Effects of Dietary Fiber Supplementation on Modulating Uremic Toxins and Inflammation in Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Results
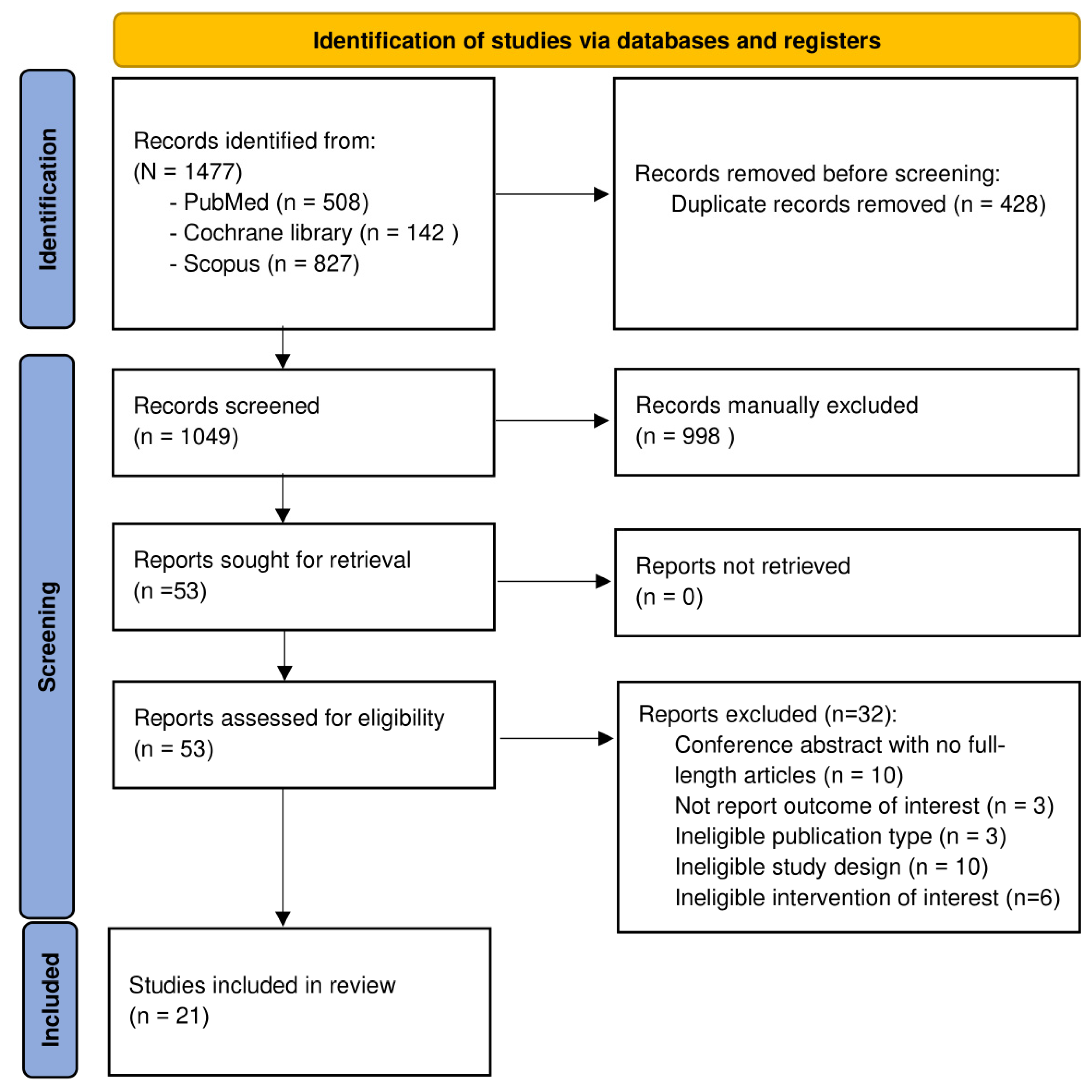
2.1. Search Results
2.2. Study Characteristics
2.3. Risk of Bias Assessment
2.4. Effects of Dietary Fiber Supplementation on Uremic Toxins Outcomes (Table 2)
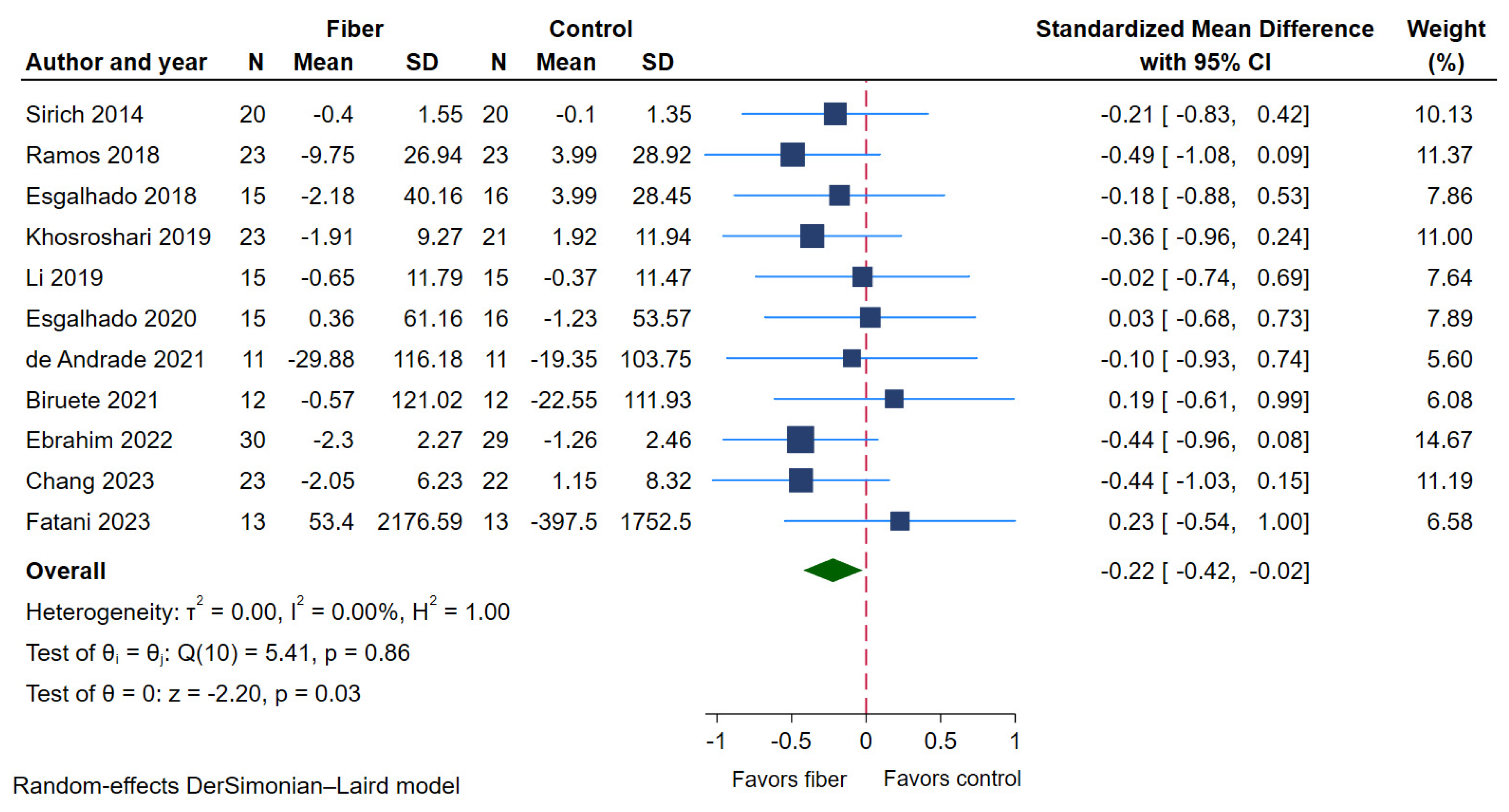
2.4.1. Serum p-Cresyl Sulfate (PCS)
| Outcomes | No. of Studies | No. of Patients | SMD (95% CI) | I2 | GRADE Evidence | ||
|---|---|---|---|---|---|---|---|
| Total | Intervention | Control | |||||
| Primary Outcomes: Uremic toxins | |||||||
| Serum PCS | 11 | 398 | 200 | 198 | −0.22 (−0.42 to −0.02) | 0% | ⨁⨁⨁⨁ High |
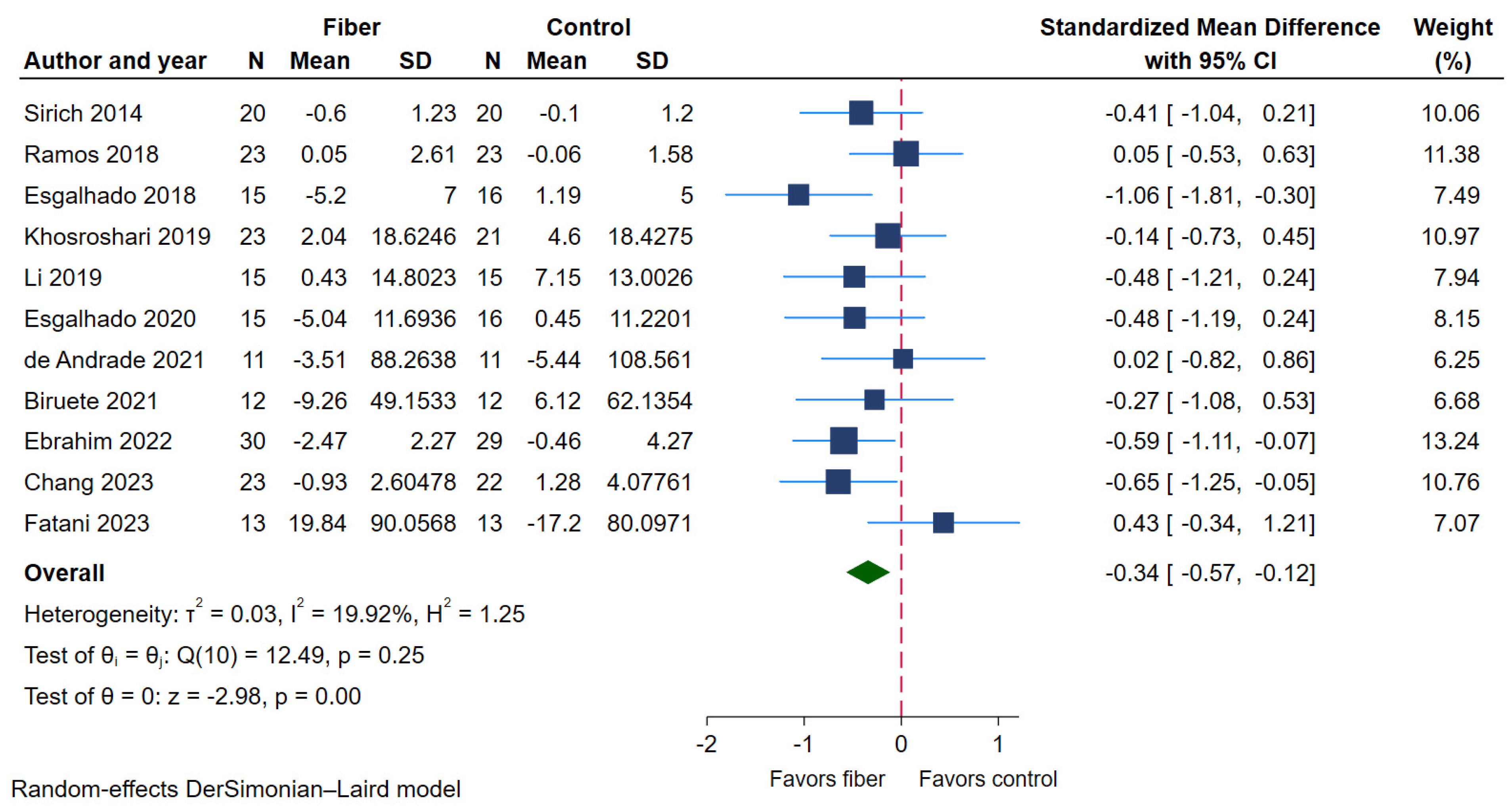
| Serum IS | 11 | 398 | 200 | 198 | −0.34 (−0.57 to −0.12) | 19.92% | ⨁⨁⨁⨁ High |
| BUN | 11 | 398 | 200 | 198 | −0.25 (−0.48 to −0.03) | 21.39% | ⨁⨁◯◯ Low |
| Serum TMAO | 4 | 135 | 67 | 68 | +0.05 (−0.29 to 0.39) | 0% | ⨁◯◯◯ Very low |
| Serum uric acid | 4 | 118 | 60 | 58 | −0.18 (−0.61 to 0.25) | 25.37% | ⨁◯◯◯ Very low |
| Secondary Outcomes: Inflammatory markers | |||||||
| Serum IL-6 | 7 | 265 | 131 | 134 | −0.44 (−0.73 to −0.16) | 24.47% | ⨁⨁⨁◯ Moderate |
| Serum hs-CRP | 5 | 218 | 110 | 108 | −0.01 (−0.38 to 0.36) | 47.76% | ⨁◯◯◯ Very low |
| Serum TNF-α | 4 | 157 | 78 | 79 | −0.34 (−0.66 to −0.02) | 0% | ⨁◯◯◯ Very low |
| Subgroup Analyses | No. of Studies | SMD (95% CI) | p-Values | Assessment of Heterogeneity | p for Interaction | |
|---|---|---|---|---|---|---|
| I2 Index | p-Value | |||||
| CKD status | 0.08 | |||||
| NDD-CKD | 3 | −0.45 (−0.78 to −0.13) | 0.01 | 0% | 0.99 | |
| DD-CKD | 8 | −0.08 (−0.33 to 0.17) | 0.51 | 0% | 0.95 | |
| Study design | 0.04 | |||||
| Crossover | 5 | 0.06 (−0.28 to 0.40) | 0.71 | 0% | 0.98 | |
| Parallel | 6 | −0.37 (−0.61 to −0.13) | <0.01 | 0% | 0.97 | |
| Fiber dosage | 0.75 | |||||
| ≤15 g/day | 7 | −0.24 (−0.49 to 0.00) | 0.05 | 0% | 0.6 | |
| >15 g/day | 4 | −0.18 (−0.52 to 0.17) | 0.32 | 0% | 0.87 | |
| Race | 0.60 | |||||
| Asian | 3 | −0.30 (−0.67 to 0.06) | 0.10 | 0% | 0.67 | |
| Non-Asian | 8 | −0.19 (−0.42 to 0.05) | 0.12 | 0% | 0.74 | |
| Study risk of bias | 0.72 | |||||
| Low | 7 | −0.22 (−0.46 to 0.02) | 0.07 | 0% | 0.68 | |
| Some concerns | 2 | −0.08 (−0.57 to 0.42) | 0.77 | 0% | 0.69 | |
| High | 2 | −0.36 (−0.84 to 0.12) | 0.14 | 0% | 0.45 | |
| Intervention duration | 0.08 | |||||
| <8 week | 6 | −0.03 (−0.32 to 0.27) | 0.86 | 0% | 0.94 | |
| ≥8 week | 5 | −0.38 (−0.64 to −0.11) | 0.03 | 0% | 0.88 | |
| Type of fiber | 0.55 | |||||
| NSP | 1 | −0.44 (−0.96 to 0.08) | 0.10 | - | - | |
| RO | 4 | −0.26 (−0.59 to 0.07) | 0.12 | 0% | 0.46 | |
| RS | 5 | −0.18 (−0.49 to 0.12) | 0.24 | 0% | 0.95 | |
| Mixed | 1 | 0.23 (−0.54 to 1.00) | 0.56 | - | - | |
| Fiber solubility | 0.36 | |||||
| Non-water soluble | 6 | −0.13 (−0.41 to 0.15) | 0.37 | 0% | 0.89 | |
| Water soluble | 5 | −0.31 (−0.59 to −0.04) | 0.03 | 0% | 0.86 | |
2.4.2. Serum Indoxyl Sulfate (IS)
2.4.3. Serum Trimethylamine N-Oxide (TMAO)
2.4.4. Blood Urea Nitrogen (BUN)
2.4.5. Serum Uric Acid
2.5. Effects of Dietary Fiber Supplementation on Inflammatory Markers (Table 2)
2.5.1. Serum Interleukin-6 (IL-6)
2.5.2. Serum Tumor Necrotic Factor Alpha (TNF-α)
2.5.3. Serum High-Sensitivity C-Reactive Protein (hs-CRP)
2.6. Sensitivity Analysis
2.7. Meta-Regression
2.8. Assessment of Publication Bias and Strength of Evidence
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Searching Strategy
5.2. Eligibility Criteria
5.3. Study Outcomes
5.4. Data Extraction
5.5. Assessments of Quality and Risk of Bias
5.6. Data Synthesis
5.7. Statistical Analysis
5.8. Grading the Strength of Evidence
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Management of protein-energy wasting in non-dialysis-dependent chronic kidney disease: Reconciling low protein intake with nutritional therapy. Am. J. Clin. Nutr. 2013, 97, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Wathanavasin, W.; Kittiskulnam, P.; Johansen, K.L. Plant-based diets in patients with chronic kidney disease. Asian Biomed. 2024, 18, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Qin, X.; Yang, C.; Sabatino, A.; Kelly, J.T.; Avesani, C.M.; Carrero, J.J. Fiber intake and health in people with chronic kidney disease. Clin. Kidney J. 2022, 15, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Romina Alina, M.; Crina Carmen, M.; Anamaria, P.; Georgiana Smaranda, M.; Andruța Elena, M.; Andreea, P.; Alina Narcisa, P.; Florina, S.; Ioana Cristina, C.; Ionuț-Dumitru, V.; et al. Dietary Fibers and Their Importance in the Diet. In New Insights in Dietary Fibers; Ing. Romina Alina Vlaic, M., Crina Carmen, M., Eds.; IntechOpen: Rijeka, Croatia, 2024; Chapter 1. [Google Scholar] [CrossRef]
- Agostoni, C.; Bresson, J.L.; Fairweather-Tait, S.; Flynn, A.; Golly, I.; Korhonen, H.; Lagiou, P.; Løvik, M.; Marchelli, R.; Martin, A.; et al. Scientific opinion on dietary reference values for carbohydrates and dietary fibre. EFSA J. 2010, 8, 1462. [Google Scholar]
- Bergen, W.G.; Wu, G. Intestinal nitrogen recycling and utilization in health and disease. J. Nutr. 2009, 139, 821–825. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef]
- Tang, W.H.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef]
- Wong, J.; Piceno, Y.M.; DeSantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am. J. Nephrol. 2014, 39, 230–237. [Google Scholar] [CrossRef]
- Lin, C.J.; Wu, V.; Wu, P.C.; Wu, C.J. Meta-Analysis of the Associations of p-Cresyl Sulfate (PCS) and Indoxyl Sulfate (IS) with Cardiovascular Events and All-Cause Mortality in Patients with Chronic Renal Failure. PLoS ONE 2015, 10, e0132589. [Google Scholar] [CrossRef]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease-Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef]
- Xie, L.M.; Ge, Y.Y.; Huang, X.; Zhang, Y.Q.; Li, J.X. Effects of fermentable dietary fiber supplementation on oxidative and inflammatory status in hemodialysis patients. Int. J. Clin. Exp. Med. 2015, 8, 1363–1369. [Google Scholar] [PubMed]
- Krishnamurthy, V.M.; Wei, G.; Baird, B.C.; Murtaugh, M.; Chonchol, M.B.; Raphael, K.L.; Greene, T.; Beddhu, S. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. 2012, 81, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, J.; Zhang, P.; Zhong, F.; Cai, J.; Ma, A. Association of dietary fiber intake with hyperuricemia in U.S. adults. Food Funct. 2019, 10, 4932–4940. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Yu, L.; Li, Y.; Man, Q.; Jia, S.; Zhou, Y.; Zuo, H.; Zhang, J. Association between Dietary Fiber Intake and Hyperuricemia among Chinese Adults: Analysis of the China Adult Chronic Disease and Nutrition Surveillance (2015). Nutrients 2022, 14, 1433. [Google Scholar] [CrossRef]
- Slavin, J.L. Position of the American Dietetic Association: Health implications of dietary fiber. J. Am. Diet. Assoc. 2008, 108, 1716–1731. [Google Scholar]
- Kwon, Y.J.; Lee, H.S.; Park, G.E.; Lee, J.W. Association Between Dietary Fiber Intake and All-Cause and Cardiovascular Mortality in Middle Aged and Elderly Adults With Chronic Kidney Disease. Front. Nutr. 2022, 9, 863391. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Bliss, D.Z.; Stein, T.P.; Schleifer, C.R.; Settle, R.G. Supplementation with gum arabic fiber increases fecal nitrogen excretion and lowers serum urea nitrogen concentration in chronic renal failure patients consuming a low-protein diet. Am. J. Clin. Nutr. 1996, 63, 392–398. [Google Scholar] [CrossRef]
- Poesen, R.; Evenepoel, P.; de Loor, H.; Delcour, J.A.; Courtin, C.M.; Kuypers, D.; Augustijns, P.; Verbeke, K.; Meijers, B. The Influence of Prebiotic Arabinoxylan Oligosaccharides on Microbiota Derived Uremic Retention Solutes in Patients with Chronic Kidney Disease: A Randomized Controlled Trial. PLoS ONE 2016, 11, e0153893. [Google Scholar] [CrossRef]
- Ramos, C.I.; Armani, R.G.; Canziani, M.E.F.; Dalboni, M.A.; Dolenga, C.J.R.; Nakao, L.S.; Campbell, K.L.; Cuppari, L. Effect of prebiotic (fructooligosaccharide) on uremic toxins of chronic kidney disease patients: A randomized controlled trial. Nephrol. Dial. Transpl. 2019, 34, 1876–1884. [Google Scholar] [CrossRef]
- Ebrahim, Z.; Proost, S.; Tito, R.Y.; Raes, J.; Glorieux, G.; Moosa, M.R.; Blaauw, R. The Effect of ß-Glucan Prebiotic on Kidney Function, Uremic Toxins and Gut Microbiome in Stage 3 to 5 Chronic Kidney Disease (CKD) Predialysis Participants: A Randomized Controlled Trial. Nutrients 2022, 14, 805. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Tian, R.; Guo, Z.; He, L.; Li, Y.; Xu, Y.; Zhang, H. Low-protein diet supplemented with inulin lowers protein-bound toxin levels in patients with stage 3b-5 chronic kidney disease: A randomized controlled study. Nutr. Hosp. 2023, 40, 819–828. [Google Scholar] [CrossRef]
- Headley, S.A.; Chapman, D.J.; Germain, M.J.; Evans, E.E.; Madsen, K.L.; Miele, E.M.; Kirton, K.; Loseke, J.; Cornelius, A.; Martin, B.; et al. Effects of High Amylose-Resistant Starch on Gut Microbiota and Uremic Toxin Levels in Patients with Stage-G3a-G4 Chronic Kidney Disease: A Randomized Trial. J. Ren. Nutr. 2024. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Kuang, D.; Wang, J.; Huo, S.; Li, P.; Lu, L.; Wei, Y.; Wang, L.; Zhong, X.; Zhao, Y. Effect of soluble dietary fiber on gut microbiota and derived metabolites in stage 3 to 5 chronic kidney disease patients: A randomized controlled trial. J. Funct. Foods 2024, 116, 106181. [Google Scholar] [CrossRef]
- Ali, A.A.; Ali, K.E.; Fadlalla, A.E.; Khalid, K.E. The effects of gum arabic oral treatment on the metabolic profile of chronic renal failure patients under regular haemodialysis in Central Sudan. Nat. Prod. Res. 2008, 22, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Sirich, T.L.; Plummer, N.S.; Gardner, C.D.; Hostetter, T.H.; Meyer, T.W. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1603–1610. [Google Scholar] [CrossRef]
- Esgalhado, M.; Kemp, J.A.; Azevedo, R.; Paiva, B.R.; Stockler-Pinto, M.B.; Dolenga, C.J.; Borges, N.A.; Nakao, L.S.; Mafra, D. Could resistant starch supplementation improve inflammatory and oxidative stress biomarkers and uremic toxins levels in hemodialysis patients? A pilot randomized controlled trial. Food Funct. 2018, 9, 6508–6516. [Google Scholar] [CrossRef]
- Khosroshahi, H.T.; Abedi, B.; Ghojazadeh, M.; Samadi, A.; Jouyban, A. Effects of fermentable high fiber diet supplementation on gut derived and conventional nitrogenous product in patients on maintenance hemodialysis: A randomized controlled trial. Nutr. Metab. 2019, 16, 18. [Google Scholar] [CrossRef]
- Laffin, M.R.; Tayebi Khosroshahi, H.; Park, H.; Laffin, L.J.; Madsen, K.; Kafil, H.S.; Abedi, B.; Shiralizadeh, S.; Vaziri, N.D. Amylose resistant starch (HAM-RS2) supplementation increases the proportion of Faecalibacterium bacteria in end-stage renal disease patients: Microbial analysis from a randomized placebo-controlled trial. Hemodial. Int. 2019, 23, 343–347. [Google Scholar] [CrossRef]
- Esgalhado, M.; Kemp, J.A.; Paiva, B.R.; Brito, J.S.; Cardozo, L.; Azevedo, R.; Cunha, D.B.; Nakao, L.S.; Mafra, D. Resistant starch type-2 enriched cookies modulate uremic toxins and inflammation in hemodialysis patients: A randomized, double-blind, crossover and placebo-controlled trial. Food Funct. 2020, 11, 2617–2625. [Google Scholar] [CrossRef]
- Biruete, A.; Cross, T.L.; Allen, J.M.; Kistler, B.M.; de Loor, H.; Evenepoel, P.; Fahey, G.C., Jr.; Bauer, L.; Swanson, K.S.; Wilund, K.R. Effect of Dietary Inulin Supplementation on the Gut Microbiota Composition and Derived Metabolites of Individuals Undergoing Hemodialysis: A Pilot Study. J. Ren. Nutr. 2021, 31, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Kemp, J.A.; Dos Santos, H.F.; de Jesus, H.E.; Esgalhado, M.; de Paiva, B.R.; Azevedo, R.; Stenvinkel, P.; Bergman, P.; Lindholm, B.; Ribeiro-Alves, M.; et al. Resistant Starch Type-2 Supplementation Does Not Decrease Trimethylamine N-Oxide (TMAO) Plasma Level in Hemodialysis Patients. J. Am. Nutr. Assoc. 2022, 41, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Fatani, A.M.N.; Suh, J.H.; Auger, J.; Alabasi, K.M.; Wang, Y.; Segal, M.S.; Dahl, W.J. Pea hull fiber supplementation does not modulate uremic metabolites in adults receiving hemodialysis: A randomized, double-blind, controlled trial. Front. Nutr. 2023, 10, 1179295. [Google Scholar] [CrossRef] [PubMed]
- Meksawan, K.; Chaotrakul, C.; Leeaphorn, N.; Gonlchanvit, S.; Eiam-Ong, S.; Kanjanabuch, T. Effects of Fructo-Oligosaccharide Supplementation on Constipation in Elderly Continuous Ambulatory Peritoneal Dialysis Patients. Perit. Dial. Int. 2016, 36, 60–66. [Google Scholar] [CrossRef]
- Li, L.; Xiong, Q.; Zhao, J.; Lin, X.; He, S.; Wu, N.; Yao, Y.; Liang, W.; Zuo, X.; Ying, C. Inulin-type fructan intervention restricts the increase in gut microbiome-generated indole in patients with peritoneal dialysis: A randomized crossover study. Am. J. Clin. Nutr. 2020, 111, 1087–1099. [Google Scholar] [CrossRef]
- de Andrade, L.S.; Sardá, F.A.H.; Pereira, N.B.F.; Teixeira, R.R.; Rodrigues, S.D.; de Lima, J.D.; Dalboni, M.A.; Aoike, D.T.; Nakao, L.S.; Cuppari, L. Effect of Unripe Banana Flour on Gut-Derived Uremic Toxins in Individuals Undergoing Peritoneal Dialysis: A Randomized, Double-Blind, Placebo-Controlled, Crossover Trial. Nutrients 2021, 13, 646. [Google Scholar] [CrossRef]
- He, S.; Xiong, Q.; Tian, C.; Li, L.; Zhao, J.; Lin, X.; Guo, X.; He, Y.; Liang, W.; Zuo, X.; et al. Inulin-type prebiotics reduce serum uric acid levels via gut microbiota modulation: A randomized, controlled crossover trial in peritoneal dialysis patients. Eur. J. Nutr. 2022, 61, 665–677. [Google Scholar] [CrossRef]
- Xiong, Q.; Li, L.; Xiao, Y.; He, S.; Zhao, J.; Lin, X.; He, Y.; Wang, J.; Guo, X.; Liang, W.; et al. The Effect of Inulin-Type Fructans on Plasma Trimethylamine N-Oxide Levels in Peritoneal Dialysis Patients: A Randomized Crossover Trial. Mol. Nutr. Food Res. 2023, 67, e2200531. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Kantartzi, K.; Tsigalou, C.; Konstantinidis, T.; Romanidou, G.; Voidarou, C.; Bezirtzoglou, E. Focus on the Gut-Kidney Axis in Health and Disease. Front. Med. 2020, 7, 620102. [Google Scholar] [CrossRef]
- Lauriola, M.; Farré, R.; Evenepoel, P.; Overbeek, S.A.; Meijers, B. Food-Derived Uremic Toxins in Chronic Kidney Disease. Toxins 2023, 15, 116. [Google Scholar] [CrossRef] [PubMed]
- Nallu, A.; Sharma, S.; Ramezani, A.; Muralidharan, J.; Raj, D. Gut microbiome in chronic kidney disease: Challenges and opportunities. Transl. Res. 2017, 179, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, G.; Nigam, S.K.; Vanholder, R.; Verbeke, F. Role of the Microbiome in Gut-Heart-Kidney Cross Talk. Circ. Res. 2023, 132, 1064–1083. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, S.; Wu, Q.J.; Xiao, J.; Wang, Z.H.; Mu, X.W.; Zhang, Y.; Wang, X.N.; You, L.L.; Wang, S.N.; et al. Serum total indoxyl sulfate levels and all-cause and cardiovascular mortality in maintenance hemodialysis patients: A prospective cohort study. BMC Nephrol. 2022, 23, 231. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-J.; Chuang, C.-K.; Jayakumar, T.; Liu, H.-L.; Pan, C.-F.; Wang, T.-J.; Chen, H.-H.; Wu, C.-J. Serum p-cresyl sulfate predicts cardiovascular disease and mortality in elderly hemodialysis patients. Arch. Med. Sci. 2013, 9, 662–668. [Google Scholar] [CrossRef]
- Jourde-Chiche, N.; Dou, L.; Cerini, C.; Dignat-George, F.; Vanholder, R.; Brunet, P. Protein-bound toxins—Update 2009. Semin. Dial. 2009, 22, 334–339. [Google Scholar] [CrossRef]
- Wu, M.; Cai, X.; Lin, J.; Zhang, X.; Scott, E.M.; Li, X. Association between fibre intake and indoxyl sulphate/P-cresyl sulphate in patients with chronic kidney disease: Meta-analysis and systematic review of experimental studies. Clin. Nutr. 2019, 38, 2016–2022. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Petersen, K.; Barger, K.; Hansen, K.E.; Anderson, C.A.M.; Baer, D.J.; Lampe, J.W.; Rasmussen, H.; Matthan, N.R. Perspective: Design and Conduct of Human Nutrition Randomized Controlled Trials. Adv. Nutr. 2021, 12, 4–20. [Google Scholar] [CrossRef]
- Zhou, Z.; Jin, H.; Ju, H.; Sun, M.; Chen, H.; Li, L. Circulating Trimethylamine-N-Oxide and Risk of All-Cause and Cardiovascular Mortality in Patients With Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Front. Med. 2022, 9, 828343. [Google Scholar] [CrossRef]
- Thomas, M.S.; Fernandez, M.L. Trimethylamine N-oxide (TMAO), diet and cardiovascular disease. Curr. Atheroscler. Rep. 2021, 23, 1–7. [Google Scholar] [CrossRef]
- Gruppen, E.G.; Garcia, E.; Connelly, M.A.; Jeyarajah, E.J.; Otvos, J.D.; Bakker, S.J.; Dullaart, R.P. TMAO is associated with mortality: Impact of modestly impaired renal function. Sci. Rep. 2017, 7, 13781. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Du, J.; Chen, Z.; Shi, D.; Qu, H. Effects of microbiota-driven therapy on circulating trimethylamine-N-oxide metabolism: A Systematic review and meta-analysis. Front. Cardiovasc. Med. 2021, 8, 710567. [Google Scholar] [CrossRef] [PubMed]
- Kühn, T.; Rohrmann, S.; Sookthai, D.; Johnson, T.; Katzke, V.; Kaaks, R.; von Eckardstein, A.; Müller, D. Intra-individual variation of plasma trimethylamine-N-oxide (TMAO), betaine and choline over 1 year. Clin. Chem. Lab. Med. 2017, 55, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.; Rox, K.; Kleine Bardenhorst, S.; Schminke, U.; Dörr, M.; Mayerle, J.; Frost, F.; Lerch, M.M.; Karch, A.; Brönstrup, M.; et al. Higher Trimethylamine-N-Oxide Plasma Levels with Increasing Age Are Mediated by Diet and Trimethylamine-Forming Bacteria. mSystems 2021, 6, e0094521. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, A.; Massy, Z.A.; Meijers, B.; Evenepoel, P.; Vanholder, R.; Raj, D.S. Role of the Gut Microbiome in Uremia: A Potential Therapeutic Target. Am. J. Kidney Dis. 2016, 67, 483–498. [Google Scholar] [CrossRef]
- Ikee, R.; Sasaki, N.; Yasuda, T.; Fukazawa, S. Chronic Kidney Disease, Gut Dysbiosis, and Constipation: A Burdensome Triplet. Microorganisms 2020, 8, 1862. [Google Scholar] [CrossRef]
- Smith, E.A.; Macfarlane, G.T. Enumeration of human colonic bacteria producing phenolic and indolic compounds: Effects of pH, carbohydrate availability and retention time on dissimilatory aromatic amino acid metabolism. J. Appl. Bacteriol. 1996, 81, 288–302. [Google Scholar] [CrossRef]
- Smith, E.A.; Macfarlane, G.T. Formation of Phenolic and Indolic Compounds by Anaerobic Bacteria in the Human Large Intestine. Microb. Ecol. 1997, 33, 180–188. [Google Scholar] [CrossRef]
- Melekoglu, E.; Samur, F.G. Dietary strategies for gut-derived protein-bound uremic toxins and cardio-metabolic risk factors in chronic kidney disease: A focus on dietary fibers. Crit. Rev. Food Sci. Nutr. 2023, 63, 3994–4008. [Google Scholar] [CrossRef]
- Johnson, R.J.; Sanchez Lozada, L.G.; Lanaspa, M.A.; Piani, F.; Borghi, C. Uric Acid and Chronic Kidney Disease: Still More to Do. Kidney Int. Rep. 2023, 8, 229–239. [Google Scholar] [CrossRef]
- Koguchi, T.; Nakajima, H.; Wada, M.; Yamamoto, Y.; Innami, S.; Maekawa, A.; Tadokor, T. Dietary fiber suppresses elevations of uric acid and allantoin in serum and urine induced by dietary RNA and increases its excretion to feces in rats. J. Nutr. Sci. Vitaminol. 2002, 48, 184–193. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koguchi, T.; Koguchi, H.; Nakajima, H.; Takano, S.; Yamamoto, Y.; Innami, S.; Maekawa, A.; Tadokoro, T. Dietary fiber suppresses elevation of uric acid and urea nitrogen concentrations in serum of rats with renal dysfunction induced by dietary adenine. Int. J. Vitam. Nutr. Res. 2004, 74, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Teng, G.G.; Pan, A.; Yuan, J.M.; Koh, W.P. Food Sources of Protein and Risk of Incident Gout in the Singapore Chinese Health Study. Arthritis Rheumatol. 2015, 67, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef]
- Wathanavasin, W.; Banjongjit, A.; Avihingsanon, Y.; Praditpornsilpa, K.; Tungsanga, K.; Eiam-Ong, S.; Susantitaphong, P. Prevalence of Sarcopenia and Its Impact on Cardiovascular Events and Mortality Among Dialysis Patients: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4077. [Google Scholar] [CrossRef]
- Qi, L.; Rimm, E.; Liu, S.; Rifai, N.; Hu, F.B. Dietary glycemic index, glycemic load, cereal fiber, and plasma adiponectin concentration in diabetic men. Diabetes Care 2005, 28, 1022–1028. [Google Scholar] [CrossRef]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Ma, Y.; Hébert, J.R.; Li, W.; Bertone-Johnson, E.R.; Olendzki, B.; Pagoto, S.L.; Tinker, L.; Rosal, M.C.; Ockene, I.S.; Ockene, J.K.; et al. Association between dietary fiber and markers of systemic inflammation in the Women’s Health Initiative Observational Study. Nutrition 2008, 24, 941–949. [Google Scholar] [CrossRef]
- Stedman, M.R.; Curtin, F.; Elbourne, D.R.; Kesselheim, A.S.; Brookhart, M.A. Meta-analyses involving cross-over trials: Methodological issues. Int. J. Epidemiol. 2011, 40, 1732–1734. [Google Scholar] [CrossRef]
- Minozzi, S.; Cinquini, M.; Gianola, S.; Gonzalez-Lorenzo, M.; Banzi, R. The revised Cochrane risk of bias tool for randomized trials (RoB 2) showed low interrater reliability and challenges in its application. J. Clin. Epidemiol. 2020, 126, 37–44. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Holger Schünemann, J.B.; Guyatt, G.; Oxman, A. Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach. Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 3 August 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]


| No. | Author | Publication Year | Country | Design | No. of Patients | Mean Age (Year) | Men (%) | CKD Status | Dialysis Vintage (Year) | Intervention of Interest | Comparator | Outcomes of Interest | Risk of Bias | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fiber Use | Type of Fiber | Daily Dose (g/day) | Duration of Intervention (Week) | Uremic Toxins | Inflammatory Markers | ||||||||||||
| 1 | Bliss DZ. | 1996 | US | Crossover | 20 | 20–72 | 65 | NDD | NA | Gum arabic | NSP | 50 | 4 | Pectin | BUN | NA | High |
| 2 | Ali AA. | 2007 | Sudan | Parallel | 36 | 44.1 | 69.4 | HD | 19.2 | Gum arabic | NSP | 50 | 12 | FF + folic | BUN, Uric | NA | High |
| 3 | Sirich TL. | 2014 | US | Parallel | 40 | 56 | 60 | HD | 48 | High-amylose corn starch | RS | 15 | 6 | Waxy corn starch | BUN, PCS, IS | NA | Low |
| 4 | Meksawan K. | 2016 | Thailand | Crossover | 9 | 71.2 | 55.6 | PD | 17.8 | FOS | RO | 20 | 4 | Sucrose | BUN | NA | High |
| 5 | Poesen R. | 2016 | Belgium | Crossover | 40 | 70 | 70 | NDD | NA | AXOS | RO | 20 | 4 | Maltodextrin | PCS, IS, TMAO | NA | Low |
| 6 | Ramos CI. | 2018 | Brazil | Parallel | 50 | 57.3 | 54 | NDD | NA | Short-chain prebiotic FOS (NutraFlora®) | RO | 12 | 12 | Maltodextrin | PCS, IS, BUN | hs-CRP, IL-6 | High |
| 7 | Esgalhado M. | 2018 | Brazil | Parallel | 43 | 54.7 | 58.1 | HD | 47.1 | Hi-Maize® | RS | 16 | 4 | Manioc flour | PCS, IS, BUN | hs-CRP, IL-6 | Some concerns |
| 8 | Khosroshari HT. | 2019 | Iran | Parallel | 50 | 55.5 | 58 | HD | 59.4 | HAM-RS2 | RS | 1st 4 week; 20, 2nd 4 week; 25 | 8 | Waxy corn starch | PCS, IS, BUN, Uric | hs-CRP | Low |
| 9 | Laffin MR. | 2019 | Iran | Parallel | 20 | 55.89 | 65 | HD | NA | HAM-RS2 | RS | 1st 4 week; 20, 2nd 4 week; 25 | 8 | Wheat flour | BUN, Uric, | IL-6 | High |
| 10 | Li L. | 2019 | China | Crossover | 21 | 35.48 | 60 | PD | 23.01 | Inulin-type fructans | RO | 10 | 12 | Maltodextrin | PCS, IS | NA | Low |
| 11 | Esgalhado M. | 2020 | Brazil | Crossover | 26 | 54.71 | NA | HD | 47.06 | Hi-Maize® | RS | 16 | 4 | Manioc flour | PCS, IS | NA | Some concerns |
| 12 | de Andrade LS. | 2021 | Brazil | Crossover | 43 | 52 | 53.5 | PD | 22.25 | Unripe banana flour | RS | 21 | 4 | Corn starch | PCS, IS, BUN | hs-CRP, IL-6, TNF-α | High |
| 13 | Biruete A. | 2021 | Belgium | Crossover | 12 | 55 | 50 | HD | NA | Inulin (91% inulin, 9% short chain FOS) | RO | Female 10, Male 15 | 4 | Maltodextrin | PCS, IS | NA | Low |
| 14 | He S. | 2022 | China | Crossover | 16 | 37 | 62.5 | PD | 25 | Mixture of inulin and oligofructose | RO | 10 | 12 | Maltodextrin | Uric | NA | Low |
| 15 | Ebrahim Z. | 2022 | South Africa | Parallel | 59 | 41 | 42.4 | NDD | NA | B-glucan prebiotics | NSP | 6 | 14 | Nil | PCS, IS | NA | Low |
| 16 | Kemp JA. | 2022 | Brazil | Parallel | 25 | 53.8 | 56 | HD | 45.84 | Hi-Maize® | RS | 16 | 4 | Manioc flour | TMAO | NA | Some concerns |
| 17 | Xiong Q. | 2023 | China | Crossover | 22 | 37.3 | 54.5 | PD | 28 | Inulin-type Fructans | RO | 10 | 12 | Maltodextrin | TMAO | NA | High |
| 18 | Chang L. | 2023 | China | Parallel | 45 | 51.4 | 51.1 | NDD | NA | Inulin | RO | 10 | 12 | Wheat starch | BUN, PCS, IS | hs-CRP, IL-6, TNF-α | Low |
| 19 | Fatani AMN. | 2023 | US | Crossover | 18 | 39.04 | 55.6 | HD | NA | Pea hull fiber | Mixed | 9 | 4 | Control muffin | PCS, IS, TMAO | NA | Low |
| 20 | Headley SA. | 2024 | US | Parallel | 65 | 60.91 | 48 | NDD | NA | HAM-RS2 | RS | 1st week; 15 then 2nd–16th; up to 33 | 16 | Waxy corn starch | PCS, IS | IL-6, hs-CRP | Low |
| 21 | Cui Y. | 2024 | China | Parallel | 40 | 68.35 | 55 | NDD | NA | Soluble dietary fiber * | Mixed | Initial 5 g/d in daily increment to a final dose of 15 g/d | 4 | Maltodextrin | BUN, TMAO | IL-6, TNF-α | High |
| Subgroup Analyses | No. of Studies | SMD (95% CI) | p-Values | Assessment of Heterogeneity | p for Interaction | |
|---|---|---|---|---|---|---|
| I2 Index | p-Value | |||||
| CKD status | 0.73 | |||||
| NDD-CKD | 3 | −0.40 (−0.83 to 0.03) | 0.07 | 43.09% | 0.17 | |
| DD-CKD | 8 | −0.31 (−0.59 to −0.02) | 0.03 | 20.17% | 0.27 | |
| Study design | 0.27 | |||||
| Crossover | 5 | −0.18 (−0.52 to 0.16) | 0.30 | 0.30% | 0.40 | |
| Parallel | 6 | −0.44 (−0.73 to −0.14) | <0.01 | 29.97% | 0.21 | |
| Fiber dosage | 0.72 | |||||
| ≤15 g/d | 7 | −0.31 (−0.59 to −0.04) | 0.03 | 21.85% | 0.26 | |
| >15 g/d | 4 | −0.41 (−0.85 to 0.04) | 0.07 | 36.09% | 0.20 | |
| Race | 0.66 | |||||
| Asian | 3 | −0.41 (−0.78 to −0.05) | 0.03 | 0% | 0.48 | |
| Non-Asian | 8 | −0.31 (−0.61 to 0.00) | 0.05 | 35.41% | 0.15 | |
| Study risk of bias | 0.10 | |||||
| Low | 7 | −0.35 (−0.60 to −0.10) | 0.01 | 5.83% | 0.38 | |
| Some concerns | 2 | −0.76 (−1.32 to −0.19) | 0.01 | 15.89% | 0.28 | |
| High | 2 | +0.04 (−0.43 to 0.52) | 0.87 | 0% | 0.95 | |
| Intervention duration | 0.84 | |||||
| <8 week | 6 | −0.31 (−0.70 to 0.08) | 0.12 | 39.17% | 0.14 | |
| ≥8 week | 5 | −0.36 (−0.63 to −0.09) | 0.01 | 5.55% | 0.38 | |
| Type of fiber | 0.18 | |||||
| NSP | 1 | −0.59 (−1.11 to −0.07) | - | - | ||
| RO | 4 | −0.32 (−0.65 to 0.00) | 0.05 | 0% | 0.40 | |
| RS | 5 | −0.40 (−0.74 to −0.07) | 0.02 | 14.81% | 0.32 | |
| Mixed | 1 | 0.43 (−0.34 to 1.21) | - | - | ||
| Fiber solubility | 0.62 | |||||
| Non-water soluble | 6 | −0.28 (−0.66 to 0.09) | 0.14 | 41.31% | 0.13 | |
| Water soluble | 5 | −0.40 (−0.68 to −0.12) | <0.01 | 0% | 0.25 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wathanavasin, W.; Cheungpasitporn, W.; Thongprayoon, C.; Fülöp, T. Effects of Dietary Fiber Supplementation on Modulating Uremic Toxins and Inflammation in Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Toxins 2025, 17, 57. https://doi.org/10.3390/toxins17020057
Wathanavasin W, Cheungpasitporn W, Thongprayoon C, Fülöp T. Effects of Dietary Fiber Supplementation on Modulating Uremic Toxins and Inflammation in Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Toxins. 2025; 17(2):57. https://doi.org/10.3390/toxins17020057
Chicago/Turabian StyleWathanavasin, Wannasit, Wisit Cheungpasitporn, Charat Thongprayoon, and Tibor Fülöp. 2025. "Effects of Dietary Fiber Supplementation on Modulating Uremic Toxins and Inflammation in Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Toxins 17, no. 2: 57. https://doi.org/10.3390/toxins17020057
APA StyleWathanavasin, W., Cheungpasitporn, W., Thongprayoon, C., & Fülöp, T. (2025). Effects of Dietary Fiber Supplementation on Modulating Uremic Toxins and Inflammation in Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Toxins, 17(2), 57. https://doi.org/10.3390/toxins17020057







