Neurotoxins Acting on TRPV1—Building a Molecular Template for the Study of Pain and Thermal Dysfunctions
Abstract
1. Introduction
1.1. TRPV1: Structure and Function
1.2. TRPV1 Modulation
1.3. TRPV1 Expression in Mammals
- (i)
- Pruritogen receptor-enriched sensory neurons (involved in itch-sensing);
- (ii)
- Putative C low-threshold mechanoreceptors involved in gentle touch (C-LTMRs);
- (iii)
- TRPA1-enriched nociceptors (involved in cold, itch pain, and detection of irritants);
- (iv)
- Putative silent nociceptors [51,52]—Sensoryomics website https://paincenter.utdallas.edu/sensoryomics/ accessed on 26 January 2025—which correspond to a subset of C-fibers that innervate joints, viscera, and skin, often referred to mechanoinsensitive C-fibers [53]. The latter are unresponsive to noxious mechanical stimuli under normal conditions but can be sensitized after inflammatory stimulation.
1.4. Role in Inflammation
1.5. TRPV1 Channelopathies
| Variant | Position in Protein | Human Disease | Clinical Features | Pharmacological Signature | Ref. |
|---|---|---|---|---|---|
| N331K | N-terminal 5th ankyrin finger | Pain insensitivity | Loss of function in pain syndroms: heat hyposensitivity and cold hypersensitivity, extensive sweating, no aversion for CAP-containing food | Insensitivity to CAP/RTX/DkTx, acidic pH, heat | [68] |
| N394del | N-terminal domain | MH | Generalized hyperthermia after exposure to VAs | Insensitivity to CAP, impaired Ca2+ homeostasis after VA | [71] |
| T612M | Pore turret | MH | Generalized hyperthermia after exposure to VAs | Insensitivity to CAP, impaired Ca2+ homeostasis after VA | [71] |
| G684V | S6 transmembrane domain | EHS | Generalized hyperthermia after prolonged and intense activity | Insensitivity to CAP, in vitro muscle contracture after halothane or caffeine | [70] |
| K710N | TRP domain | Pain insensitivity | Loss of function in pain syndrome (mouse): CAP-induced hyposensitivity, reduced neuropathic pain, no aversion for CAP-containing food | Insensitivity to CAP | [69] |
| R772C | CaM binding domain | EHS | Generalized hyperthermia after prolonged and intense activity | In vitro muscle contracture after halothane | [70] |
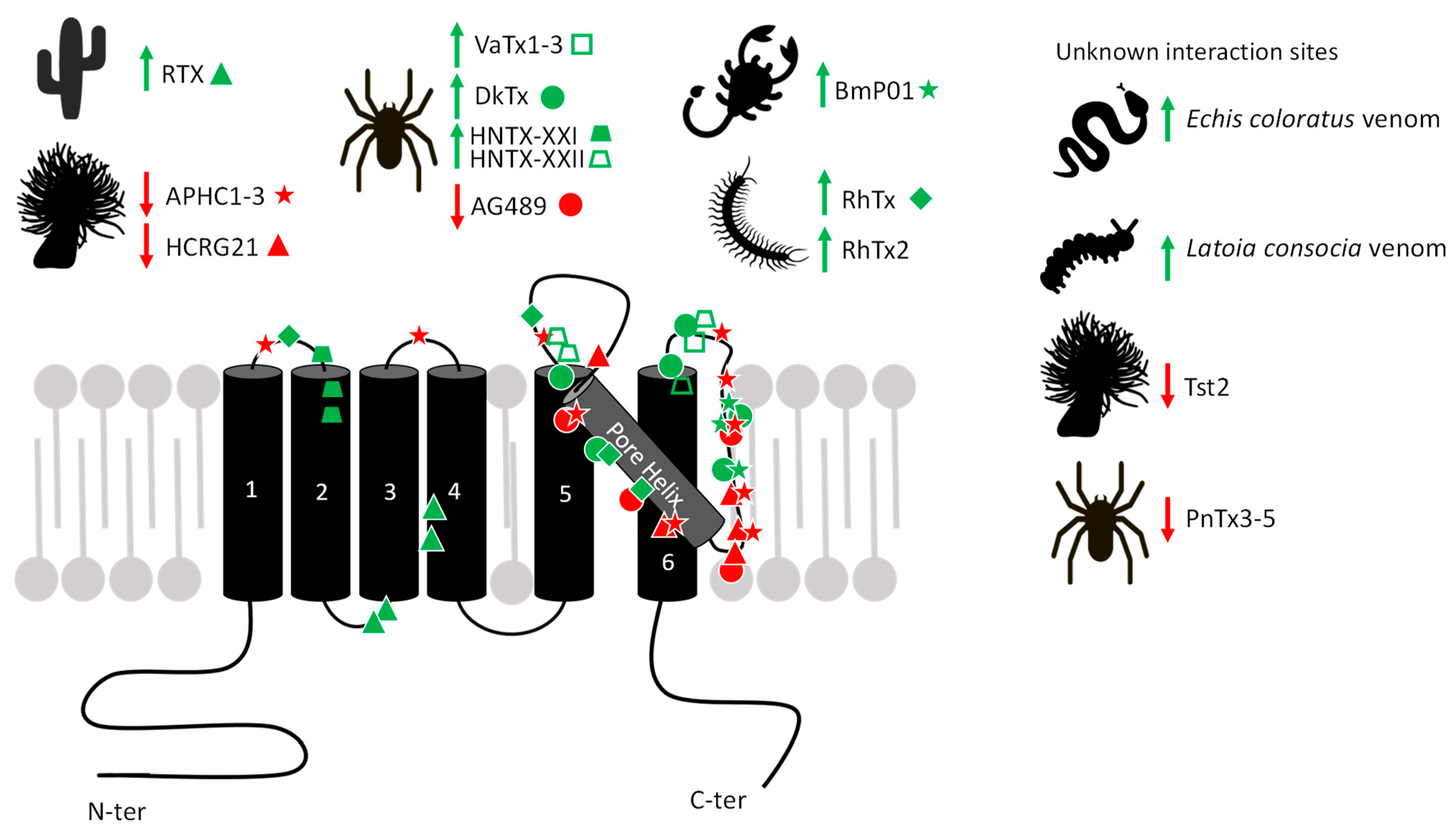
2. Toxins Acting on TRPV1
2.1. Agonists
2.1.1. RTX
2.1.2. Vanillotoxins (VaTx)
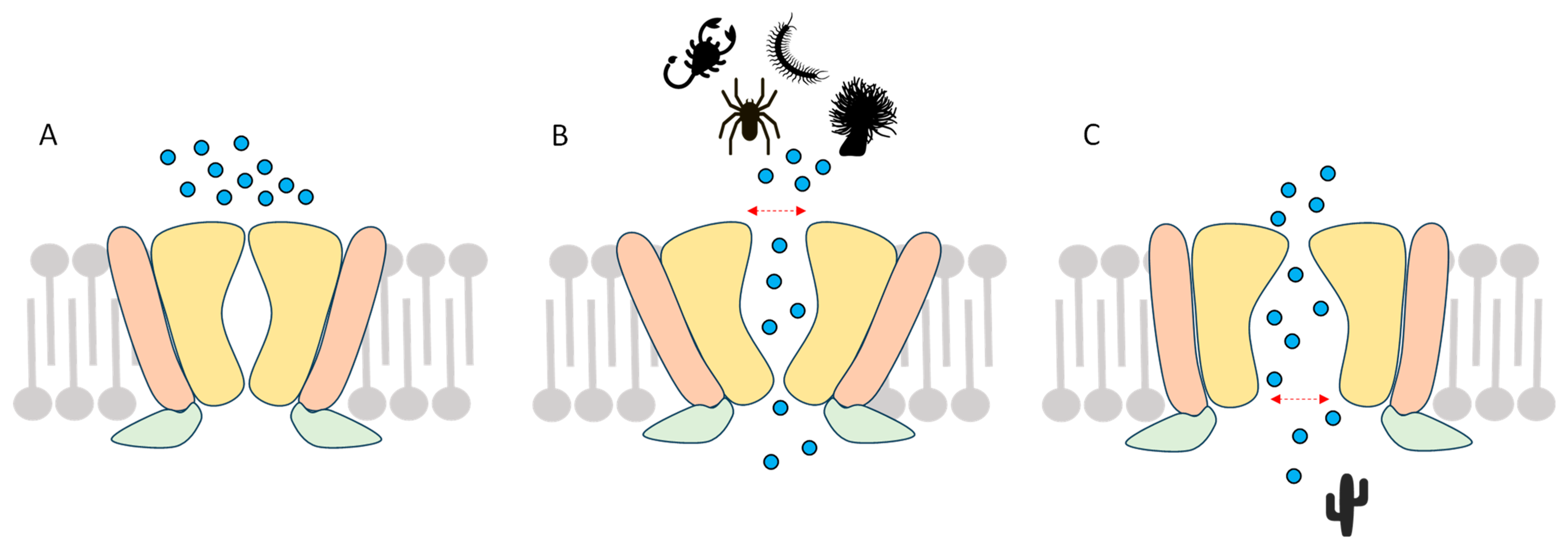
2.1.3. Double-Knot Toxin (DkTx)
2.1.4. BmP01
 ), [92] for DkTx (●), [99] for BmP01 (
), [92] for DkTx (●), [99] for BmP01 ( ), [100,101] for RhTx (♦), and [102] for HNTX-XXI (
), [100,101] for RhTx (♦), and [102] for HNTX-XXI ( ) and HNTX-XXII (
) and HNTX-XXII ( ). Latoia consocia is from ©Daniel Ruyle. All other images are from Wikimedia commons.
). Latoia consocia is from ©Daniel Ruyle. All other images are from Wikimedia commons.
 ), [92] for DkTx (●), [99] for BmP01 (
), [92] for DkTx (●), [99] for BmP01 ( ), [100,101] for RhTx (♦), and [102] for HNTX-XXI (
), [100,101] for RhTx (♦), and [102] for HNTX-XXI ( ) and HNTX-XXII (
) and HNTX-XXII ( ). Latoia consocia is from ©Daniel Ruyle. All other images are from Wikimedia commons.
). Latoia consocia is from ©Daniel Ruyle. All other images are from Wikimedia commons.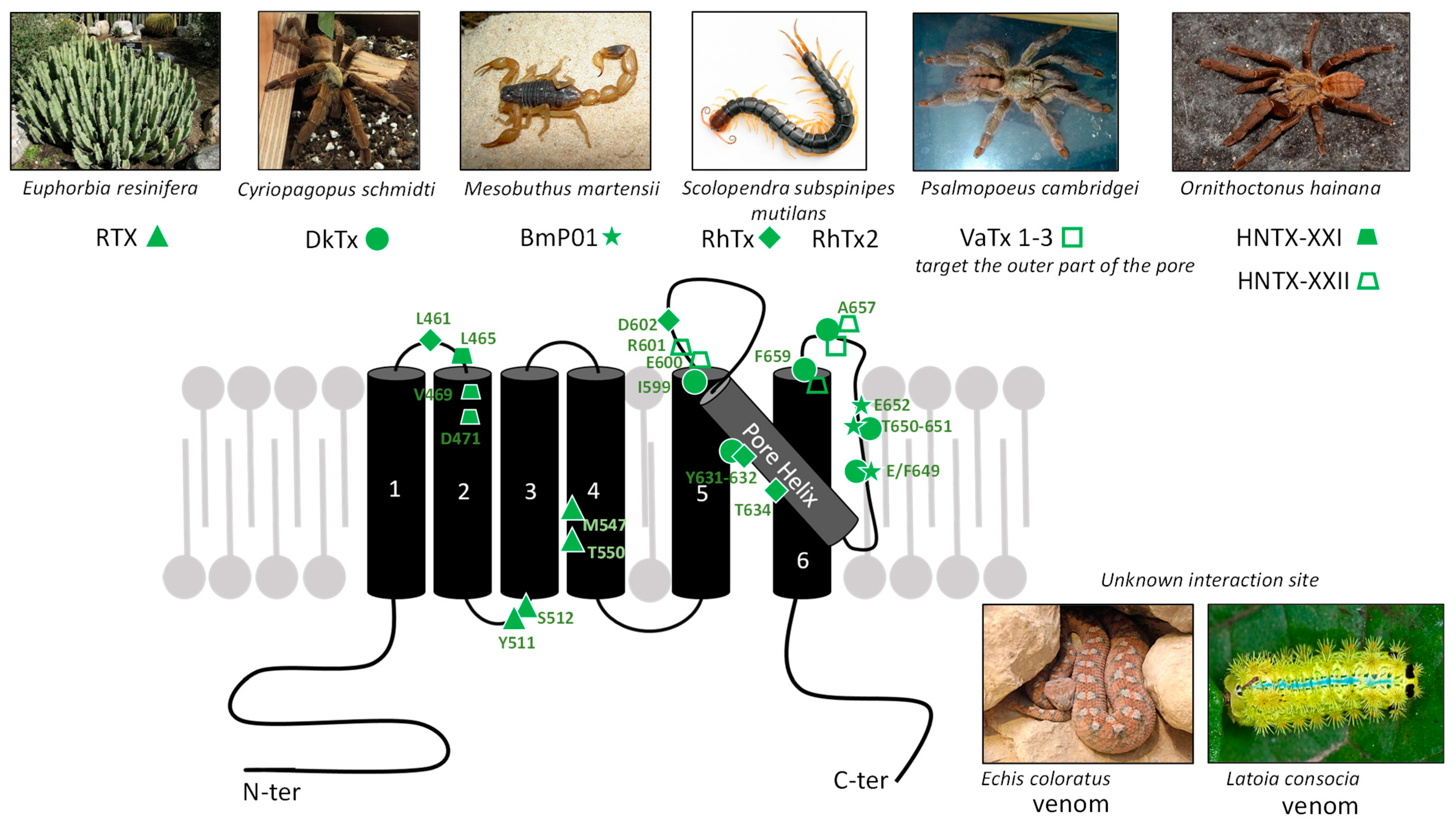
2.1.5. RhTx Toxin
2.1.6. Hainantoxins (HNTX-XXI and HNTX-XXII)
2.1.7. Viperidae Venom
2.1.8. Caterpillar Venom
| Molecule | Effect on TRPV1 | EC50/IC50 | Mode of Action | Site of Action | Identification or Prediction of the Site of Action | Ref. |
|---|---|---|---|---|---|---|
| CAP | Agonist | 712 nM | Stabilizes the open state | S3, S4 | Cryoelectromicroscopy | [9,25] |
| RTX | 39 nM | Stabilizes the open state through expansion of the lower gate | S3, S4 | Cryoelectromicroscopy | [9,25] | |
| DkTx | 230 nM | Widens the upper pore | Outer pore domain | Cryoelectromicroscopy, site-directed mutagenesis | [25,92,94] | |
| VaTx1 VaTx2 VaTx3 | 9.9 µM 1.35 µM 0.45 µM | Widens the upper pore | Outer pore domain | Site-directed mutagenesis | [87,92] | |
| BmP01 | 131.8 µM 40.4 µM | Widens the upper pore in acidic pH | Outer pore domain | Site-directed mutagenesis | [98,99] | |
| RhTx RhTx2 | 470–521.5 nM 38.3 µM | Widens the upper pore in a T°-dependent manner | Outer pore domain + S1/S2 loop | Site-directed mutagenesis | [100,101] | |
| HNTX-XXI HNTX-XXII | 3.6 µM 0.86 μM | Widens the upper pore | Outer pore domain | Site-directed mutagenesis | [102] | |
| AG489 | Antagonist | 300 nM | Pore blocking | Pore-forming extracellular loop | Site-directed mutagenesis | [107] |
| PnTx3-5 | 30 nM | nd | nd | nd | [108] | |
| APHC1 APHC2 APHC3 | 60 nM 23 NM 18 nM | Stabilize an intermediate state at receptor activation | Pore helix and outer pore domain; S1/S2 and S3/S4 external loops | Molecular modeling | [109] | |
| HCRG21 | 6.9 µM | nd | Outer pore domain | Molecular modelling | [110] | |
| Tst2 | 100 nM | nd | nd | nd | [111] |
2.2. Antagonists
2.2.1. AG489
2.2.2. PnTx3-5
2.2.3. APHC1-3 Polypeptides
2.2.4. HCRG21
2.2.5. Tst2
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AG489 | Agelenopsis aperta toxin; |
| APHC | Analgesic Polypeptide from Heteractis crispa; |
| ARD | Ankyrin Repeated Domain; |
| ASICs | Acid-Sensing Ion Channels; |
| BmP01 | Mesobutus martensii toxin; |
| CAP | Capsaicin; |
| CPZ | Capsazepin; |
| CB1 | Cannabinoid Receptor 1; |
| CFA | Complete Freund’s Adjuvant; |
| DkTx | Double-Knot Toxin; |
| DRG | Dorsal Root Ganglion; |
| EC50 | Half Maximal Effective Concentration; |
| EHS | Exertional Heat Stroke; |
| HCRG21 | Heteractis crispa RG 21; |
| ICK | Inhibitor Cysteine Knot; |
| IC50 | Half Maximal Inhibitory Concentration; |
| I-RTX | Iodo-resiniferatoxin; |
| LPA | Lysophosphatidic acid; |
| MH | Malignant Hyperthermia; |
| NGF | Nerve Growth Factor; |
| NMDA | N-methyl-D-aspartate; |
| PnTx | Phoneutria nigriventer toxin; |
| RhTx | Red-headed centipede toxin; |
| RTX | Resiniferatoxin; |
| TRP | Transient Receptor Potential; |
| TRPV1 | Transient Receptor Potential Vanilloid 1; |
| hTRPV1 | human TRPV1; |
| rTRPV1 | rat TRPV1; |
| TrkA | Tyrosine kinase A; |
| VA | Volatile anesthetics; |
| VaTx | Vanillotoxin; |
| wt | wild-type. |
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The Revised International Association for the Study of Pain Definition of Pain: Concepts, Challenges, and Compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.L. Pain Processing in the Human Nervous System. Prim. Care Clin. Off. Pract. 2012, 39, 561–571. [Google Scholar] [CrossRef]
- Moore, C.; Gupta, R.; Jordt, S.-E.; Chen, Y.; Liedtke, W.B. Regulation of Pain and Itch by TRP Channels. Neurosci. Bull. 2018, 34, 120–142. [Google Scholar] [CrossRef] [PubMed]
- Jardín, I.; López, J.J.; Diez, R.; Sánchez-Collado, J.; Cantonero, C.; Albarrán, L.; Woodard, G.E.; Redondo, P.C.; Salido, G.M.; Smani, T.; et al. TRPs in Pain Sensation. Front. Physiol. 2017, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Owsianik, G.; Talavera, K.; Voets, T.; Nilius, B. Permeation and Selectivity of TRP Channels. Annu. Rev. Physiol. 2006, 68, 685–717. [Google Scholar] [CrossRef]
- Cesare, P.; McNaughton, P. A Novel Heat-Activated Current in Nociceptive Neurons and Its Sensitization by Bradykinin. Proc. Natl. Acad. Sci. USA 1996, 93, 15435–15439. [Google Scholar] [CrossRef]
- Wood, J.; Winter, J.; James, I.; Rang, H.; Yeats, J.; Bevan, S. Capsaicin-Induced Ion Fluxes in Dorsal Root Ganglion Cells in Culture. J. Neurosci. 1988, 8, 3208–3220. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The Capsaicin Receptor: A Heat-Activated Ion Channel in the Pain Pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Yang, F.; Xiao, X.; Lee, B.H.; Vu, S.; Yang, W.; Yarov-Yarovoy, V.; Zheng, J. The Conformational Wave in Capsaicin Activation of Transient Receptor Potential Vanilloid 1 Ion Channel. Nat. Commun. 2018, 9, 2879. [Google Scholar] [CrossRef]
- Yang, F.; Zheng, J. Understand Spiciness: Mechanism of TRPV1 Channel Activation by Capsaicin. Protein Cell 2017, 8, 169–177. [Google Scholar] [CrossRef]
- Julius, D. TRP Channels and Pain. Annu. Rev. Cell Dev. Biol. 2013, 29, 355–384. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Hoon, M.A. Ablation of TrpV1 Neurons Reveals Their Selective Role in Thermal Pain Sensation. Mol. Cell. Neurosci. 2010, 43, 157–163. [Google Scholar] [CrossRef]
- Zhang, F.; Jara-Oseguera, A.; Chang, T.-H.; Bae, C.; Hanson, S.M.; Swartz, K.J. Heat Activation Is Intrinsic to the Pore Domain of TRPV1. Proc. Natl. Acad. Sci. USA 2018, 115, E317–E324. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, A.; Uzzell, V.; Dubin, A.E.; Mathur, J.; Petrus, M.; Bandell, M.; Patapoutian, A. TRPV1 Is Activated by Both Acidic and Basic pH. J. Neurosci. 2009, 29, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Ugawa, S.; Ueda, T.; Ishida, Y.; Nishigaki, M.; Shibata, Y.; Shimada, S. Amiloride-Blockable Acid-Sensing Ion Channels Are Leading Acid Sensors Expressed in Human Nociceptors. J. Clin. Invest. 2002, 110, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.G.; Slater, R.; Cadiou, H.; McNaughton, P.; McMahon, S.B. Acid-Induced Pain and Its Modulation in Humans. J. Neurosci. 2004, 24, 10974–10979. [Google Scholar] [CrossRef]
- Heber, S.; Ciotu, C.I.; Hartner, G.; Gold-Binder, M.; Ninidze, N.; Gleiss, A.; Kress, H.-G.; Fischer, M.J.M. TRPV1 Antagonist BCTC Inhibits pH 6.0-Induced Pain in Human Skin. Pain 2020, 161, 1532–1541. [Google Scholar] [CrossRef]
- Baumann, T.K.; Burchiel, K.J.; Ingram, S.L.; Martenson, M.E. Responses of Adult Human Dorsal Root Ganglion Neurons in Culture to Capsaicin and Low pH. Pain 1996, 65, 31–38. [Google Scholar] [CrossRef]
- Baumann, T.K.; Chaudhary, P.; Martenson, M.E. Background Potassium Channel Block and TRPV1 Activation Contribute to Proton Depolarization of Sensory Neurons from Humans with Neuropathic Pain. Eur. J. Neurosci. 2004, 19, 1343–1351. [Google Scholar] [CrossRef]
- Latorre, R.; Zaelzer, C.; Brauchi, S. Structure–Functional Intimacies of Transient Receptor Potential Channels. Quart. Rev. Biophys. 2009, 42, 201–246. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Cao, E.; Julius, D.; Cheng, Y. Structure of the TRPV1 Ion Channel Determined by Electron Cryo-Microscopy. Nature 2013, 504, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Gavva, N.R.; Klionsky, L.; Qu, Y.; Shi, L.; Tamir, R.; Edenson, S.; Zhang, T.J.; Viswanadhan, V.N.; Toth, A.; Pearce, L.V.; et al. Molecular Determinants of Vanilloid Sensitivity in TRPV1. J. Biol. Chem. 2004, 279, 20283–20295. [Google Scholar] [CrossRef] [PubMed]
- Bohlen, C.J.; Julius, D. Receptor-Targeting Mechanisms of Pain-Causing Toxins: How Ow? Toxicon 2012, 60, 254–264. [Google Scholar] [CrossRef]
- Cao, E.; Liao, M.; Cheng, Y.; Julius, D. TRPV1 Structures in Distinct Conformations Reveal Activation Mechanisms. Nature 2013, 504, 113–118. [Google Scholar] [CrossRef]
- Gao, Y.; Cao, E.; Julius, D.; Cheng, Y. TRPV1 Structures in Nanodiscs Reveal Mechanisms of Ligand and Lipid Action. Nature 2016, 534, 347–351. [Google Scholar] [CrossRef]
- Garcia-Sanz, N. Identification of a Tetramerization Domain in the C Terminus of the Vanilloid Receptor. J. Neurosci. 2004, 24, 5307–5314. [Google Scholar] [CrossRef]
- Jordt, S.-E.; Tominaga, M.; Julius, D. Acid Potentiation of the Capsaicin Receptor Determined by a Key Extracellular Site. Proc. Natl. Acad. Sci. USA 2000, 97, 8134–8139. [Google Scholar] [CrossRef]
- Ryu, S.; Liu, B.; Yao, J.; Fu, Q.; Qin, F. Uncoupling Proton Activation of Vanilloid Receptor TRPV1. J. Neurosci. 2007, 27, 12797–12807. [Google Scholar] [CrossRef]
- Aneiros, E.; Cao, L.; Papakosta, M.; Stevens, E.B.; Phillips, S.; Grimm, C. The Biophysical and Molecular Basis of TRPV1 Proton Gating: Biophysical and Molecular Basis of TRPV1 Proton Gating. EMBO J. 2011, 30, 994–1002. [Google Scholar] [CrossRef]
- Grycova, L.; Lansky, Z.; Friedlova, E.; Vlachova, V.; Kubala, M.; Obsilova, V.; Obsil, T.; Teisinger, J. ATP Binding Site on the C-Terminus of the Vanilloid Receptor. Arch. Biochem. Biophys. 2007, 465, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Lishko, P.V.; Procko, E.; Jin, X.; Phelps, C.B.; Gaudet, R. The Ankyrin Repeats of TRPV1 Bind Multiple Ligands and Modulate Channel Sensitivity. Neuron 2007, 54, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Nersesyan, Y.; Demirkhanyan, L.; Cabezas-Bratesco, D.; Oakes, V.; Kusuda, R.; Dawson, T.; Sun, X.; Cao, C.; Cohen, A.M.; Chelluboina, B.; et al. Oxytocin Modulates Nociception as an Agonist of Pain-Sensing TRPV1. Cell Rep. 2017, 21, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.; Prescott, E.D.; Kong, H.; Shields, S.; Jordt, S.-E.; Basbaum, A.I.; Chao, M.V.; Julius, D. Bradykinin and Nerve Growth Factor Release the Capsaicin Receptor from PtdIns(4,5)P2-Mediated Inhibition. Nature 2001, 411, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Prescott, E.D.; Julius, D. A Modular PIP2 Binding Site as a Determinant of Capsaicin Receptor Sensitivity. Science 2003, 300, 1284–1288. [Google Scholar] [CrossRef]
- Numazaki, M.; Tominaga, T.; Toyooka, H.; Tominaga, M. Direct Phosphorylation of Capsaicin Receptor VR1 by Protein Kinase Cε and Identification of Two Target Serine Residues. J. Biol. Chem. 2002, 277, 13375–13378. [Google Scholar] [CrossRef]
- Bhave, G.; Zhu, W.; Wang, H.; Brasier, D.J.; Oxford, G.S.; Gereau, R.W. cAMP-Dependent Protein Kinase Regulates Desensitization of the Capsaicin Receptor (VR1) by Direct Phosphorylation. Neuron 2002, 35, 721–731. [Google Scholar] [CrossRef]
- Starowicz, K.; Nigam, S.; Di Marzo, V. Biochemistry and Pharmacology of Endovanilloids. Pharmacol. Ther. 2007, 114, 13–33. [Google Scholar] [CrossRef]
- Martins, D.; Tavares, I.; Morgado, C. “Hotheaded”: The Role OF TRPV1 in Brain Functions. Neuropharmacology 2014, 85, 151–157. [Google Scholar] [CrossRef]
- Jordt, S.-E.; Julius, D. Molecular Basis for Species-Specific Sensitivity to “Hot” Chili Peppers. Cell 2002, 108, 421–430. [Google Scholar] [CrossRef]
- Arora, V.; Campbell, J.N.; Chung, M.-K. Fight Fire with Fire: Neurobiology of Capsaicin-Induced Analgesia for Chronic Pain. Pharmacol. Ther. 2021, 220, 107743. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Blair, N.T.; Clapham, D.E. Camphor Activates and Strongly Desensitizes the Transient Receptor Potential Vanilloid Subtype 1 Channel in a Vanilloid-Independent Mechanism. J. Neurosci. 2005, 25, 8924–8937. [Google Scholar] [CrossRef] [PubMed]
- Frias, B.; Merighi, A. Capsaicin, Nociception and Pain. Molecules 2016, 21, 797. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, A.; Hochgerner, H.; Lönnerberg, P.; Johnsson, A.; Memic, F.; Van Der Zwan, J.; Häring, M.; Braun, E.; Borm, L.E.; La Manno, G.; et al. Molecular Architecture of the Mouse Nervous System. Cell 2018, 174, 999–1014.e22. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired Nociception and Pain Sensation in Mice Lacking the Capsaicin Receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef]
- Montell, C.; Caterina, M.J. Thermoregulation: Channels That Are Cool to the Core. Curr. Biol. 2007, 17, R885–R887. [Google Scholar] [CrossRef]
- Szolcsányi, J. Effect of Capsaicin on Thermoregulation: An Update with New Aspects. Temperature 2015, 2, 277–296. [Google Scholar] [CrossRef]
- Szallasi, A. Autoradiographic Visualization and Pharmacological Characterization of Vanilloid (Capsaicin) Receptors in Several Species, Including Man. Acta Physiol. Scand. Suppl. 1995, 629, 1–68. [Google Scholar]
- Papalampropoulou-Tsiridou, M.; Shiers, S.; Wang, F.; Godin, A.G.; Price, T.J.; De Koninck, Y. Distribution of Acid-Sensing Ion Channel Subunits in Human Sensory Neurons Contrasts with That in Rodents. Brain Commun. 2022, 4, fcac256. [Google Scholar] [CrossRef]
- Shiers, S.; Klein, R.M.; Price, T.J. Quantitative Differences in Neuronal Subpopulations between Mouse and Human Dorsal Root Ganglia Demonstrated with RNAscope in Situ Hybridization. Pain 2020, 161, 2410–2424. [Google Scholar] [CrossRef]
- Ray, P.; Torck, A.; Quigley, L.; Wangzhou, A.; Neiman, M.; Rao, C.; Lam, T.; Kim, J.-Y.; Kim, T.H.; Zhang, M.Q.; et al. Comparative Transcriptome Profiling of the Human and Mouse Dorsal Root Ganglia: An RNA-Seq–Based Resource for Pain and Sensory Neuroscience Research. Pain 2018, 159, 1325–1345. [Google Scholar] [CrossRef] [PubMed]
- Tavares-Ferreira, D.; Shiers, S.; Ray, P.R.; Wangzhou, A.; Jeevakumar, V.; Sankaranarayanan, I.; Cervantes, A.M.; Reese, J.C.; Chamessian, A.; Copits, B.A.; et al. Spatial Transcriptomics of Dorsal Root Ganglia Identifies Molecular Signatures of Human Nociceptors. Sci. Transl. Med. 2022, 14, eabj8186. [Google Scholar] [CrossRef] [PubMed]
- Prato, V.; Taberner, F.J.; Hockley, J.R.F.; Callejo, G.; Arcourt, A.; Tazir, B.; Hammer, L.; Schad, P.; Heppenstall, P.A.; Smith, E.S.; et al. Functional and Molecular Characterization of Mechanoinsensitive “Silent” Nociceptors. Cell Rep. 2017, 21, 3102–3115. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, D.J.; Chesler, A.T.; Jackson, A.C.; Sigal, Y.M.; Yamanaka, H.; Grant, R.; O’Donnell, D.; Nicoll, R.A.; Shah, N.M.; Julius, D.; et al. Trpv1 Reporter Mice Reveal Highly Restricted Brain Distribution and Functional Expression in Arteriolar Smooth Muscle Cells. J. Neurosci. 2011, 31, 5067–5077. [Google Scholar] [CrossRef]
- Fernandes, E.; Fernandes, M.; Keeble, J. The Functions of TRPA1 and TRPV1: Moving Away from Sensory Nerves. Br. J. Pharmacol. 2012, 166, 510–521. [Google Scholar] [CrossRef]
- Luo, Z.; Ma, L.; Zhao, Z.; He, H.; Yang, D.; Feng, X.; Ma, S.; Chen, X.; Zhu, T.; Cao, T.; et al. TRPV1 Activation Improves Exercise Endurance and Energy Metabolism through PGC-1α Upregulation in Mice. Cell Res. 2012, 22, 551–564. [Google Scholar] [CrossRef]
- Zhong, B.; Ma, S.; Wang, D.H. TRPV1 Mediates Glucose-Induced Insulin Secretion Through Releasing Neuropeptides. Vivo 2019, 33, 1431–1437. [Google Scholar] [CrossRef]
- Panchal, S.K.; Bliss, E.; Brown, L. Capsaicin in Metabolic Syndrome. Nutrients 2018, 10, 630. [Google Scholar] [CrossRef]
- Gallego-Sandín, S.; Rodríguez-García, A.; Alonso, M.T.; García-Sancho, J. The Endoplasmic Reticulum of Dorsal Root Ganglion Neurons Contains Functional TRPV1 Channels. J. Biol. Chem. 2009, 284, 32591–32601. [Google Scholar] [CrossRef]
- Ellis, A.; Bennett, D.L.H. Neuroinflammation and the Generation of Neuropathic Pain. Br. J. Anaesth. 2013, 111, 26–37. [Google Scholar] [CrossRef]
- Palazzo, E.; Luongo, L.; De Novellis, V.; Rossi, F.; Marabese, I.; Maione, S. Transient Receptor Potential Vanilloid Type 1 and Pain Development. Curr. Opin. Pharmacol. 2012, 12, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.Y.; Jung, J.Y.; Hwang, S.W.; Lee, W.T.; Oh, U. A Capsaicin-Receptor Antagonist, Capsazepine, Reduces Inflammation-Induced Hyperalgesic Responses in the Rat: Evidence for an Endogenous Capsaicin-like Substance. Neuroscience 1998, 86, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.B.; Gray, J.; Gunthorpe, M.J.; Hatcher, J.P.; Davey, P.T.; Overend, P.; Harries, M.H.; Latcham, J.; Clapham, C.; Atkinson, K.; et al. Vanilloid Receptor-1 Is Essential for Inflammatory Thermal Hyperalgesia. Nature 2000, 405, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Geppetti, P.; Trevisani, M. Activation and Sensitisation of the Vanilloid Receptor: Role in Gastrointestinal Inflammation and Function. Br. J. Pharmacol. 2004, 141, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Morales-Lázaro, S.L.; Rosenbaum, T. A Painful Link between the TRPV1 Channel and Lysophosphatidic Acid. Life Sci. 2015, 125, 15–24. [Google Scholar] [CrossRef]
- Bujak, J.K.; Kosmala, D.; Szopa, I.M.; Majchrzak, K.; Bednarczyk, P. Inflammation, Cancer and Immunity—Implication of TRPV1 Channel. Front. Oncol. 2019, 9, 1087. [Google Scholar] [CrossRef]
- Szallasi, A.; Cortright, D.N.; Blum, C.A.; Eid, S.R. The Vanilloid Receptor TRPV1: 10 Years from Channel Cloning to Antagonist Proof-of-Concept. Nat. Rev. Drug Discov. 2007, 6, 357–372. [Google Scholar] [CrossRef]
- Katz, B.; Zaguri, R.; Edvardson, S.; Maayan, C.; Elpeleg, O.; Lev, S.; Davidson, E.; Peters, M.; Kfir-Erenfeld, S.; Berger, E.; et al. Nociception and Pain in Humans Lacking a Functional TRPV1 Channel. J. Clin. Investig. 2023, 133, e153558. [Google Scholar] [CrossRef]
- He, S.; Zambelli, V.O.; Sinharoy, P.; Brabenec, L.; Bian, Y.; Rwere, F.; Hell, R.C.R.; Stein Neto, B.; Hung, B.; Yu, X.; et al. A Human TRPV1 Genetic Variant within the Channel Gating Domain Regulates Pain Sensitivity in Rodents. J. Clin. Investig. 2023, 133, e163735. [Google Scholar] [CrossRef]
- Bosson, C.; Rendu, J.; Pelletier, L.; Abriat, A.; Chatagnon, A.; Brocard, J.; Brocard, J.; Figarella-Branger, D.; Ducreux, S.; Van Coppenolle, F.; et al. Variations in the TRPV1 Gene Are Associated to Exertional Heat Stroke. J. Sci. Med. Sport. 2020, 23, 1021–1027. [Google Scholar] [CrossRef]
- Vanden Abeele, F.; Lotteau, S.; Ducreux, S.; Dubois, C.; Monnier, N.; Hanna, A.; Gkika, D.; Romestaing, C.; Noyer, L.; Flourakis, M.; et al. TRPV1 Variants Impair Intracellular Ca2+ Signaling and May Confer Susceptibility to Malignant Hyperthermia. Genet. Med. 2019, 21, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Iftinca, M.; Defaye, M.; Altier, C. TRPV1-Targeted Drugs in Development for Human Pain Conditions. Drugs 2021, 81, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Min, J.-W.; Liu, W.-H.; He, X.-H.; Peng, B.-W. Different Types of Toxins Targeting TRPV1 in Pain. Toxicon 2013, 71, 66–75. [Google Scholar] [CrossRef]
- Aghazadeh Tabrizi, M.; Baraldi, P.G.; Baraldi, S.; Gessi, S.; Merighi, S.; Borea, P.A. Medicinal Chemistry, Pharmacology, and Clinical Implications of TRPV1 Receptor Antagonists. Med. Res. Rev. 2017, 37, 936–983. [Google Scholar] [CrossRef] [PubMed]
- Gavva, N.R. Body-Temperature Maintenance as the Predominant Function of the Vanilloid Receptor TRPV1. Trends Pharmacol. Sci. 2008, 29, 550–557. [Google Scholar] [CrossRef]
- Nagy, I.; Sántha, P.; Jancsó, G.; Urbán, L. The Role of the Vanilloid (Capsaicin) Receptor (TRPV1) in Physiology and Pathology. Eur. J. Pharmacol. 2004, 500, 351–369. [Google Scholar] [CrossRef]
- Carnevale, V.; Rohacs, T. TRPV1: A Target for Rational Drug Design. Pharmaceuticals 2016, 9, 52. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology Modelling of Protein Structures and Complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Raisinghani, M.; Pabbidi, R.M.; Premkumar, L.S. Activation of Transient Receptor Potential Vanilloid 1 (TRPV1) by Resiniferatoxin. J. Physiol. 2005, 567, 771–786. [Google Scholar] [CrossRef]
- Rigoni, M.; Trevisani, M.; Gazzieri, D.; Nadaletto, R.; Tognetto, M.; Creminon, C.; Davis, J.B.; Campi, B.; Amadesi, S.; Geppetti, P.; et al. Neurogenic Responses Mediated by Vanilloid Receptor-1 (TRPV1) Are Blocked by the High Affinity Antagonist, Iodo-resiniferatoxin. Br. J. Pharmacol. 2003, 138, 977–985. [Google Scholar] [CrossRef]
- Szallasi, A.; Blumberg, P.M. Resiniferatoxin, a Phorbol-Related Diterpene, Acts as an Ultrapotent Analog of Capsaicin, the Irritant Constituent in Red Pepper. Neuroscience 1989, 30, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Karai, L.; Brown, D.C.; Mannes, A.J.; Connelly, S.T.; Brown, J.; Gandal, M.; Wellisch, O.M.; Neubert, J.K.; Olah, Z.; Iadarola, M.J. Deletion of Vanilloid Receptor 1_expressing Primary Afferent Neurons for Pain Control. J. Clin. Investig. 2004, 113, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Leo, M.; Schulte, M.; Schmitt, L.-I.; Schäfers, M.; Kleinschnitz, C.; Hagenacker, T. Intrathecal Resiniferatoxin Modulates TRPV1 in DRG Neurons and Reduces TNF-Induced Pain-Related Behavior. Mediat. Inflamm. 2017, 2017, 2786427. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.Z.; Mtui, T.; Gao, Y.-D.; Kohler, M.; Middleton, R.E. Resiniferatoxin Binds to the Capsaicin Receptor (TRPV1) near the Extracellular Side of the S4 Transmembrane Domain. Biochemistry 2004, 43, 2501–2511. [Google Scholar] [CrossRef] [PubMed]
- Elokely, K.; Velisetty, P.; Delemotte, L.; Palovcak, E.; Klein, M.L.; Rohacs, T.; Carnevale, V. Understanding TRPV1 Activation by Ligands: Insights from the Binding Modes of Capsaicin and Resiniferatoxin. Proc. Natl. Acad. Sci. USA 2016, 113, E137–E145. [Google Scholar] [CrossRef]
- Szallasi, A. Resiniferatoxin: Nature’s Precision Medicine to Silence TRPV1-Positive Afferents. Int. J. Mol. Sci. 2023, 24, 15042. [Google Scholar] [CrossRef]
- Siemens, J.; Zhou, S.; Piskorowski, R.; Nikai, T.; Lumpkin, E.A.; Basbaum, A.I.; King, D.; Julius, D. Spider Toxins Activate the Capsaicin Receptor to Produce Inflammatory Pain. Nature 2006, 444, 208–212. [Google Scholar] [CrossRef]
- Takahashi, H.; Kim, J.I.; Min, H.J.; Sato, K.; Swartz, K.J.; Shimada, I. Solution Structure of Hanatoxin1, a Gating Modifier of Voltage-Dependent K+ Channels: Common Surface Features of Gating Modifier Toxins. J. Mol. Biol. 2000, 297, 771–780. [Google Scholar] [CrossRef]
- Craik, D.J.; Daly, N.L.; Waine, C. The Cystine Knot Motif in Toxins and Implications for Drug Design. Toxicon 2001, 39, 43–60. [Google Scholar] [CrossRef]
- Redaelli, E.; Cassulini, R.R.; Silva, D.F.; Clement, H.; Schiavon, E.; Zamudio, F.Z.; Odell, G.; Arcangeli, A.; Clare, J.J.; Alagón, A.; et al. Target Promiscuity and Heterogeneous Effects of Tarantula Venom Peptides Affecting Na+ and K+ Ion Channels. J. Biol. Chem. 2010, 285, 4130–4142. [Google Scholar] [CrossRef]
- Swartz, K.J.; MacKinnon, R. An Inhibitor of the Kv2.1 Potassium Channel Isolated from the Venom of a Chilean Tarantula. Neuron 1995, 15, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Bohlen, C.J.; Priel, A.; Zhou, S.; King, D.; Siemens, J.; Julius, D. A Bivalent Tarantula Toxin Activates the Capsaicin Receptor, TRPV1, by Targeting the Outer Pore Domain. Cell 2010, 141, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Escoubas, P.; De Weille, J.R.; Lecoq, A.; Diochot, S.; Waldmann, R.; Champigny, G.; Moinier, D.; Ménez, A.; Lazdunski, M. Isolation of a Tarantula Toxin Specific for a Class of Proton-Gated Na+ Channels. J. Biol. Chem. 2000, 275, 25116–25121. [Google Scholar] [CrossRef] [PubMed]
- Bae, C.; Kalia, J.; Song, I.; Yu, J.; Kim, H.H.; Swartz, K.J.; Kim, J.I. High Yield Production and Refolding of the Double-Knot Toxin, an Activator of TRPV1 Channels. PLoS ONE 2012, 7, e51516. [Google Scholar] [CrossRef]
- Bae, C.; Anselmi, C.; Kalia, J.; Jara-Oseguera, A.; Schwieters, C.D.; Krepkiy, D.; Won Lee, C.; Kim, E.-H.; Kim, J.I.; Faraldo-Gómez, J.D.; et al. Structural Insights into the Mechanism of Activation of the TRPV1 Channel by a Membrane-Bound Tarantula Toxin. eLife 2016, 5, e11273. [Google Scholar] [CrossRef]
- Geron, M.; Kumar, R.; Zhou, W.; Faraldo-Gómez, J.D.; Vásquez, V.; Priel, A. TRPV1 Pore Turret Dictates Distinct DkTx and Capsaicin Gating. Proc. Natl. Acad. Sci. USA 2018, 115, E11837–E11846. [Google Scholar] [CrossRef]
- Diochot, S. Pain-Related Toxins in Scorpion and Spider Venoms: A Face to Face with Ion Channels. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, e20210026. [Google Scholar] [CrossRef]
- Hakim, M.; Jiang, W.; Luo, L.; Li, B.; Yang, S.; Song, Y.; Lai, R. Scorpion Toxin, BmP01, Induces Pain by Targeting TRPV1 Channel. Toxins 2015, 7, 3671–3687. [Google Scholar] [CrossRef]
- Yang, S.; Yang, F.; Zhang, B.; Lee, B.H.; Li, B.; Luo, L.; Zheng, J.; Lai, R. A Bimodal Activation Mechanism Underlies Scorpion Toxin–Induced Pain. Sci. Adv. 2017, 3, e1700810. [Google Scholar] [CrossRef]
- Yang, S.; Yang, F.; Wei, N.; Hong, J.; Li, B.; Luo, L.; Rong, M.; Yarov-Yarovoy, V.; Zheng, J.; Wang, K.; et al. A Pain-Inducing Centipede Toxin Targets the Heat Activation Machinery of Nociceptor TRPV1. Nat. Commun. 2015, 6, 8297. [Google Scholar] [CrossRef]
- Zhu, A.; Aierken, A.; Yao, Z.; Vu, S.; Tian, Y.; Zheng, J.; Yang, S.; Yang, F. A Centipede Toxin Causes Rapid Desensitization of Nociceptor TRPV1 Ion Channel. Toxicon 2020, 178, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Hu, Z.; Chen, X.; Zeng, X. Molecular Mechanisms of Two Novel and Selective TRPV1 Channel Activators. Int. J. Biol. Macromol. 2024, 275, 133658. [Google Scholar] [CrossRef] [PubMed]
- Geron, M.; Kumar, R.; Matzner, H.; Lahiani, A.; Gincberg, G.; Cohen, G.; Lazarovici, P.; Priel, A. Protein Toxins of the Echis Coloratus Viper Venom Directly Activate TRPV1. Biochim. Et. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Battisti, A.; Holm, G.; Fagrell, B.; Larsson, S. Urticating Hairs in Arthropods: Their Nature and Medical Significance. Annu. Rev. Entomol. 2011, 56, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Kamau, P.M.; Han, Y.; Hu, J.; Luo, A.; Luo, L.; Zheng, J.; Tian, Y.; Lai, R. The Latoia Consocia Caterpillar Induces Pain by Targeting Nociceptive Ion Channel TRPV1. Toxins 2019, 11, 695. [Google Scholar] [CrossRef]
- Gewehr, C.; Oliveira, S.M.; Rossato, M.F.; Trevisan, G.; Dalmolin, G.D.; Rigo, F.K.; De Castro Júnior, C.J.; Cordeiro, M.N.; Ferreira, J.; Gomez, M.V. Mechanisms Involved in the Nociception Triggered by the Venom of the Armed Spider Phoneutria Nigriventer. PLoS Negl. Trop. Dis. 2013, 7, e2198. [Google Scholar] [CrossRef]
- Kitaguchi, T.; Swartz, K.J. An Inhibitor of TRPV1 Channels Isolated from Funnel Web Spider Venom. Biochemistry 2005, 44, 15544–15549. [Google Scholar] [CrossRef]
- Rita Pereira, E.M.; Souza, J.M.; Carobin, N.V.; Silva, J.F.; Santos, D.C.; Silva Júnior, C.A.; Binda, N.S.; Borges, M.H.; Pinto Nagem, R.A.; Kushmerick, C.; et al. Phoneutria Toxin PnTx3-5 Inhibits TRPV1 Channel with Antinociceptive Action in an Orofacial Pain Model. Neuropharmacology 2020, 162, 107826. [Google Scholar] [CrossRef]
- Nikolaev, M.V.; Dorofeeva, N.A.; Komarova, M.S.; Korolkova, Y.V.; Andreev, Y.A.; Mosharova, I.V.; Grishin, E.V.; Tikhonov, D.B.; Kozlov, S.A. TRPV1 Activation Power Can Switch an Action Mode for Its Polypeptide Ligands. PLoS ONE 2017, 12, e0177077. [Google Scholar] [CrossRef]
- Monastyrnaya, M.; Peigneur, S.; Zelepuga, E.; Sintsova, O.; Gladkikh, I.; Leychenko, E.; Isaeva, M.; Tytgat, J.; Kozlovskaya, E. Kunitz-Type Peptide HCRG21 from the Sea Anemone Heteractis Crispa Is a Full Antagonist of the TRPV1 Receptor. Mar. Drugs 2016, 14, 229. [Google Scholar] [CrossRef]
- Elnahriry, K.A.; Wai, D.C.C.; Ashwood, L.M.; Naseem, M.U.; Szanto, T.G.; Guo, S.; Panyi, G.; Prentis, P.J.; Norton, R.S. Structural and Functional Characterisation of Tst2, a Novel TRPV1 Inhibitory Peptide from the Australian Sea Anemone Telmatactis Stephensoni. Biochim. Et. Biophys. Acta (BBA) Proteins Proteom. 2024, 1872, 140952. [Google Scholar] [CrossRef]
- Kiskin, N.I.; Chizhmakov, I.V.; Tsyndrenko, A.Y.; Mueller, A.L.; Jackson, H.; Krishtal, O.A. A Highly Potent and Selective Receptor Antagonist from the Venom of the Agelenopsis Aperta Spider. Neuroscience 1992, 51, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.M.; Silva, C.R.; Trevisan, G.; Villarinho, J.G.; Cordeiro, M.N.; Richardson, M.; Borges, M.H.; Castro, C.J.; Gomez, M.V.; Ferreira, J. Antinociceptive Effect of a Novel Armed Spider Peptide Tx3-5 in Pathological Pain Models in Mice. Pflug. Arch. Eur. J. Physiol. 2016, 468, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Andreev, Y.A.; Kozlov, S.A.; Koshelev, S.G.; Ivanova, E.A.; Monastyrnaya, M.M.; Kozlovskaya, E.P.; Grishin, E.V. Analgesic Compound from Sea Anemone Heteractis Crispa Is the First Polypeptide Inhibitor of Vanilloid Receptor 1 (TRPV1). J. Biol. Chem. 2008, 283, 23914–23921. [Google Scholar] [CrossRef] [PubMed]
- Zelepuga, E.A.; Tabakmakher, V.M.; Chausova, V.E.; Monastyrnaya, M.M.; Isaeva, M.P.; Kozlovskaya, E.P. Interaction of Sea Anemone Heteractis Crispa Kunitz Type Polypeptides with Pain Vanilloid Receptor TRPV1: In Silico Investigation. Russ. J. Bioorg Chem. 2012, 38, 159–170. [Google Scholar] [CrossRef]
- Andreev, Y.; Kozlov, S.; Korolkova, Y.; Dyachenko, I.; Bondarenko, D.; Skobtsov, D.; Murashev, A.; Kotova, P.; Rogachevskaja, O.; Kabanova, N.; et al. Polypeptide Modulators of TRPV1 Produce Analgesia without Hyperthermia. Mar. Drugs 2013, 11, 5100–5115. [Google Scholar] [CrossRef]
- Sintsova, O.; Gladkikh, I.; Klimovich, A.; Palikova, Y.; Palikov, V.; Styshova, O.; Monastyrnaya, M.; Dyachenko, I.; Kozlov, S.; Leychenko, E. TRPV1 Blocker HCRG21 Suppresses TNF-α Production and Prevents the Development of Edema and Hypersensitivity in Carrageenan-Induced Acute Local Inflammation. Biomedicines 2021, 9, 716. [Google Scholar] [CrossRef]
- Peigneur, S.; Paiva, A.L.B.; Cordeiro, M.N.; Borges, M.H.; Diniz, M.R.V.; De Lima, M.E.; Tytgat, J. Phoneutria Nigriventer Spider Toxin PnTx2-1 (δ-Ctenitoxin-Pn1a) Is a Modulator of Sodium Channel Gating. Toxins 2018, 10, 337. [Google Scholar] [CrossRef]
- Schmidtko, A.; Lötsch, J.; Freynhagen, R.; Geisslinger, G. Ziconotide for Treatment of Severe Chronic Pain. Lancet 2010, 375, 1569–1577. [Google Scholar] [CrossRef]
- Garami, A.; Shimansky, Y.P.; Rumbus, Z.; Vizin, R.C.L.; Farkas, N.; Hegyi, J.; Szakacs, Z.; Solymar, M.; Csenkey, A.; Chiche, D.A.; et al. Hyperthermia Induced by Transient Receptor Potential Vanilloid-1 (TRPV1) Antagonists in Human Clinical Trials: Insights from Mathematical Modeling and Meta-Analysis. Pharmacol. Ther. 2020, 208, 107474. [Google Scholar] [CrossRef]
- Yue, W.W.S.; Yuan, L.; Braz, J.M.; Basbaum, A.I.; Julius, D. TRPV1 Drugs Alter Core Body Temperature via Central Projections of Primary Afferent Sensory Neurons. eLife 2022, 11, e80139. [Google Scholar] [CrossRef] [PubMed]
- Logashina, Y.A.; Palikova, Y.A.; Palikov, V.A.; Kazakov, V.A.; Smolskaya, S.V.; Dyachenko, I.A.; Tarasova, N.V.; Andreev, Y.A. Anti-Inflammatory and Analgesic Effects of TRPV1 Polypeptide Modulator APHC3 in Models of Osteo- and Rheumatoid Arthritis. Mar. Drugs 2021, 19, 39. [Google Scholar] [CrossRef] [PubMed]


 ), and [110] for HCRG21 (▲). All images are from Wikimedia commons.
), and [110] for HCRG21 (▲). All images are from Wikimedia commons.
 ), and [110] for HCRG21 (▲). All images are from Wikimedia commons.
), and [110] for HCRG21 (▲). All images are from Wikimedia commons.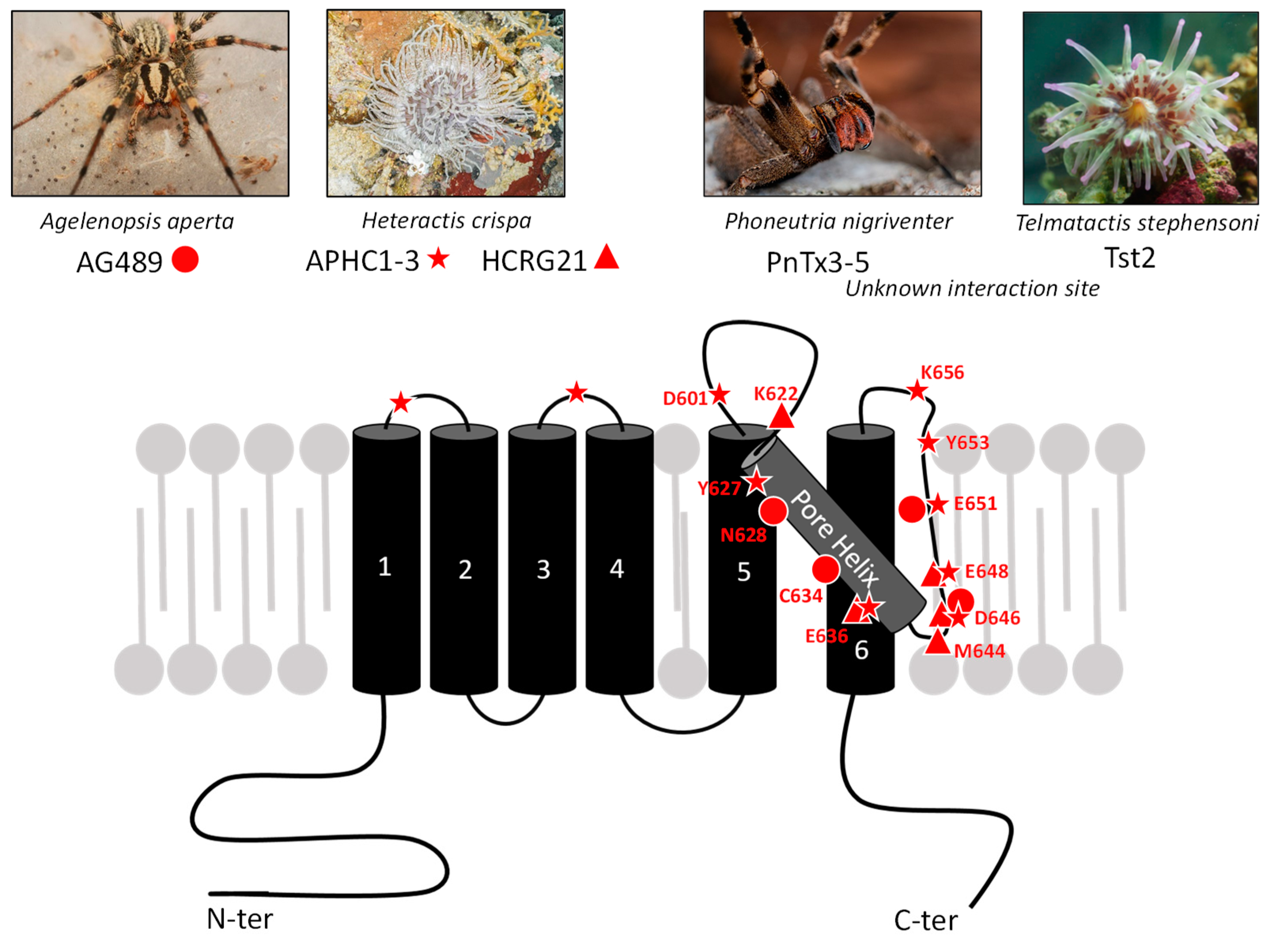
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beignon, F.; Notais, M.; Diochot, S.; Baron, A.; Fajloun, Z.; Tricoire-Leignel, H.; Lenaers, G.; Mattei, C. Neurotoxins Acting on TRPV1—Building a Molecular Template for the Study of Pain and Thermal Dysfunctions. Toxins 2025, 17, 64. https://doi.org/10.3390/toxins17020064
Beignon F, Notais M, Diochot S, Baron A, Fajloun Z, Tricoire-Leignel H, Lenaers G, Mattei C. Neurotoxins Acting on TRPV1—Building a Molecular Template for the Study of Pain and Thermal Dysfunctions. Toxins. 2025; 17(2):64. https://doi.org/10.3390/toxins17020064
Chicago/Turabian StyleBeignon, Florian, Margaux Notais, Sylvie Diochot, Anne Baron, Ziad Fajloun, Hélène Tricoire-Leignel, Guy Lenaers, and César Mattei. 2025. "Neurotoxins Acting on TRPV1—Building a Molecular Template for the Study of Pain and Thermal Dysfunctions" Toxins 17, no. 2: 64. https://doi.org/10.3390/toxins17020064
APA StyleBeignon, F., Notais, M., Diochot, S., Baron, A., Fajloun, Z., Tricoire-Leignel, H., Lenaers, G., & Mattei, C. (2025). Neurotoxins Acting on TRPV1—Building a Molecular Template for the Study of Pain and Thermal Dysfunctions. Toxins, 17(2), 64. https://doi.org/10.3390/toxins17020064







