Bite First, Bleed Later: How Philippine Trimeresurus Pit Viper Venoms Hijack Blood Clotting
Abstract
1. Introduction
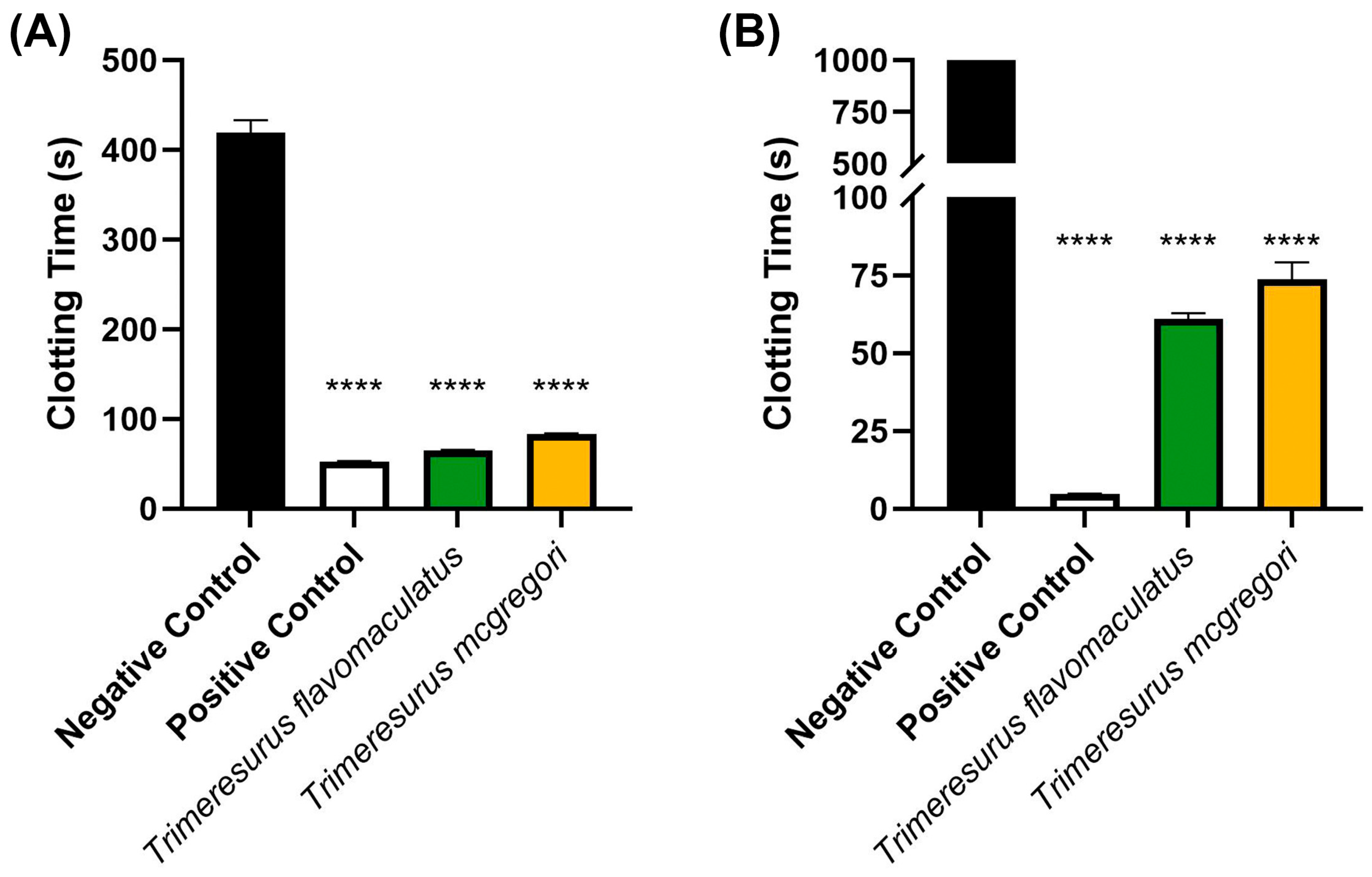
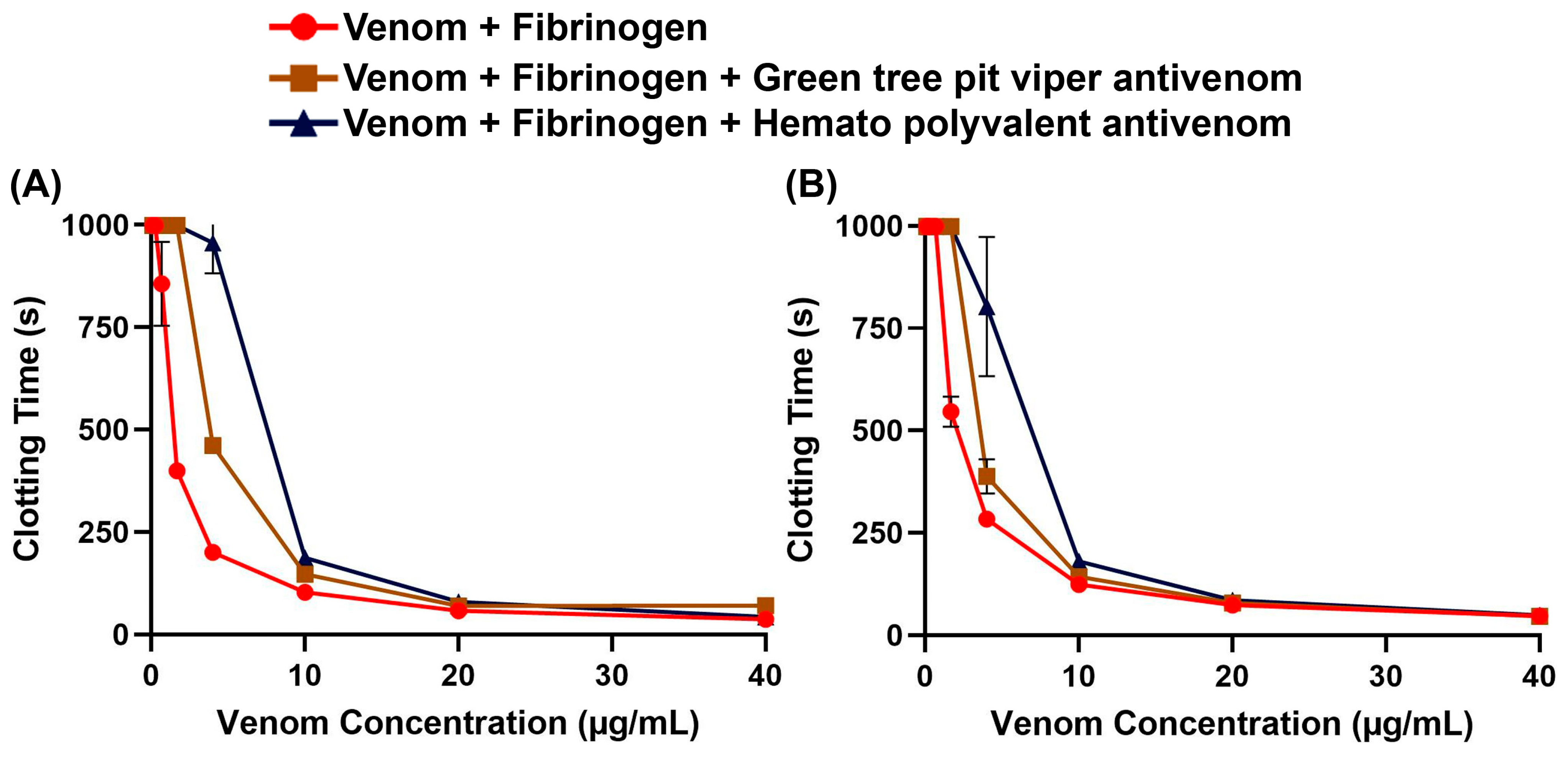
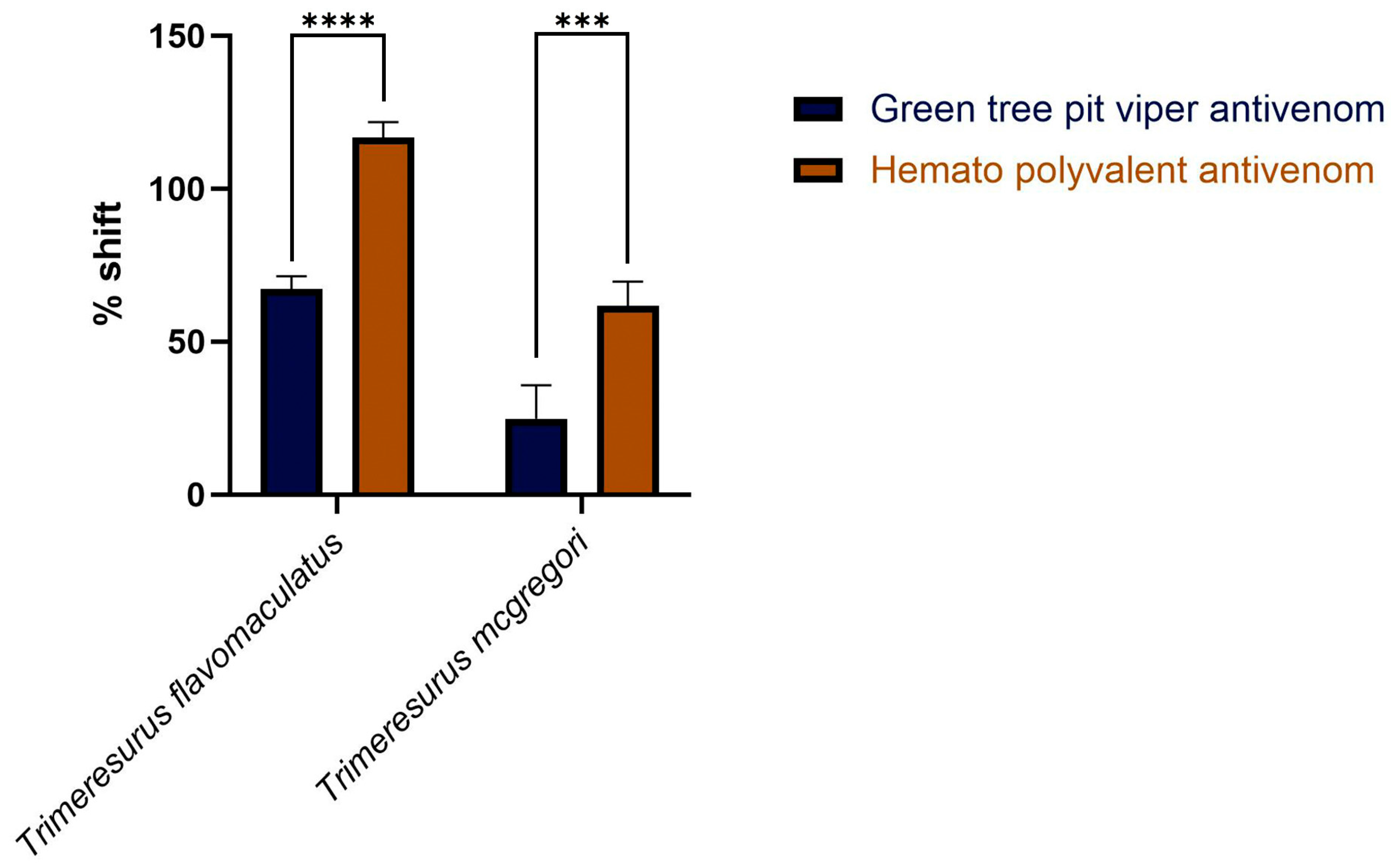
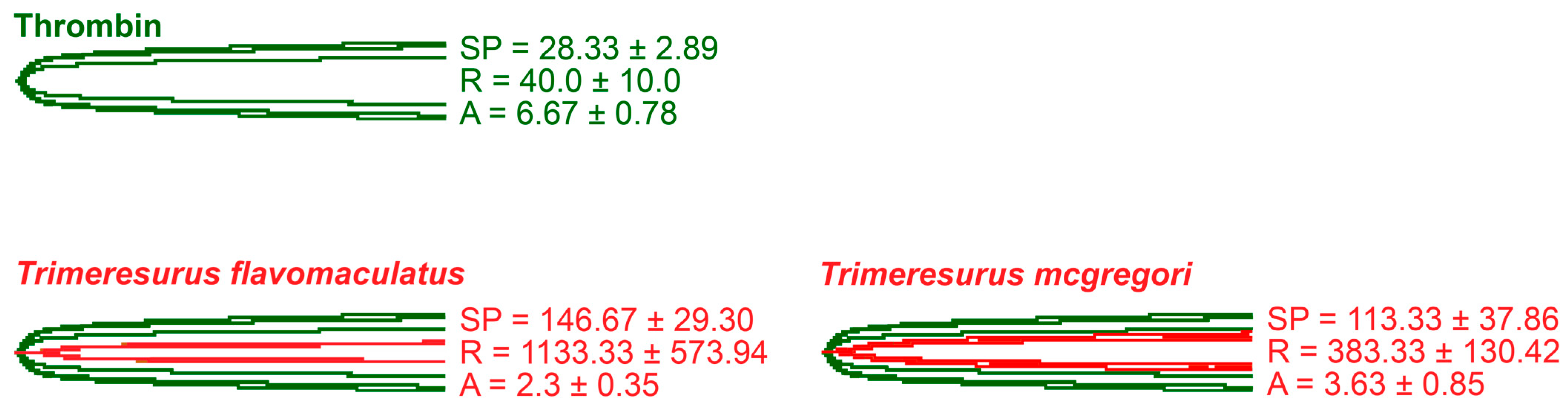
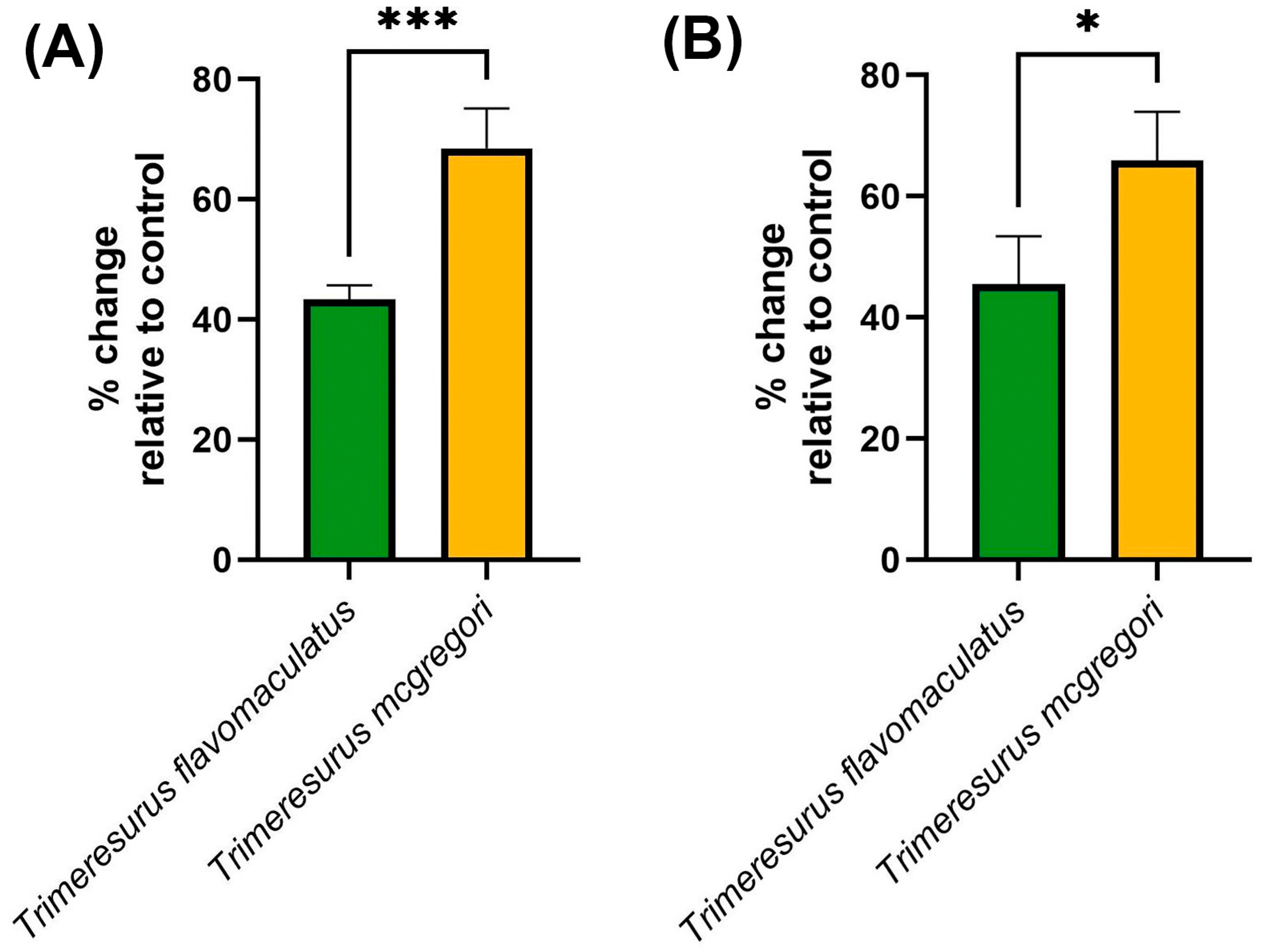
2. Results
3. Discussion
4. Materials and Methods
4.1. Venom Preparation
4.2. Antivenom Preparation
4.3. Plasma and Fibrinogen Preparation
4.4. Coagulation Analysis and Antivenom Cross-Neutralization
4.5. Clotting Factor Inhibition
4.6. Thromboelastography
4.7. Data Analysis and Visualization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jackson, T.N.W.; Fry, B.G. A tricky trait: Applying the fruits of the “function debate” in the philosophy of biology to the “venom debate” in the science of toxinology. Toxins 2016, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- von Reumont, B.M.; Campbell, L.I.; Jenner, R.A. Quo Vadis Venomics? A Roadmap to Neglected Venomous Invertebrates. Toxins 2014, 6, 3488–3551. [Google Scholar] [CrossRef]
- Calvete, J.J. Venomics: Integrative venom proteomics and beyond. Biochem. J. 2017, 474, 611–634. [Google Scholar] [CrossRef]
- Gremski, L.H.; Trevisan-Silva, D.; Ferrer, V.P.; Matsubara, F.H.; Meissner, G.O.; Wille, A.C.M.; Vuitika, L.; Dias-Lopes, C.; Ullah, A.; De Moraes, F.R.; et al. Recent advances in the understanding of brown spider venoms: From the biology of spiders to the molecular mechanisms of toxins. Toxicon 2014, 83, 91–120. [Google Scholar] [CrossRef]
- Tilbury, C.R.; Verster, J. A fatal bite from the burrowing asp Atractaspis corpulenta (Hallowell 1854). Toxicon 2016, 118, 21–26. [Google Scholar] [CrossRef]
- Senthilkumaran, S.; Sampath, S.; Almeida, J.R.; Williams, J.; Williams, H.F.; Patel, K.; Thirumalaikolundusubramanian, P.; Vaiyapuri, S. Pulmonary Thromboembolism following Russell’s Viper Bites. Toxins 2024, 16, 222. [Google Scholar] [CrossRef]
- Harris, R.J.; Fry, B.G. Electrostatic resistance to alpha-neurotoxins conferred by charge reversal mutations in nicotinic acetylcholine receptors. Proc. R. Soc. B Biol. Sci. 2021, 288, 7–9. [Google Scholar] [CrossRef]
- von Reumont, B.M.; Anderluh, G.; Antunes, A.; Ayvazyan, N.; Beis, D.; Caliskan, F.; Crnković, A.; Damm, M.; Dutertre, S.; Ellgaard, L.; et al. Modern venomics—Current insights, novel methods, and future perspectives in biological and applied animal venom research. GigaScience 2022, 11, giac048. [Google Scholar]
- Yong, M.Y.; Tan, K.Y.; Tan, C.H. Potential para-specific and geographical utility of Thai Green Pit Viper (Trimeresurus albolabris) Monovalent Antivenom: Neutralization of procoagulant and hemorrhagic activities of diverse Trimeresurus pit viper venoms. Toxicon 2021, 203, 85–92. [Google Scholar] [CrossRef]
- Abouyannis, M.; Esmail, H.; Hamaluba, M.; Ngama, M.; Mwangudzah, H.; Mumba, N.; Yeri, B.K.; Mwalukore, S.; Alphan, H.J.; Aggarwal, D.; et al. A global core outcome measurement set for snakebite clinical trials. Lancet Glob. Health 2023, 11, e296–e300. [Google Scholar] [CrossRef]
- World Health Organization. Recommendation for the Adoption of an Additional Disease as a Neglected Tropical Disease: The Case for Snakebite Envenoming; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Williams, D.J.; Faiz, M.A.; Abela-Ridder, B.; Ainsworth, S.; Bulfone, T.C.; Nickerson, A.D.; Habib, A.G.; Junghanss, T.; Fan, H.W.; Turner, M.; et al. Strategy for a globally coordinated response to a priority neglected tropical disease: Snakebite envenoming. PLoS Negl. Trop. Dis. 2019, 13, e0007059. [Google Scholar] [CrossRef] [PubMed]
- Roberts, N.L.S.; Zeng, S.M.; Hamilton, E.B.; Abdoli, A.; Alahdab, F.; Alipour, V.; Ancuceanu, R.; Andrei, C.L.; Anvari, D.; Arabloo, J.; et al. Global mortality of snakebite envenoming between 1990 and 2019. Nat. Commun. 2022, 13, 6160. [Google Scholar] [CrossRef]
- Habib, A.G.; Kuznik, A.; Hamza, M.; Abdullahi, M.I.; Chedi, B.A.; Chippaux, J.-P.; Warrell, D.A. Snakebite is Under Appreciated: Appraisal of Burden from West Africa. PLoS Negl. Trop. Dis. 2015, 9, e0004088. [Google Scholar] [CrossRef]
- Chippaux, J.-P. Snakebite envenomation turns again into a neglected tropical disease! J. Venom. Anim. Toxins Trop. Dis. 2017, 23, 38. [Google Scholar] [CrossRef]
- Leviton, A.E.; Brown, R.M.; Siler, C.D. The Dangerously Venomous Snakes of the Philippine Archipelago with Identification Keys and Species Accounts. In The Coral Triangle: The 2011 Hearst Philippine Biodiversity Expedition; Williams, G.C., Gosliner, T.M., Eds.; California Academy of Sciences: San Francisco, CA, USA, 2014; pp. 473–530. [Google Scholar]
- Eriksson, S. Medical geography views on snakebites in Southeast Asia: A case study from Vietnam. Asian Geogr. 2011, 28, 123–134. [Google Scholar] [CrossRef]
- Patikorn, C.; Blessmann, J.; Nwe, M.T.; Tiglao, P.J.G.; Vasaruchapong, T.; Maharani, T.; Doan, U.V.; Zainal Abidin, S.A.; Ismail, A.K.; Othman, I.; et al. Estimating economic and disease burden of snakebite in ASEAN countries using a decision analytic model. PLoS Negl. Trop. Dis. 2022, 16, e0010775. [Google Scholar] [CrossRef]
- Arrieta, R.; Aoki, Y.; Tan, M.A.; Sarsalijo, M.S.; Sarmiento, M.J.; Paghubasan, J.; Tiglao, P.J.; Yoshimura, K.; Sakai, A.; Agosto, L.C. A fatal snakebite envenomation due to King Cobra (Ophiophagus hannah) in the Eastern Visayas, Philippines. Toxicon 2024, 244, 107751. [Google Scholar] [CrossRef]
- Clark, R.F.; Davidson, T.M. Intraarticular envenomation by Trimeresurus flavomaculatus mcgregori resulting in joint destruction. Toxicon 1997, 35, 837–842. [Google Scholar] [CrossRef]
- De Leon, W.; Salafranca, E. Cobra Anti-Venom Serum Production at the Alabang Serum and Vaccine Laboratories. Philipp. J. Sci. 1956, 85, 477–486. [Google Scholar]
- Scheske, L.; Ruitenberg, J.; Bissumbhar, B. Needs and availability of snake antivenoms: Relevance and application of international guidelines. Int. J. Health Policy Manag. 2015, 4, 447–457. [Google Scholar] [CrossRef]
- Patikorn, C.; Ismail, A.K.; Abidin, S.A.Z.; Blanco, F.B.; Blessmann, J.; Choumlivong, K.; Comandante, J.D.; Doan, U.V.; Mohamed Ismail, Z.; Khine, Y.Y.; et al. Situation of snakebite, antivenom market and access to antivenoms in ASEAN countries. BMJ Glob. Health 2022, 7, e007639. [Google Scholar] [CrossRef] [PubMed]
- Bleicher, S.S.; Brown, J.S.; Embar, K.; Kotler, B.P. Novel predator recognition by Allenby’s gerbil (Gerbillus andersoni allenbyi): Do gerbils learn to respond to a snake that can “see” in the dark? Isr. J. Ecol. Evol. 2016, 62, 178–185. [Google Scholar] [CrossRef]
- Francis, S.; Khandelwal, S.; Straight, R.; Welton, L.; Liang, P.; Yang, H.; Gerardo, C.J.; Arepally, G. Platelet and red cell responses to three North American pit vipers. Toxicon 2024, 247, 107798. [Google Scholar] [CrossRef]
- Cao, D.; Domanski, K.; Hodgman, E.; Cardenas, C.; Weinreich, M.; Hutto, J.; AbdelFattah, K.R.; Chen, C. Thromboelastometry analysis of severe North American pit viper-induced coagulopathy: A case report. Toxicon 2018, 151, 29–33. [Google Scholar] [CrossRef]
- Qamruddin, R.M.; Safferi, R.S.; Mohamed@Ismail, Z.; Salleh, M.S.; Abd Hamid, M.N.H.; Ng, V.E.R.F.; Goh, W.C.; Ismail, A.K. Frequency, geographical distribution and outcomes of pit viper bites in Malaysia consulted to Remote Envenomation Consultancy Services (RECS) from 2017 to 2020. PLoS Negl. Trop. Dis. 2023, 17, e0011569. [Google Scholar] [CrossRef]
- Miller, A.; Parsh, B. Caring for patients with venomous Crotalinae snakebites. Nursing 2020, 50, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Diniz-Sousa, R.; Moraes, J.d.N.; Rodrigues-da-Silva, T.M.; Oliveira, C.S.; Caldeira, C.A.d.S. A brief review on the natural history, venomics and the medical importance of bushmaster (Lachesis) pit viper snakes. Toxicon X 2020, 7, 100053. [Google Scholar] [CrossRef]
- Weinell, J.L.; Hooper, E.; Leviton, A.E.; Brown, R.M. Illustrated Key to the Snakes of the Philippines. Proc. Calif. Acad. Sci. 2019, 66, 1–49. [Google Scholar]
- World Health Organization; WHO Expert Committee on Biological Standardization. WHO Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins; WHO Press: Geneva, Switzerland, 2017; Volume 1004, pp. 197–388. [Google Scholar]
- Le Roux, G.; Grenet, G.; Schmitt, C.; French Poison Control Centers Research Group; Larréché, S.; Descatha, A. Bites by Non-Native Reptiles in France: Species, Circumstances and Outcome. Toxins 2022, 14, 570. [Google Scholar] [CrossRef]
- Ilagan, V.A.D.; Alejandro, G.J.D.; Paraguison, D.J.B.; Perolina, S.M.W.; Mendoza, G.R.; Bolina, A.B.; Raterta, R.; Vales, M.B.; Suarez, G.J.D.; Blasco, F.A. Ethnopharmacological documentation and molecular authentication of medicinal plants used by the Manobo and Mamanwa tribes of Surigao del Sur, Philippines. Biodiversitas J. Biol. Divers. 2022, 23. [Google Scholar] [CrossRef]
- Litschka-Koen, T.; Pons, J.; Tiglao, P.J.; Comandante, J.D.; Santamaria, E.; Sarmiento, M.J.; Whitaker, R.; Jesudasan, A.; Kartik, A.; Ch, G.; et al. Case Reports of Tropical Snakebite Victims Illustrate the Vital Humanitarian Role and Challenges of Community Action Groups: RSTMH Special Report on Snakebite 2019. Available online: https://www.rstmh.org/sites/rstmh/files/content/attachments/2021-04-01/RSTMH%20%E2%80%93%20Snakebite%20Report%202019%20v2.1.pdf (accessed on 28 January 2025).
- Panagides, N.; Jackson, T.N.W.; Ikonomopoulou, M.P.; Arbuckle, K.; Pretzler, R.; Yang, D.C.; Ali, S.A.; Koludarov, I.; Dobson, J.; Sanker, B.; et al. How the cobra got its flesh-eating venom: Cytotoxicity as a defensive innovation and its co-evolution with hooding, aposematic marking, and spitting. Toxins 2017, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Patikorn, C.; Ismail, A.K.; Zainal Abidin, S.A.; Othman, I.; Chaiyakunapruk, N.; Taychakhoonavudh, S. Potential economic and clinical implications of improving access to snake antivenom in five ASEAN countries: A cost-effectiveness analysis. PLoS Negl. Trop. Dis. 2022, 16, e0010915. [Google Scholar] [CrossRef]
- Sanchez, E.F.; Flores-Ortiz, R.J.; Alvarenga, V.G.; Eble, J.A. Direct fibrinolytic snake venom metalloproteinases affecting hemostasis: Structural, biochemical features and therapeutic potential. Toxins 2017, 9, 392. [Google Scholar] [CrossRef]
- Bustillo, S.; Van de Velde, A.C.; Matzner Perfumo, V.; Gay, C.C.; Leiva, L.C. Apoptosis induced by a snake venom metalloproteinase from Bothrops alternatus venom in C2C12 muscle cells. Apoptosis 2017, 22, 491–501. [Google Scholar] [CrossRef]
- Emswiler, M.P.; Griffith, F.P.; Cumpston, K.L. Clinically Significant Envenomation from Postmortem Copperhead (Agkistrodon contortrix). Wilderness Environ. Med. 2017, 28, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Slagboom, J.; Kool, J.; Harrison, R.A.; Casewell, N.R. Haemotoxic snake venoms: Their functional activity, impact on snakebite victims and pharmaceutical promise. Br. J. Haematol. 2017, 177, 947–959. [Google Scholar] [CrossRef]
- Rucavado, A.; Chacón, M.; Villalobos, D.; Argüello, I.; Campos, M.; Guerrero, G.; Méndez, M.L.; Escalante, T.; Gutiérrez, J.M. Coagulopathy induced by viperid snake venoms in a murine model: Comparison of standard coagulation tests and rotational thromboelastometry. Toxicon 2022, 214, 121–129. [Google Scholar] [CrossRef]
- Kim, Y.; Bae, H.; Yu, D. The in vitro effects of acidemia and acidemia reversal on coagulation in dogs. Front. Vet. Sci. 2024, 11, 1427237. [Google Scholar] [CrossRef]
- Abou Khalil, E.; Gaines, B.A.; Morgan, K.M.; Leeper, C.M. Admission maximum amplitude–reaction time ratio: Association between thromboelastography values predicts poor outcome in injured children. J. Trauma Acute Care Surg. 2023, 94, 212–219. [Google Scholar] [CrossRef]
- Al-Amer, O.M. The role of thrombin in haemostasis. Blood Coagul. Fibrinolysis 2022, 33, 145–148. [Google Scholar] [CrossRef]
- Bourke, L.A.; Youngman, N.J.; Zdenek, C.N.; op den Brouw, B.; Violette, A.; Fourmy, R.; Fry, B.G. Trimeresurus albolabris snakebite treatment implications arising from ontogenetic venom comparisons of anticoagulant function, and antivenom efficacy. Toxicol. Lett. 2020, 327, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Youngman, N.J.; Peng, Y.H.; Harris, R.J.; Jones, L.; Llinas, J.; Haworth, M.; Gillett, A.; Fry, B.G. Differential coagulotoxic and neurotoxic venom activity from species of the arboreal viperid snake genus Bothriechis (palm-pitvipers). Comp. Biochem. Physiol.-Part C Toxicol. Pharmacol. 2022, 256, 109326. [Google Scholar] [CrossRef]
- Youngman, N.J.; Lewin, M.R.; Carter, R.; Naude, A.; Fry, B.G. Efficacy and Limitations of Chemically Diverse Small-Molecule Enzyme-Inhibitors against the Synergistic Coagulotoxic Activities of Bitis Viper Venoms. Molecules 2022, 27, 1733. [Google Scholar] [CrossRef] [PubMed]
- Kant, A.; Ng, M.; Tan, M.J.E.; Ponampalam, R. Successful use of anti-venom cross-neutralization effects in the clinical management of Shore Pit Viper envenomation. Am. J. Emerg. Med. 2024, 84, 190.e1–190.e5. [Google Scholar] [CrossRef]
- Yong, M.Y.; Tan, K.Y.; Tan, C.H. A genus-wide study on venom proteome variation and phospholipase A2 inhibition in Asian lance-headed pit vipers (genus: Trimeresurus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2025, 288, 110077. [Google Scholar] [CrossRef] [PubMed]
- Debono, J.; Bos, M.H.A.; Frank, N.; Fry, B. Clinical implications of differential antivenom efficacy in neutralising coagulotoxicity produced by venoms from species within the arboreal viperid snake genus Trimeresurus. Toxicol. Lett. 2019, 316, 35–48. [Google Scholar] [CrossRef]
- World Health Organization Guidelines for the Management of Snakebites; World Health Organization Regional Office for South-East Asia: New Delhi, India, 2016; ISBN 978-92-9022-530-0.
- World Health Organization. WHO Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins. In WHO Technical Report Series, No. 1004; WHO Expert Committee on Biological Standardization, Ed.; WHO Press: Geneva, Switzerland, 2018; pp. 197–388. ISBN 2105-0678. (Print)r2105-0678 (Linking). [Google Scholar]
- Leong, P.K.; Sim, S.M.; Fung, S.Y.; Sumana, K.; Sitprija, V.; Tan, N.H. Cross neutralization of Afro-Asian cobra and Asian Krait Venoms by a Thai polyvalent snake antivenom (Neuro Polyvalent Snake Antivenom). PLoS Negl. Trop. Dis. 2012, 6, e1672. [Google Scholar] [CrossRef]
- Leong, P.K.; Tan, C.H.; Sim, S.M.; Fung, S.Y.; Sumana, K.; Sitprija, V.; Tan, N.H. Cross neutralization of common Southeast Asian viperid venoms by a Thai polyvalent snake antivenom (Hemato Polyvalent Snake Antivenom). Acta Trop. 2014, 132, 7–14. [Google Scholar] [CrossRef]
- Tan, C.H.; Tan, K.Y.; Lim, S.E.; Tan, N.H. Venomics of the beaked sea snake, Hydrophis schistosus: A minimalist toxin arsenal and its cross-neutralization by heterologous antivenoms. J. Proteomics 2015, 126, 121–130. [Google Scholar] [CrossRef]
- Yang, D.C.; Dobson, J.; Cochran, C.; Dashevsky, D.; Arbuckle, K.; Benard, M.; Boyer, L.; Alagón, A.; Hendrikx, I.; Hodgson, W.C.; et al. The Bold and the Beautiful: A Neurotoxicity Comparison of New World Coral Snakes in the Micruroides and Micrurus Genera and Relative Neutralization by Antivenom. Neurotox. Res. 2017, 32, 487–495. [Google Scholar] [CrossRef]
- Ainsworth, S.; Slagboom, J.; Alomran, N.; Pla, D.; Alhamdi, Y.; King, S.I.; Bolton, F.M.S.; Gutiérrez, J.M.; Vonk, F.J.; Toh, C.-H.; et al. The paraspecific neutralisation of snake venom induced coagulopathy by antivenoms. Commun. Biol. 2018, 1, 34. [Google Scholar] [CrossRef]
- Williams, D.J.; Gutiérrez, J.M.; Calvete, J.J.; Wüster, W.; Ratanabanangkoon, K.; Paiva, O.; Brown, N.I.; Casewell, N.R.; Harrison, R.A.; Rowley, P.D.; et al. Ending the drought: New strategies for improving the flow of affordable, effective antivenoms in Asia and Africa. J. Proteomics 2011, 74, 1735–1767. [Google Scholar] [CrossRef]
- Tan, C.H.; Palasuberniam, P.; Blanco, F.B.; Tan, K.Y. Immunoreactivity and neutralization capacity of Philippine cobra antivenom against Naja philippinensis and Naja samarensis venoms. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Palasuberniam, P.; Chan, Y.W.; Tan, K.Y.; Tan, C.H. Snake Venom Proteomics of Samar Cobra (Naja samarensis) from the Southern Philippines: Short Alpha-Neurotoxins as the Dominant Lethal Component Weakly Cross-Neutralized by the Philippine Cobra Antivenom. Front. Pharmacol. 2021, 12, 727756. [Google Scholar] [CrossRef]
- Department of Health. Philippine Health Advisories; Department of Health: Manila, Philippines, 2012. [Google Scholar]
- Chan, Y.W.; Tan, K.Y.; Tan, C.H. Preclinical assessment of VPEAV, a new trivalent antivenom for elapid snakebite envenoming in the Philippines: Proteomics, immunoreactivity and toxicity neutralization. Toxicon 2022, 220, 106942. [Google Scholar] [CrossRef] [PubMed]
- Chaisakul, J.; Rusmili, M.R.A.; Alsolaiss, J.; Albulescu, L.-O.; Harrison, R.A.; Othman, I.; Casewell, N.R. In Vitro Immunological Cross-Reactivity of Thai Polyvalent and Monovalent Antivenoms with Asian Viper Venoms. Toxins 2020, 12, 766. [Google Scholar] [CrossRef]
- Chuang, P.-C.; Chen, J.-W.; Chan, Y.-Y.; Tse, T.-C.; Chiang, Y.-W.; Tsai, T.-S. In vitro immunoreactivity and in vivo neutralization of Trimeresurus gracilis venom with antivenoms targeting four pit viper species. PLoS Negl. Trop. Dis. 2024, 18, e0012070. [Google Scholar] [CrossRef]
- Thakur, S.; Giri, S.; Lalremsanga, H.T.; Doley, R. Indian green pit vipers: A lesser-known snake group of north-east India. Toxicon 2024, 242, 107689. [Google Scholar] [CrossRef]
- Mao, Y.-C.; Liu, P.-Y.; Lai, K.-L.; Luo, Y.; Chen, K.-T.; Lai, C.-S. Clinical Characteristics of Snakebite Envenomings in Taiwan. Toxins 2025, 17, 14. [Google Scholar] [CrossRef]
- Alvitigala, B.Y.; Dissanayake, H.A.; Weeratunga, P.N.; Padmaperuma, P.A.C.D.; Gooneratne, L.V.; Gnanathasan, C.A. Haemotoxicity of snakes: A review of pathogenesis, clinical manifestations, novel diagnostics and challenges in management. Trans. R. Soc. Trop. Med. Hyg. 2025, 119, 283–303. [Google Scholar] [CrossRef]
- Bittenbinder, M.A.; Zdenek, C.N.; op den Brouw, B.; Youngman, N.J.; Dobson, J.S.; Naude, A.; Vonk, F.J.; Fry, B.G. Coagulotoxic Cobras: Clinical Implications of Strong Anticoagulant Actions of African Spitting Naja Venoms That Are Not Neutralised by Antivenom but Are by LY315920 (Varespladib). Toxins 2019, 10, 516. [Google Scholar] [CrossRef]
- Chowdhury, A.; Zdenek, C.N.; Dobson, J.S.; Bourke, L.A.; Soria, R.; Fry, B.G. Clinical implications of differential procoagulant toxicity of the palearctic viperid genus Macrovipera, and the relative neutralization efficacy of antivenoms and enzyme inhibitors. Toxicol. Lett. 2021, 340, 77–88. [Google Scholar] [CrossRef]
- Zdenek, C.N.; Youngman, N.J.; Hay, C.; Dobson, J.; Dunstan, N.; Allen, L.; Milanovic, L.; Fry, B.G. Anticoagulant activity of black snake (Elapidae: Pseudechis) venoms: Mechanisms, potency, and antivenom efficacy. Toxicol. Lett. 2020, 330, 176–184. [Google Scholar] [CrossRef]
- Bourke, L.A.; Zdenek, C.N.; Huynh, T.M.; Hodgson, W.C.; Alagón, A.; Castro, E.N.; Jones, J.; Fry, B.G. Fangs and foliage: Unearthing the haemotoxic secrets of cannabis-dwelling rattlesnakes. Toxicon 2024, 244, 107756. [Google Scholar] [CrossRef]
- Youngman, N.J.; Debono, J.; Dobson, J.S.; Zdenek, C.N.; Harris, R.J.; op den Brouw, B.; Coimbra, F.C.P.; Naude, A.; Coster, K.; Sundman, E.; et al. Venomous Landmines: Clinical Implications of Extreme Coagulotoxic Diversification and Differential Neutralization by Antivenom of Venoms within the Viperid Snake Genus Bitis. Toxins 2019, 11, 422. [Google Scholar] [CrossRef]
- Grashof, D.; Zdenek, C.N.; Dobson, J.S.; Youngman, N.J.; Coimbra, F.; Benard-Valle, M.; Alagon, A.; Fry, B.G. A web of coagulotoxicity: Failure of antivenom to neutralize the destructive (non-clotting) fibrinogenolytic activity of Loxosceles and Sicarius spider venoms. Toxins 2020, 12, 91. [Google Scholar] [CrossRef]
- Chowdhury, A.; Youngman, N.J.; Liu, J.; Lewin, M.R.; Carter, R.W.; Fry, B.G. The relative efficacy of chemically diverse small-molecule enzyme-inhibitors against anticoagulant activities of Black Snake (Pseudechis spp.) venoms. Toxicol. Lett. 2022, 366, 26–32. [Google Scholar] [CrossRef]

|
Venom Concentration | Trimeresurus flavomaculatus | Trimeresurus mcgregori | ||||
|---|---|---|---|---|---|---|
| MAv | HPAv | MAv vs. HPAv | MAv | HPAv | MAv vs. HPAv | |
| 40.00 µg/mL | 0.0067 | 0.0282 | 0.0039 * | 0.1455 | 0.2252 | 0.0527 |
| 20.00 µg/mL | 0.0128 | 0.0193 | 0.1669 | 0.6678 | 0.1997 | 0.5933 |
| 10.00 µg/mL | 0.0057 | 0.0160 | 0.0903 | 0.2804 | 0.0189 | 0.0470 * |
| 4.00 µg/mL | <0.0001 | 0.0043 | 0.0095 ^ | 0.0827 | 0.0606 | 0.0825 |
| 1.67 µg/mL | <0.0001 | <0.0001 | N/A | 0.0036 | 0.0036 | N/A |
| 0.67 µg/mL | 0.2391 | 0.2391 | N/A | N/A | N/A | N/A |
| 0.25 µg/mL | N/A | N/A | N/A | N/A | N/A | N/A |
| 0.125 µg/mL | N/A | N/A | N/A | N/A | N/A | N/A |
| 0.05 µg/mL | N/A | N/A | N/A | N/A | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo, D.A.E.; Seneci, L.; Chowdhury, A.; Rimando, M.G.; Fry, B.G. Bite First, Bleed Later: How Philippine Trimeresurus Pit Viper Venoms Hijack Blood Clotting. Toxins 2025, 17, 185. https://doi.org/10.3390/toxins17040185
Castillo DAE, Seneci L, Chowdhury A, Rimando MG, Fry BG. Bite First, Bleed Later: How Philippine Trimeresurus Pit Viper Venoms Hijack Blood Clotting. Toxins. 2025; 17(4):185. https://doi.org/10.3390/toxins17040185
Chicago/Turabian StyleCastillo, Daniel Albert E., Lorenzo Seneci, Abhinandan Chowdhury, Marilyn G. Rimando, and Bryan G. Fry. 2025. "Bite First, Bleed Later: How Philippine Trimeresurus Pit Viper Venoms Hijack Blood Clotting" Toxins 17, no. 4: 185. https://doi.org/10.3390/toxins17040185
APA StyleCastillo, D. A. E., Seneci, L., Chowdhury, A., Rimando, M. G., & Fry, B. G. (2025). Bite First, Bleed Later: How Philippine Trimeresurus Pit Viper Venoms Hijack Blood Clotting. Toxins, 17(4), 185. https://doi.org/10.3390/toxins17040185







