Catalysis. FEBS J. 2011, 278, 4544–4576. [Google Scholar] [CrossRef]
- Gasanov, S.E.; Dagda, R.K.; Rael, E.D. Snake Venom Cytotoxins, Phospholipase A2s, and Zn2+-Dependent Metalloproteinases: Mechanisms of Action and Pharmacological Relevance. J. Clin. Toxicol. 2014, 4, 217–218. [Google Scholar] [CrossRef]
- Jin Hiu, J.; Khai Khun Yap, M.; Michelle Khai Khun, Y.; Hiu, J.J.; Yap, M.K.K. Cytotoxicity of Snake Venom Enzymatic Toxins: Phospholipase A 2 and L-Amino Acid Oxidase. Biochem. Soc. Trans. 2020, 48, 719. [Google Scholar] [CrossRef]
- Bittenbinder, M.A.; van Thiel, J.; Cardoso, F.C.; Casewell, N.R.; Gutiérrez, J.M.; Kool, J.; Vonk, F.J. Tissue Damaging Toxins in Snake Venoms: Mechanisms of Action, Pathophysiology and Treatment Strategies. Commun. Biol. 2024, 7, 358. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M.; Rao, V.S.; Joseph, J.S. Procoagulant Proteins from Snake Venoms. Haemostasis 2001, 31, 218–224. [Google Scholar] [CrossRef]
- Kini, R.M.; Evans, H.J. Structure-Function Relationships of Phospholipases the Anticoagulant Region of Phospholipases A. J. Biol. Chem. 1987, 262, 14402–14407. [Google Scholar] [CrossRef]
- Casewell, N.R.; Jackson, T.N.W.; Laustsen, A.H.; Sunagar, K. Causes and Consequences of Snake Venom Variation. Trends Pharmacol. Sci. 2020, 41, 570–581. [Google Scholar] [CrossRef]
- Seifert, S.A.; Armitage, J.O.; Sanchez, E.E. Snake Envenomation. N. Engl. J. Med. 2022, 386, 68–78. [Google Scholar] [CrossRef]
- Bénard-Valle, M.; Neri-Castro, E.E.; Fry, B.G.; Boyer, L.; Cochran, C.; Alam, M.; Jackson, T.N.W.; Paniagua, D.; Olvera-Rodríguez, F.; Koludarov, I.; et al. Antivenom Research and Development. In Venomous Reptiles and Their Toxins: Evolution, Pathophysiology, and Biodiscovery; Oxford University Press: New York, NY, USA, 2015; pp. 61–72. ISBN 9780199309405. [Google Scholar]
- Habibid, A.G.; Musa, M.; Iliyasuid, G.; Hamza, M.; Kuznik, A.; Chippauxid, J.-P. Challenges and Prospects of Snake Antivenom Supply in Sub-Saharan Africa. PLoS Negl. Trop. Dis. 2020, 14, e0008374. [Google Scholar] [CrossRef]
- Carter, R.W.; Gerardo, C.J.; Samuel, S.P.; Kumar, S.; Kotehal, S.D.; Mukherjee, P.P.; Shirazi, F.M.; Akpunonu, P.D.; Bammigatti, C.; Bhalla, A.; et al. The BRAVO Clinical Study Protocol: Oral Varespladib for Inhibition of Secretory Phospholipase A2 in the Treatment of Snakebite Envenoming. Toxins 2023, 15, 22. [Google Scholar] [CrossRef]
- Bittenbinder, M.A.; Dobson, J.S.; Zdenek, C.N.; op den Brouw, B.; Naude, A.; Vonk, F.J.; Fry, B.G. Differential Destructive (Non-Clotting) Fibrinogenolytic Activity in Afro-Asian Elapid Snake Venoms and the Links to Defensive Hooding Behavior. Toxicol. Vitr. 2019, 60, 330–335. [Google Scholar] [CrossRef]
- Jagadeesha, D.K.; Shashidhara murthy, R.; Girish, K.S.; Kemparaju, K. A Non-Toxic Anticoagulant Metalloprotease: Purification and Characterization from Indian Cobra (Naja Naja Naja) Venom. Toxicon 2002, 40, 667–675. [Google Scholar] [CrossRef]
- Evans, H.J. Cleavage of the Aα-Chain of Fibrinogen and the α-Polymer of Fibrin by the Venom of Spitting Cobra (Naja Nigricollis). Biochim. Biophys. Acta Enzymol. 1981, 660, 219–226. [Google Scholar] [CrossRef]
- Guan, H.H.; Goh, K.S.; Davamani, F.; Wu, P.L.; Huang, Y.W.; Jeyakanthan, J.; Wu, W.G.; Chen, C.J. Structures of Two Elapid Snake Venom Metalloproteases with Distinct Activities Highlight the Disulfide Patterns in the D Domain of ADAMalysin Family Proteins. J. Struct. Biol. 2010, 169, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.S.; Devaraj, V.R.; Vishwanath, B.S.; Kemparaju, K. Anti-Coagulant Activity of a Metalloprotease: Further Characterization from the Indian Cobra (Naja Naja) Venom. J. Thromb. Thrombolysis 2010, 29, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.M.; Vinogradov, D.V.; Andrews, R.K.; Berndt, M.C. Characterization of Mocarhagin, a Cobra Venom Metalloproteinase from Naja Mocambique Mocambique, and Related Proteins from Other Elapidae Venoms. Toxicon 1996, 34, 1203–1206. [Google Scholar] [CrossRef]
- Román-Domínguez, L.; Neri-Castro, E.; Vázquez López, H.; García-Osorio, B.; Archundia, I.G.; Ortiz-Medina, J.A.; Petricevich, V.L.; Alagón, A.; Bénard-Valle, M. Biochemical and Immunochemical Characterization of Venoms from Snakes of the Genus Agkistrodon. Toxicon X 2019, 4, 100013. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, F.C.P.; Sanchez, E.; María Gutiérrez, J.; Calvete, J.J.; Fry, B.G. Blood Lines: Intraspecific and Interspecific Variations in Anticoagulant Actions of Agkistrodon Viperid Venoms. Toxins 2024, 16, 291. [Google Scholar] [CrossRef]
- Greenbaum, E.; Galeva, N.; Jorgensen, M. Venom Variation and Chemoreception of the Viperid Agkistrodon Contortrix: Evidence for Adaptation? J. Chem. Ecol. 2003, 29, 1741–1755. [Google Scholar] [CrossRef]
- House, L.M.L.; Lewin, M.R.; Naidu, R.K.; Beqaj, H. Complex Regional Pain Syndrome Following Southern Pacific Rattlesnake (C. Oreganus Helleri) Envenoming. Clin. Case Rep. 2021, 9, e05019. [Google Scholar] [CrossRef]
- Levine, M.; Tashman, D.; Recchio, I.; Friedman, N.; Seltzer, J.; Minns, A.; LoVecchio, F. Neurotoxicity Associated With the Southern Pacific Rattlesnake (Crotalus Helleri). Ann. Emerg. Med. 2023, 81, 318–322. [Google Scholar] [CrossRef]
- Dobson, J.; Yang, D.C.; op den Brouw, B.; Cochran, C.; Huynh, T.; Kurrupu, S.; Sánchez, E.E.; Massey, D.J.; Baumann, K.; Jackson, T.N.W.; et al. Rattling the Border Wall: Pathophysiological Implications of Functional and Proteomic Venom Variation between Mexican and US Subspecies of the Desert Rattlesnake Crotalus Scutulatus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 205, 62–69. [Google Scholar] [CrossRef]
- French, W.J.; Hayes, W.K.; Bush, S.P.; Cardwell, M.D.; Bader, J.O.; Rael, E.D. Mojave Toxin in Venom of Crotalus Helleri (Southern Pacific Rattlesnake): Molecular and Geographic Characterization. Toxicon 2004, 44, 781–791. [Google Scholar] [CrossRef]
- Sunagar, K.; Undheim, E.A.B.; Scheib, H.; Gren, E.C.K.; Cochran, C.; Person, C.E.; Koludarov, I.; Kelln, W.; Hayes, W.K.; King, G.F.; et al. Intraspecific Venom Variation in the Medically Significant Southern Pacific Rattlesnake (Crotalus Oreganus Helleri): Biodiscovery, Clinical and Evolutionary Implications. J. Proteom. 2014, 99, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, E.E.; González, R.; Lucena, S.; García, S.; Finol, H.J.; Suntravat, M.; Girón, M.E.; Fernández, I.; Rodríguez-Acosta, A. Crotamine-like from Southern Pacific Rattlesnake (Crotalus Oreganus Helleri) Venom Acts on Human Leukemia (K-562) Cell Lines and Produces Ultrastructural Changes on Mice Adrenal Gland. Ultrastruct. Pathol. 2018, 42, 116–123. [Google Scholar] [CrossRef]
- Salazar, A.M.; Guerrero, B.; Cantu, B.; Cantu, E.; Rodríguez-Acosta, A.; Pérez, J.C.; Galán, J.A.; Tao, A.; Sánchez, E.E. Venom Variation in Hemostasis of the Southern Pacific Rattlesnake (Crotalus Oreganus Helleri): Isolation of Hellerase. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 149, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Franco-Servín, C.; Neri-Castro, E.; Bénard-Valle, M.; Alagón, A.; Rosales-García, R.A.; Guerrero-Alba, R.; Poblano-Sánchez, J.E.; Silva-Briano, M.; Guerrero-Barrera, A.L.; Sigala-Rodríguez, J.J. Biological and Biochemical Characterization of Coronado Island Rattlesnake (Crotalus Helleri Caliginis) Venom and Antivenom Neutralization. Toxins 2021, 13, 582. [Google Scholar] [CrossRef] [PubMed]
- Mebs, D.; Langelüddeke, T. European Viper Venoms: Haemorrhagic and Myotoxic Activities. Toxicon 1992, 30, 1303–1306. [Google Scholar] [CrossRef]
- Karlo, R.; Dželalija, B.; Župančić, B.; Bačić, I.; Dunatov, T.; Kanjer, A.; Škarica, R.; Sabalić, S.; Bukvic, N.; Nikolić, H.; et al. Venomous Snakebites in the Croatian North Dalmatia Region. Cent. Eur. J. Med. 2011, 123, 732–737. [Google Scholar] [CrossRef]
- Lukšić, B.; Čulić, V.; Stričević, L.; Brizić, I.; Poljak, N.K.; Tadić, Z. Infant Death after Nose-Horned Viper (Vipera Ammodytes Ammodytes) Bite in Croatia: A Case Report. Toxicon 2010, 56, 1506–1509. [Google Scholar] [CrossRef]
- Leonardi, A.; Sajevic, T.; Pungerčar, J.; Križaj, I. Comprehensive Study of the Proteome and Transcriptome of the Venom of the Most Venomous European Viper: Discovery of a New Subclass of Ancestral Snake Venom Metalloproteinase Precursor-Derived Proteins. J. Proteome Res. 2019, 18, 2287–2309. [Google Scholar] [CrossRef]
- Georgieva, D.; Risch, M.; Kardas, A.; Buck, F.; Von Bergen, M.; Betzel, C. Comparative Analysis of the Venom Proteomes of Vipera Ammodytes Ammodytes and Vipera Ammodytes Meridionalis. J. Proteome Res. 2008, 7, 866–886. [Google Scholar] [CrossRef]
- İğci, N.; Nalbantsoy, A.; Erkan, L.G.; Akça, G.Y.; Yalçın, H.T.; Yalçın, M.; Göçmen, B. Screening of Cytotoxic, Anti-Angiogenic, Anti-Tumorogenic and Antimicrobial Activities of Anatolian Vipera Ammodytes (Nose-Horned Viper) Venom. Turkish J. Biochem. 2016, 41, 483–491. [Google Scholar] [CrossRef]
- Leonardi, A.; Sajevic, T.; Kovačič, L.; Pungerčar, J.; Lang Balija, M.; Halassy, B.; Trampuš Bakija, A.; Križaj, I. Hemorrhagin VaH4, a Covalent Heterodimeric P-III Metalloproteinase from Vipera Ammodytes Ammodytes with a Potential Antitumour Activity. Toxicon 2014, 77, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Latinović, Z.; Leonardi, A.; Koh, C.Y.; Kini, R.M.; Trampuš Bakija, A.; Pungerčar, J.; Križaj, I. The Procoagulant Snake Venom Serine Protease Potentially Having a Dual, Blood Coagulation Factor V and X-Activating Activity. Toxins 2020, 12, 358. [Google Scholar] [CrossRef] [PubMed]
- Kuzon, W.M.; Marcus, J.R.; Kerluke, L.D.; Phillips, J.H. African Spitting Cobra (Naja Nigricollis) Bite of the Hand. Can. J. Plast. Surg. 1994, 2, 90–92. [Google Scholar] [CrossRef]
- Panagides, N.; Jackson, T.N.W.; Ikonomopoulou, M.P.; Arbuckle, K.; Pretzler, R.; Yang, D.C.; Ali, S.A.; Koludarov, I.; Dobson, J.; Sanker, B.; et al. How the Cobra Got Its Flesh-Eating Venom: Cytotoxicity as a Defensive Innovation and Its Co-Evolution with Hooding, Aposematic Marking, and Spitting. Toxins 2017, 9, 103. [Google Scholar] [CrossRef]
- Huang, H.W.; Liu, B.S.; Chien, K.Y.; Chiang, L.C.; Huang, S.Y.; Sung, W.C.; Wu, W.G. Cobra Venom Proteome and Glycome Determined from Individual Snakes of Naja Atra Reveal Medically Important Dynamic Range and Systematic Geographic Variation. J. Proteom. 2015, 128, 92–104. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Zhang, X.; Ren, V.; Wang, N.; Zhao, K.; Chen, X.; Zhao, C.; Li, X.; Shao, J.; et al. Proteomic Characterization of Two Snake Venoms: Naja Naja Atra and Agkistrodon Halys. Biochem. J. 2004, 384, 119–127. [Google Scholar] [CrossRef]
- Méndez, I.; Gutiérrez, J.M.; Angulo, Y.; Calvete, J.J.; Lomonte, B. Comparative Study of the Cytolytic Activity of Snake Venoms from African Spitting Cobras (Naja Spp., Elapidae) and Its Neutralization by a Polyspecific Antivenom. Toxicon 2011, 58, 558–564. [Google Scholar] [CrossRef]
- Bartlett, K.E.; Hall, S.R.; Rasmussen, S.A.; Crittenden, E.; Dawson, C.A.; Albulescu, L.O.; Laprade, W.; Harrison, R.A.; Saviola, A.J.; Modahl, C.M.; et al. Dermonecrosis Caused by a Spitting Cobra Snakebite Results from Toxin Potentiation and Is Prevented by the Repurposed Drug Varespladib. Proc. Natl. Acad. Sci. USA 2024, 121, e2315597121. [Google Scholar] [CrossRef]
- Bittenbinder, M.A.; Zdenek, C.N.; op den Brouw, B.; Youngman, N.J.; Dobson, J.S.; Naude, A.; Vonk, F.J.; Fry, B.G. Coagulotoxic Cobras: Clinical Implications of Strong Anticoagulant Actions of African Spitting Naja Venoms That Are Not Neutralised by Antivenom but Are by LY315920 (Varespladib). Toxins 2018, 10, 516. [Google Scholar] [CrossRef]
- Bulfone, T.C.; Samuel, S.P.; Bickler, P.E.; Lewin, M.R. Developing Small Molecule Therapeutics for the Initial and Adjunctive Treatment of Snakebite. J. Trop. Med. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Puzari, M.U.; Fernandes, P.A.; Mukherjee, A.K. Advances in the Therapeutic Application of Small-Molecule Inhibitors and Repurposed Drugs against Snakebite. J. Med. Chem. 2021, 64, 13938–13979. [Google Scholar] [CrossRef] [PubMed]
- Salvador, G.H.M.; Gomes, A.A.S.; Bryan-Quirós, W.; Fernández, J.; Lewin, M.R.; Gutiérrez, J.M.; Lomonte, B.; Fontes, M.R.M. Structural Basis for Phospholipase A2-like Toxin Inhibition by the Synthetic Compound Varespladib (LY315920). Sci. Rep. 2019, 9, 17203. [Google Scholar] [CrossRef] [PubMed]
- Salvador, G.H.M.; Borges, R.J.; Lomonte, B.; Lewin, M.R.; Fontes, M.R.M. The Synthetic Varespladib Molecule Is a Multi-Functional Inhibitor for PLA2 and PLA2-like Ophidic Toxins. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129913. [Google Scholar] [CrossRef] [PubMed]
- Lewin, M.R.; Carter, R.W.; Matteo, I.A.; Samuel, S.P.; Rao, S.; Fry, B.G.; Bickler, P.E. Varespladib in the Treatment of Snakebite Envenoming: Development History and Preclinical Evidence Supporting Advancement to Clinical Trials in Patients Bitten by Venomous Snakes. Toxins 2022, 14, 783. [Google Scholar] [CrossRef]
- Salvador, G.H.M.; Pinto, Ê.K.R.; Ortolani, P.L.; Fortes-Dias, C.L.; Cavalcante, W.L.G.; Soares, A.M.; Lomonte, B.; Lewin, M.R.; Fontes, M.R.M. Structural Basis of the Myotoxic Inhibition of the Bothrops Pirajai PrTX-I by the Synthetic Varespladib. Biochimie 2023, 207, 1–10. [Google Scholar] [CrossRef]
- Hall, S.R.; Rasmussen, S.A.; Crittenden, E.; Dawson, C.A.; Bartlett, K.E.; Westhorpe, A.P.; Albulescu, L.O.; Kool, J.; Gutiérrez, J.M.; Casewell, N.R. Repurposed Drugs and Their Combinations Prevent Morbidity-Inducing Dermonecrosis Caused by Diverse Cytotoxic Snake Venoms. Nat. Commun. 2023, 14, 781. [Google Scholar] [CrossRef]
- Layfield, H.J.; Williams, H.F.; Ravishankar, D.; Mehmi, A.; Sonavane, M.; Salim, A.; Vaiyapuri, R.; Lakshminarayanan, K.; Vallance, T.M.; Bicknell, A.B.; et al. Repurposing Cancer Drugs Batimastat and Marimastat to Inhibit the Activity of a Group I Metalloprotease from the Venom of the Western Diamondback Rattlesnake, Crotalus Atrox. Toxins 2020, 12, 309. [Google Scholar] [CrossRef]
- Clare, R.H.; Dawson, C.A.; Westhorpe, A.; Albulescu, L.O.; Woodley, C.M.; Mosallam, N.; Chong, D.J.W.; Kool, J.; Berry, N.G.; O’Neill, P.M.; et al. Snakebite Drug Discovery: High-Throughput Screening to Identify Novel Snake Venom Metalloproteinase Toxin Inhibitors. Front. Pharmacol. 2023, 14, 1328950. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Lewin, M.R.; Williams, D.J.; Lomonte, B. Varespladib (LY315920) and Methyl Varespladib (LY333013) Abrogate or Delay Lethality Induced by Presynaptically Acting Neurotoxic Snake Venoms. Toxins 2020, 12, 131. [Google Scholar] [CrossRef]
- Lewin, M.R.; Gilliam, L.L.; Gilliam, J.; Samuel, S.P.; Bulfone, T.C.; Bickler, P.E.; Gutiérrez, J.M. Delayed LY333013 (Oral) and LY315920 (Intravenous) Reverse Severe Neurotoxicity and Rescue Juvenile Pigs from Lethal Doses of Micrurus Fulvius (Eastern Coral Snake) Venom. Toxins 2018, 10, 479. [Google Scholar] [CrossRef]
- da Silva, G.M.; Chowdhury, A. Enhancing Snakebite Management: The Role of Small Molecule Therapeutics in Complementing Antivenom Strategies. Toxicon 2024, 249, 108081. [Google Scholar] [CrossRef]
- Applied BioPhysics Publications Using ECIS. Available online: https://www.biophysics.com/publications.php (accessed on 26 August 2024).
- Applied BioPhysics Operation Manual for All ECIS Systems; Applied BioPhysics: Troy, NY, USA, 2023.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choudhury, A.; Linne, K.; Bulfone, T.C.; Hossain, T.; Sina, A.A.I.; Bickler, P.L.; Fry, B.G.; Lewin, M.R. Electrical Cell Impedance Sensing (ECIS): Feasibility of a Novel In Vitro Approach to Studying Venom Toxicity and Potential Therapeutics. Toxins 2025, 17, 193. https://doi.org/10.3390/toxins17040193
Choudhury A, Linne K, Bulfone TC, Hossain T, Sina AAI, Bickler PL, Fry BG, Lewin MR. Electrical Cell Impedance Sensing (ECIS): Feasibility of a Novel In Vitro Approach to Studying Venom Toxicity and Potential Therapeutics. Toxins. 2025; 17(4):193. https://doi.org/10.3390/toxins17040193
Chicago/Turabian StyleChoudhury, Abhinandan, Kaitlin Linne, Tommaso C. Bulfone, Tanvir Hossain, Abu Ali Ibn Sina, Philip L. Bickler, Bryan G. Fry, and Matthew R. Lewin. 2025. "Electrical Cell Impedance Sensing (ECIS): Feasibility of a Novel In Vitro Approach to Studying Venom Toxicity and Potential Therapeutics" Toxins 17, no. 4: 193. https://doi.org/10.3390/toxins17040193
APA StyleChoudhury, A., Linne, K., Bulfone, T. C., Hossain, T., Sina, A. A. I., Bickler, P. L., Fry, B. G., & Lewin, M. R. (2025). Electrical Cell Impedance Sensing (ECIS): Feasibility of a Novel In Vitro Approach to Studying Venom Toxicity and Potential Therapeutics. Toxins, 17(4), 193. https://doi.org/10.3390/toxins17040193





