Two Amino Acid Substitutions Improve the Pharmacological Profile of the Snake Venom Peptide Mambalgin
Abstract
1. Introduction
2. Results
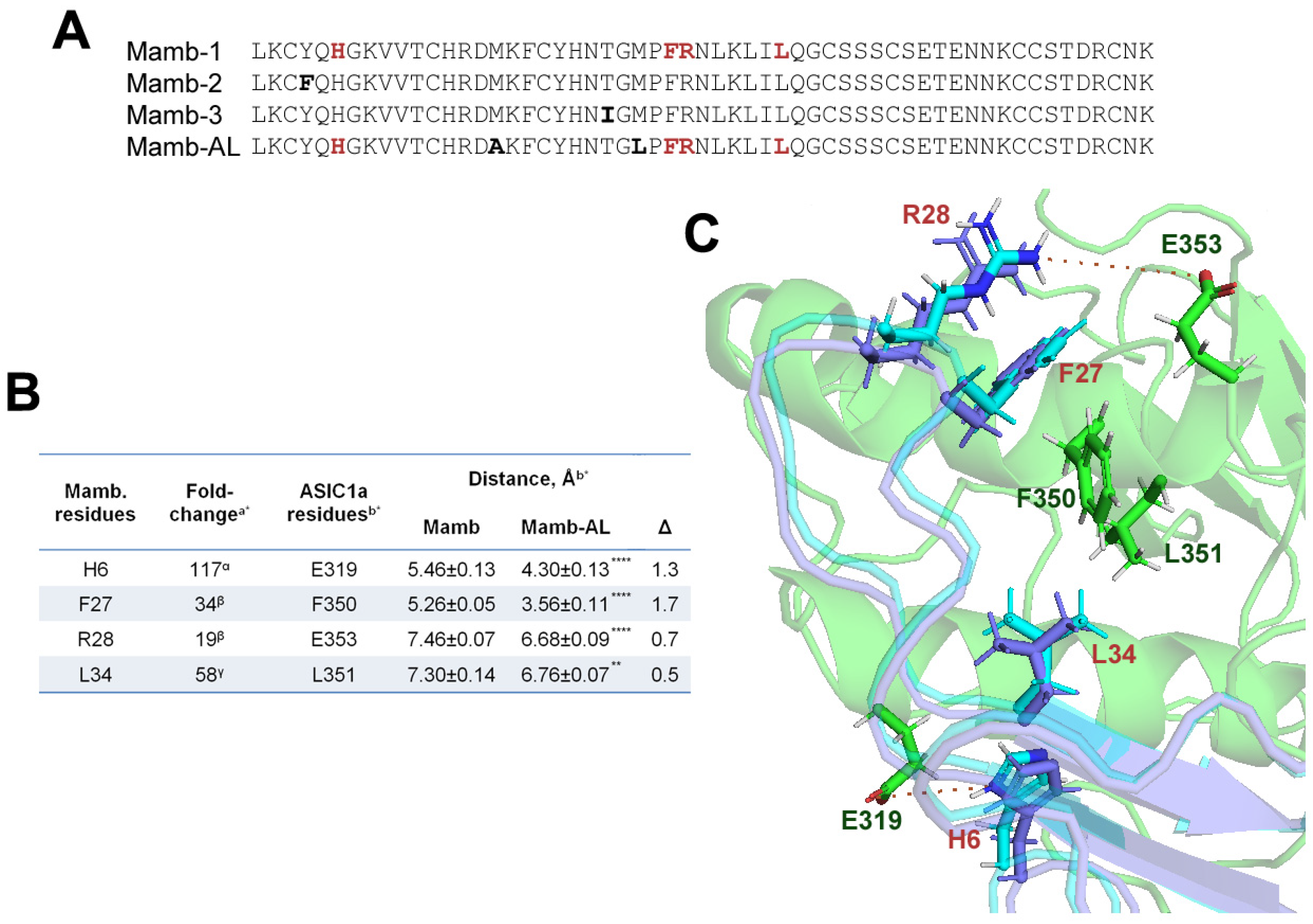
2.1. In the Interaction Models, the Mutant Analog of Mambalgin Exhibits Tighter Binding to the ASIC1a Channel than the Wild-Type Peptide
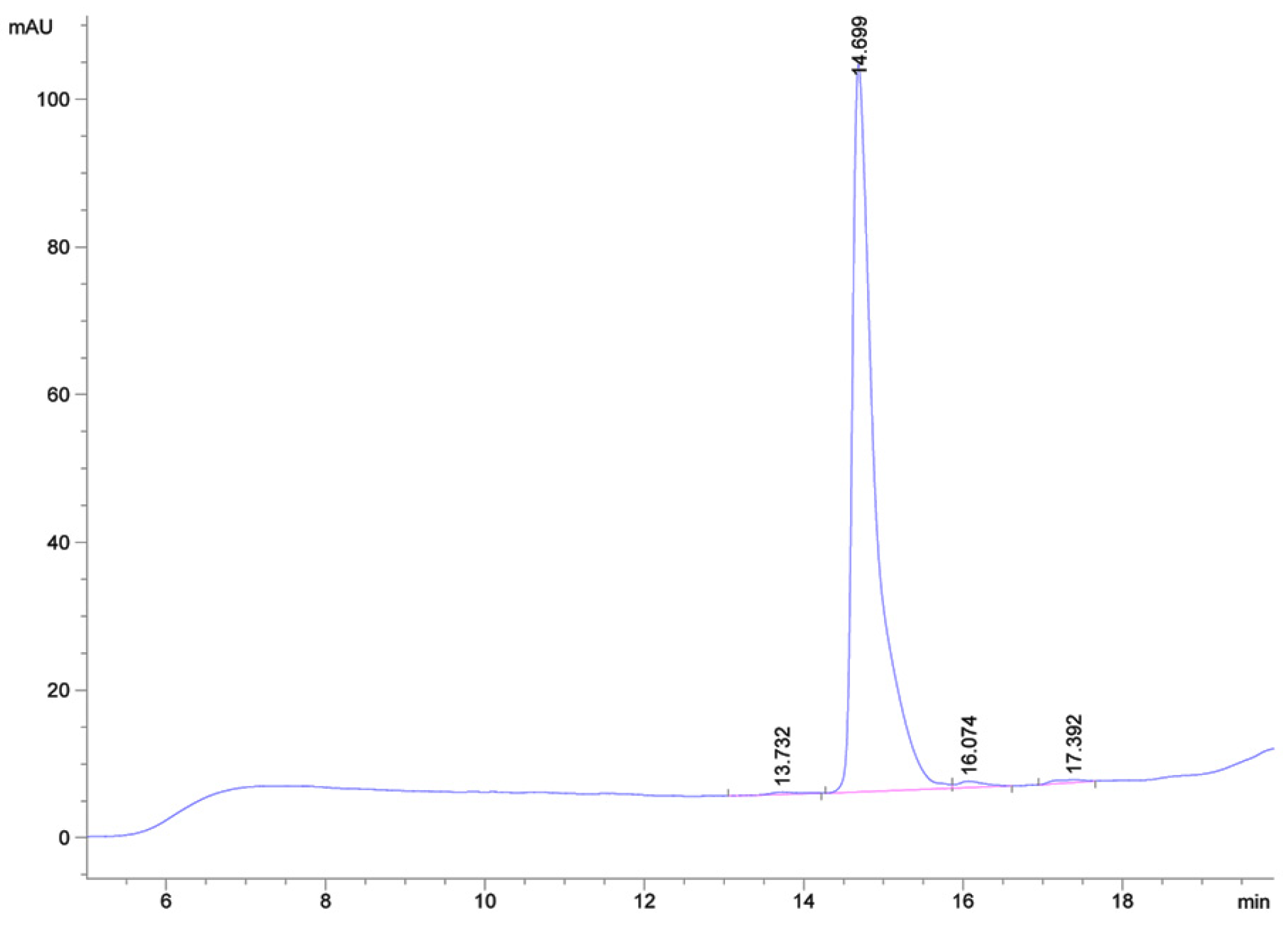
2.2. High-Yield Production of the Mutant Peptide Mamb-AL
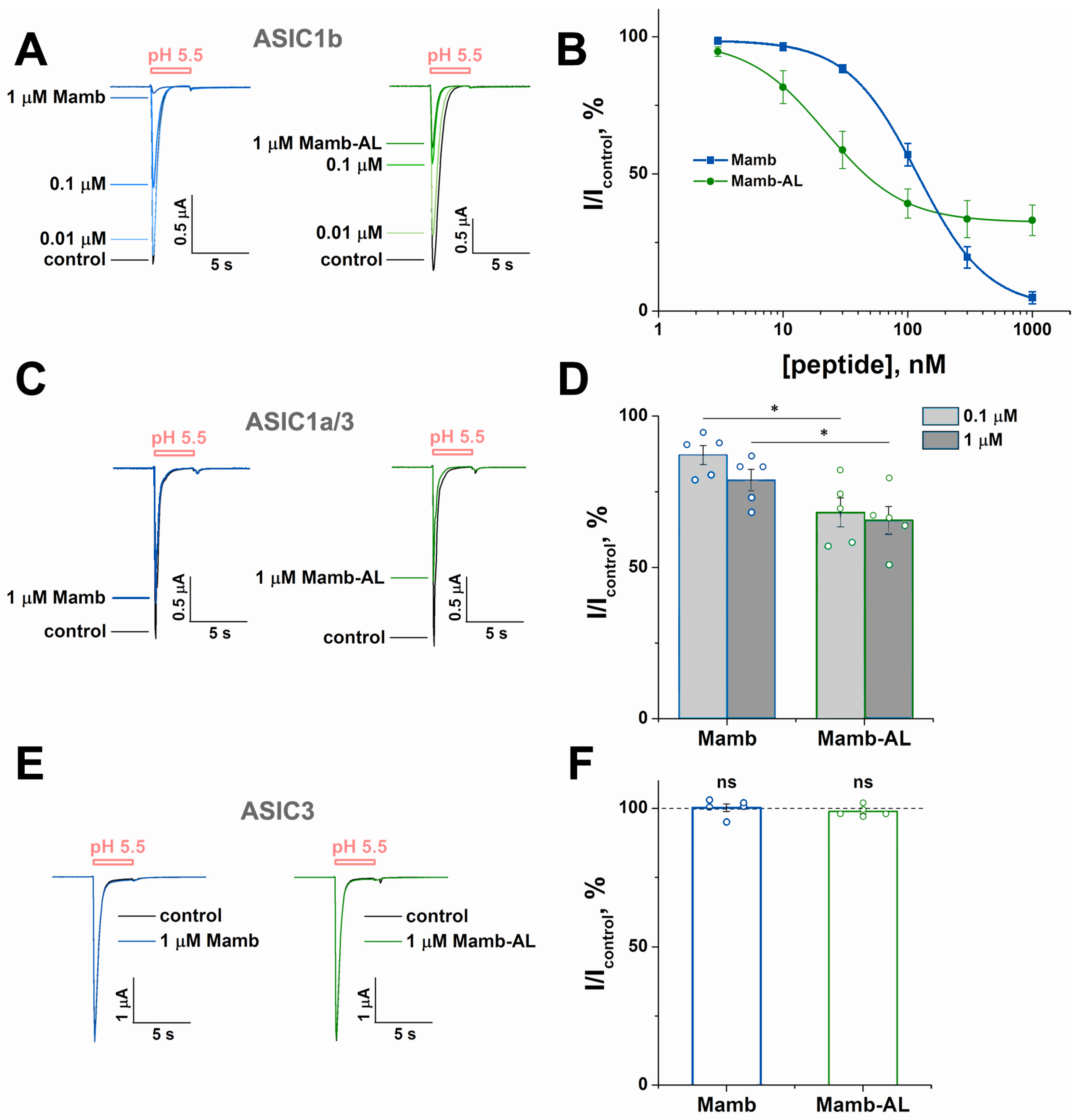
2.3. Mamb-AL Is a Stronger Negative Modulator of rASIC1a than Wild-Type Mamb
2.4. Mamb-AL Has a Stronger Effect on Other ASIC1-Containing Channels than Mamb
2.5. Mamb and Mamb-AL Exhibit Analgesic Effect
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Molecular Docking Analysis of Peptide Interactions with the Rat ASIC1a
5.3. Mamb-AL Gene Synthesis
5.4. Recombinant Mamb-AL Production
5.5. Mass Spectrometry
5.6. Expression of ASIC Channels in Xenopus laevisOocytes
5.7. Electrophysiological Assay
5.8. Acetic Acid-Induced Writhing Pain Test
5.9. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASIC1 | acid-sensing ion channel type 1 |
| CNBr | cyanogen bromide |
| cryo-EM | cryo-electron microscopy |
| HPLC | high-performance liquid chromatography |
| IC50 | half-maximal inhibitory concentration |
| Mamb | mambalgin |
| MeCN | acetonitrile |
| TEVC | two-electrode voltage clamp |
Appendix A
| Peptide | pI 1 | ε | GRAVY | AI |
|---|---|---|---|---|
| Mamb | 8.88 | 3480 | −0.696 | 44.39 |
| Mamb-AL | 8.88 | 3480 | −0.665 | 52.98 |
| Rosetta Score | Peptide | 1 | 2 | 3 | 4 | 5 | Mean ± S.E.M |
|---|---|---|---|---|---|---|---|
| Interaction score | Mamb | −33.52 | −32.25 | −31.97 | −31.98 | −31.93 | −32.33 ± 0.31 |
| Mamb-AL | −32.53 | −31.99 | −31.79 | −31.79 | −31.56 | −31.93 ± 0.16 | |
| Total score | Mamb | 2391.16 | 2391.29 | 2405.78 | 2399.36 | 2390.77 | 2395.67 ± 2.99 |
| Mamb-AL | 2387.98 | 2383.71 | 2385.39 | 2428.01 | 2353.91 | 2387.81 ± 11.81 |
References
- Boscardin, E.; Alijevic, O.; Hummler, E.; Frateschi, S.; Kellenberger, S. The function and regulation of acid-sensing ion channels (ASICs) and the epithelial Na+ channel (ENaC): IUPHAR Review 19. Br. J. Pharmacol. 2016, 173, 2671–2701. [Google Scholar] [CrossRef]
- Sherwood, T.W.; Frey, E.N.; Askwith, C.C. Structure and activity of the acid-sensing ion channels. Am. J. Physiol. Physiol. 2012, 303, C699–C710. [Google Scholar] [CrossRef] [PubMed]
- Kellenberger, S.; Schild, L. International union of basic and clinical pharmacology. XCI. Structure, function, and pharmacology of acid-sensing ion channels and the epithelial Na+ channel. Pharmacol. Rev. 2015, 67, 1–35. [Google Scholar] [CrossRef]
- Jasti, J.; Furukawa, H.; Gonzales, E.B.; Gouaux, E. Structure of acid-sensing ion channel 1 at 1.9 Å resolution and low pH. Nature 2007, 449, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, E.B.; Kawate, T.; Gouaux, E. Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature 2009, 460, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Baconguis, I.; Bohlen, C.J.; Goehring, A.; Julius, D.; Gouaux, E. X-Ray Structure of acid-sensing ion channel 1-snake toxin complex reveals open state of a Na+-selective channel. Cell 2014, 156, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Yoder, N.; Yoshioka, C.; Gouaux, E. Gating mechanisms of acid-sensing ion channels. Nature 2018, 555, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Deval, E.; Lingueglia, E. Acid-sensing ion channels and nociception in the peripheral and central nervous systems. Neuropharmacology 2015, 94, 49–57. [Google Scholar] [CrossRef]
- Kreple, C.J.; Lu, Y.; Taugher, R.J.; Schwager-Gutman, A.L.; Du, J.; Stump, M.; Wang, Y.; Ghobbeh, A.; Fan, R.; Cosme, C.V.; et al. Acid-sensing ion channels contribute to synaptic transmission and inhibit cocaine-evoked plasticity. Nat. Neurosci. 2014, 17, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Wemmie, J.A.; Chen, J.; Askwith, C.C.; Hruska-Hageman, A.M.; Price, M.P.; Nolan, B.C.; Yoder, P.G.; Lamani, E.; Hoshi, T.; Freeman, J.H.; et al. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron 2002, 34, 463–477. [Google Scholar] [CrossRef]
- Taugher, R.J.; Lu, Y.; Fan, R.; Ghobbeh, A.; Kreple, C.J.; Faraci, F.M.; Wemmie, J.A. ASIC1A in neurons is critical for fear-related behaviors. Genes Brain Behav. 2017, 16, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Kalinovskii, A.P.; Pushkarev, A.P.; Mikhailenko, A.D.; Kudryavtsev, D.S.; Belozerova, O.A.; Shmygarev, V.I.; Yatskin, O.N.; Korolkova, Y.V.; Kozlov, S.A.; Osmakov, D.I.; et al. Dual modulator of ASIC channels and GABAA receptors from thyme alters fear-related hippocampal activity. Int. J. Mol. Sci. 2023, 24, 13148. [Google Scholar] [CrossRef] [PubMed]
- Aissouni, Y.; El Guerrab, A.; Hamieh, A.M.; Ferrier, J.; Chalus, M.; Lemaire, D.; Grégoire, S.; Etienne, M.; Eschalier, A.; Ardid, D.; et al. Acid-sensing ion channel 1a in the amygdala is involved in pain and anxiety-related behaviours associated with arthritis. Sci. Rep. 2017, 7, 43617. [Google Scholar] [CrossRef]
- Lai, K.; Pritišanac, I.; Liu, Z.Q.; Liu, H.W.; Gong, L.N.; Li, M.X.; Lu, J.F.; Qi, X.; Xu, T.L.; Forman-Kay, J.; et al. Glutamate acts on acid-sensing ion channels to worsen ischaemic brain injury. Nature 2024, 631, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Gobetto, M.N.; Castellanos, L.C.S.; Contreras, N.E.; Sodero, A.O.; Cambiagno, D.A.; Malnati, G.O.M.; Montes, M.M.; Uchitel, O.D.; Weissmann, C. Segmental upregulation of ASIC1 channels in the formalin acute pain mouse model. Pharmaceuticals 2022, 15, 1539. [Google Scholar] [CrossRef] [PubMed]
- Heusser, S.A.; Pless, S.A. Acid-sensing ion channels as potential therapeutic targets. Trends Pharmacol. Sci. 2021, 42, 1035–1050. [Google Scholar] [CrossRef]
- Redd, M.A.; Yoshikawa, Y.; Khan, N.; Waqar, M.; Saez, N.J.; Outhwaite, J.E.; Russell, J.S.; Hanna, A.D.; Chiu, H.S.; Er, S.Y.; et al. Acid-sensing ion channel 1a blockade reduces myocardial injury in rodent models of myocardial infarction. Eur. Heart J. 2024, 45, 1571–1574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, D.; Zhang, J.; Cheng, K.; Zhen, F.; Li, M.; Chen, B. Pathology and physiology of acid-sensitive ion channels in the bladder. Heliyon 2024, 10, e38031. [Google Scholar] [CrossRef]
- Osmakov, D.I.; Khasanov, T.A.; Andreev, Y.A.; Lyukmanova, E.N.; Kozlov, S.A. Animal, herb, and microbial toxins for structural and pharmacological study of acid-sensing ion channels. Front. Pharmacol. 2020, 11, 991. [Google Scholar] [CrossRef] [PubMed]
- Verkest, C.; Salinas, M.; Diochot, S.; Deval, E.; Lingueglia, E.; Baron, A. Mechanisms of action of the peptide toxins targeting human and rodent acid-sensing ion channels and relevance to their in vivo analgesic effects. Toxins 2022, 14, 709. [Google Scholar] [CrossRef] [PubMed]
- Diochot, S.; Baron, A.; Salinas, M.; Douguet, D.; Scarzello, S.; Dabert-Gay, A.S.; Debayle, D.; Friend, V.; Alloui, A.; Lazdunski, M.; et al. Black mamba venom peptides target acid-sensing ion channels to abolish pain. Nature 2012, 490, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Cristofori-Armstrong, B.; Budusan, E.; Rash, L.D. Mambalgin-3 potentiates human acid-sensing ion channel 1b under mild to moderate acidosis: Implications as an analgesic lead. Proc. Natl. Acad. Sci. USA 2021, 118, e2021581118. [Google Scholar] [CrossRef]
- Sun, D.; Yu, Y.; Xue, X.; Pan, M.; Wen, M.; Li, S.; Qu, Q.; Li, X.; Zhang, L.; Li, X.; et al. Cryo-EM structure of the ASIC1a–mambalgin-1 complex reveals that the peptide toxin mambalgin-1 inhibits acid-sensing ion channels through an unusual allosteric effect. Cell Discov. 2018, 4, 27. [Google Scholar] [CrossRef]
- Sun, D.; Liu, S.; Li, S.; Zhang, M.; Yang, F.; Wen, M.; Shi, P.; Wang, T.; Pan, M.; Chang, S.; et al. Structural insights into human acid-sensing ion channel 1a inhibition by snake toxin mambalgin1. Elife 2020, 9, e57096. [Google Scholar] [CrossRef]
- Schroeder, C.I.; Rash, L.D.; Vila-Farrés, X.; Rosengren, K.J.; Mobli, M.; King, G.F.; Alewood, P.F.; Craik, D.J.; Durek, T. Chemical synthesis, 3D structure, and ASIC binding site of the toxin mambalgin-2. Angew. Chem. Int. Ed. 2014, 53, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Salinas, M.; Kessler, P.; Douguet, D.; Sarraf, D.; Tonali, N.; Thai, R.; Servent, D.; Lingueglia, E. Mambalgin-1 pain-relieving peptide locks the hinge between α4 and α5 helices to inhibit rat acid-sensing ion channel 1a. Neuropharmacology 2021, 185, 108453. [Google Scholar] [CrossRef] [PubMed]
- Cristofori-Armstrong, B.; Budusan, E.; Smith, J.J.; Reynaud, S.; Voll, K.; Chassagnon, I.R.; Durek, T.; Rash, L.D. Revealing molecular determinants governing mambalgin-3 pharmacology at acid-sensing ion channel 1 variants. Cell. Mol. Life Sci. 2024, 81, 266. [Google Scholar] [CrossRef]
- Diochot, S.; Alloui, A.; Rodrigues, P.; Dauvois, M.; Friend, V.; Aissouni, Y.; Eschalier, A.; Lingueglia, E.; Baron, A. Analgesic effects of mambalgin peptide inhibitors of acid-sensing ion channels in inflammatory and neuropathic pain. Pain 2016, 157, 552–559. [Google Scholar] [CrossRef]
- Verkest, C.; Piquet, E.; Diochot, S.; Dauvois, M.; Lanteri-Minet, M.; Lingueglia, E.; Baron, A. Effects of systemic inhibitors of acid-sensing ion channels 1 (ASIC1) against acute and chronic mechanical allodynia in a rodent model of migraine. Br. J. Pharmacol. 2018, 175, 4154–4166. [Google Scholar] [CrossRef] [PubMed]
- Salinas, M.; Besson, T.; Delettre, Q.; Diochot, S.; Boulakirba, S.; Douguet, D.; Lingueglia, E. Binding site and inhibitory mechanism of the mambalgin-2 pain-relieving peptide on acid-sensing ion channel 1a. J. Biol. Chem. 2014, 289, 13363–13373. [Google Scholar] [CrossRef] [PubMed]
- Mourier, G.; Salinas, M.; Kessler, P.; Stura, E.A.; Leblanc, M.; Tepshi, L.; Besson, T.; Diochot, S.; Baron, A.; Douguet, D.; et al. Mambalgin-1 pain-relieving peptide, stepwise solid-phase synthesis, crystal structure, and functional domain for acid-sensing ion channel 1a inhibition. J. Biol. Chem. 2016, 291, 2616–2629. [Google Scholar] [CrossRef]
- Bychkov, M.; Shulepko, M.; Osmakov, D.; Andreev, Y.; Sudarikova, A.; Vasileva, V.; Pavlyukov, M.S.; Latyshev, Y.A.; Potapov, A.A.; Kirpichnikov, M.; et al. Mambalgin-2 induces cell cycle arrest and apoptosis in glioma cells via interaction with ASIC1a. Cancers 2020, 12, 1837. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lei, X.; Li, A.; Li, J.; Li, S.; Chen, L. Scalable production of recombinant three-finger proteins: From inclusion bodies to high quality molecular probes. Microb. Cell Fact. 2024, 23, 48. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the ExPASy server. In The Proteomics Protocols Handbook; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Go, Y.-M.; Roede, J.R.; Walker, D.I.; Duong, D.M.; Seyfried, N.T.; Orr, M.; Liang, Y.; Pennell, K.D.; Jones, D.P. Selective targeting of the cysteine proteome by thioredoxin and glutathione redox systems. Mol. Cell. Proteom. 2013, 12, 3285–3296. [Google Scholar] [CrossRef] [PubMed]
- Logashina, Y.A.; Korolkova, Y.V.; Maleeva, E.E.; Osmakov, D.I.; Kozlov, S.A.; Andreev, Y.A. Refolding of disulfide containing peptides in fusion with thioredoxin. Mendeleev Commun. 2020, 30, 214–216. [Google Scholar] [CrossRef]
- Chang, C.-T.; Fong, S.W.; Lee, C.-H.; Chuang, Y.-C.; Lin, S.-H.; Chen, C.-C. Involvement of acid-sensing ion channel 1b in the development of acid-induced chronic muscle pain. Front. Neurosci. 2019, 13, 1247. [Google Scholar] [CrossRef]
- Gu, Q.; Lee, L.Y. Acid-sensing ion channels and pain. Pharmaceuticals 2010, 3, 1411–1425. [Google Scholar] [CrossRef]
- Kini, R.M.; Doley, R. Structure, function and evolution of three-finger toxins: Mini proteins with multiple targets. Toxicon 2010, 56, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Utkin, Y.N. Last decade update for three-finger toxins: Newly emerging structures and biological activities. World J. Biol. Chem. 2019, 10, 17–27. [Google Scholar] [CrossRef]
- Kalinovskii, A.P.; Osmakov, D.I.; Koshelev, S.G.; Lubova, K.I.; Korolkova, Y.V.; Kozlov, S.A.; Andreev, Y.A. Retinoic acid-differentiated neuroblastoma SH-SY5Y is an accessible in vitro model to study native human acid-sensing ion channels 1a (ASIC1a). Biology 2022, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Osmakov, D.I.; Koshelev, S.G.; Lyukmanova, E.N.; Shulepko, M.A.; Andreev, Y.A.; Illes, P.; Kozlov, S.A. Multiple modulation of acid-sensing ion channel 1a by the alkaloid daurisoline. Biomolecules 2019, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Young, G.T.; Gutteridge, A.; Fox, H.D.E.; Wilbrey, A.L.; Cao, L.; Cho, L.T.; Brown, A.R.; Benn, C.L.; Kammonen, L.R.; Friedman, J.H.; et al. Characterizing human stem cell–derived sensory neurons at the single-cell level reveals their ion channel expression and utility in pain research. Mol. Ther. 2014, 22, 1530–1543. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, K.; Kumar, R.; Singh, S.; Tuknait, A.; Gautam, A.; Mathur, D.; Anand, P.; Varshney, G.C.; Raghava, G.P.S. A Web server and mobile app for computing hemolytic potency of peptides. Sci. Rep. 2016, 6, 22843. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P. How does hormesis impact biology, toxicology, and medicine? NPJ Aging Mech. Dis. 2017, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Andreev, Y.; Osmakov, D.; Koshelev, S.; Maleeva, E.; Logashina, Y.; Palikov, V.; Palikova, Y.; Dyachenko, I.; Kozlov, S. Analgesic activity of acid-sensing ion channel 3 (ASIC3) inhibitors: Sea anemones peptides Ugr9-1 and APETx2 versus low molecular weight compounds. Mar. Drugs 2018, 16, 500. [Google Scholar] [CrossRef] [PubMed]
- Khasanov, T.A.; Maleeva, E.E.; Koshelev, S.G.; Palikov, V.A.; Palikova, Y.A.; Dyachenko, I.A.; Kozlov, S.A.; Andreev, Y.A.; Osmakov, D.I. Mutagenesis of the peptide inhibitor of ASIC3 channel introduces binding to thumb domain of ASIC1a but reduces analgesic activity. Mar. Drugs 2024, 22, 382. [Google Scholar] [CrossRef]
- Lyskov, S.; Gray, J.J. The RosettaDock server for local protein-protein docking. Nucleic Acids Res. 2008, 36, W233–W238. [Google Scholar] [CrossRef]
- Osmakov, D.I.; Koshelev, S.G.; Palikov, V.A.; Palikova, Y.A.; Shaykhutdinova, E.R.; Dyachenko, I.A.; Andreev, Y.A.; Kozlov, S.A. Alkaloid lindoldhamine inhibits acid-sensing ion channel 1a and reveals anti-inflammatory properties. Toxins 2019, 11, 542. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osmakov, D.I.; Khasanov, T.A.; Maleeva, E.E.; Pavlov, V.M.; Palikov, V.A.; Belozerova, O.A.; Koshelev, S.G.; Korolkova, Y.V.; Dyachenko, I.A.; Kozlov, S.A.; et al. Two Amino Acid Substitutions Improve the Pharmacological Profile of the Snake Venom Peptide Mambalgin. Toxins 2025, 17, 101. https://doi.org/10.3390/toxins17030101
Osmakov DI, Khasanov TA, Maleeva EE, Pavlov VM, Palikov VA, Belozerova OA, Koshelev SG, Korolkova YV, Dyachenko IA, Kozlov SA, et al. Two Amino Acid Substitutions Improve the Pharmacological Profile of the Snake Venom Peptide Mambalgin. Toxins. 2025; 17(3):101. https://doi.org/10.3390/toxins17030101
Chicago/Turabian StyleOsmakov, Dmitry I., Timur A. Khasanov, Ekaterina E. Maleeva, Vladimir M. Pavlov, Victor A. Palikov, Olga A. Belozerova, Sergey G. Koshelev, Yuliya V. Korolkova, Igor A. Dyachenko, Sergey A. Kozlov, and et al. 2025. "Two Amino Acid Substitutions Improve the Pharmacological Profile of the Snake Venom Peptide Mambalgin" Toxins 17, no. 3: 101. https://doi.org/10.3390/toxins17030101
APA StyleOsmakov, D. I., Khasanov, T. A., Maleeva, E. E., Pavlov, V. M., Palikov, V. A., Belozerova, O. A., Koshelev, S. G., Korolkova, Y. V., Dyachenko, I. A., Kozlov, S. A., & Andreev, Y. A. (2025). Two Amino Acid Substitutions Improve the Pharmacological Profile of the Snake Venom Peptide Mambalgin. Toxins, 17(3), 101. https://doi.org/10.3390/toxins17030101





