Venom Peptides Across Asian and American Tarantulas Utilize Dual Pharmacology to Target Activation and Fast Inactivation of Voltage-Gated Sodium Channels
Abstract
1. Introduction
2. Results
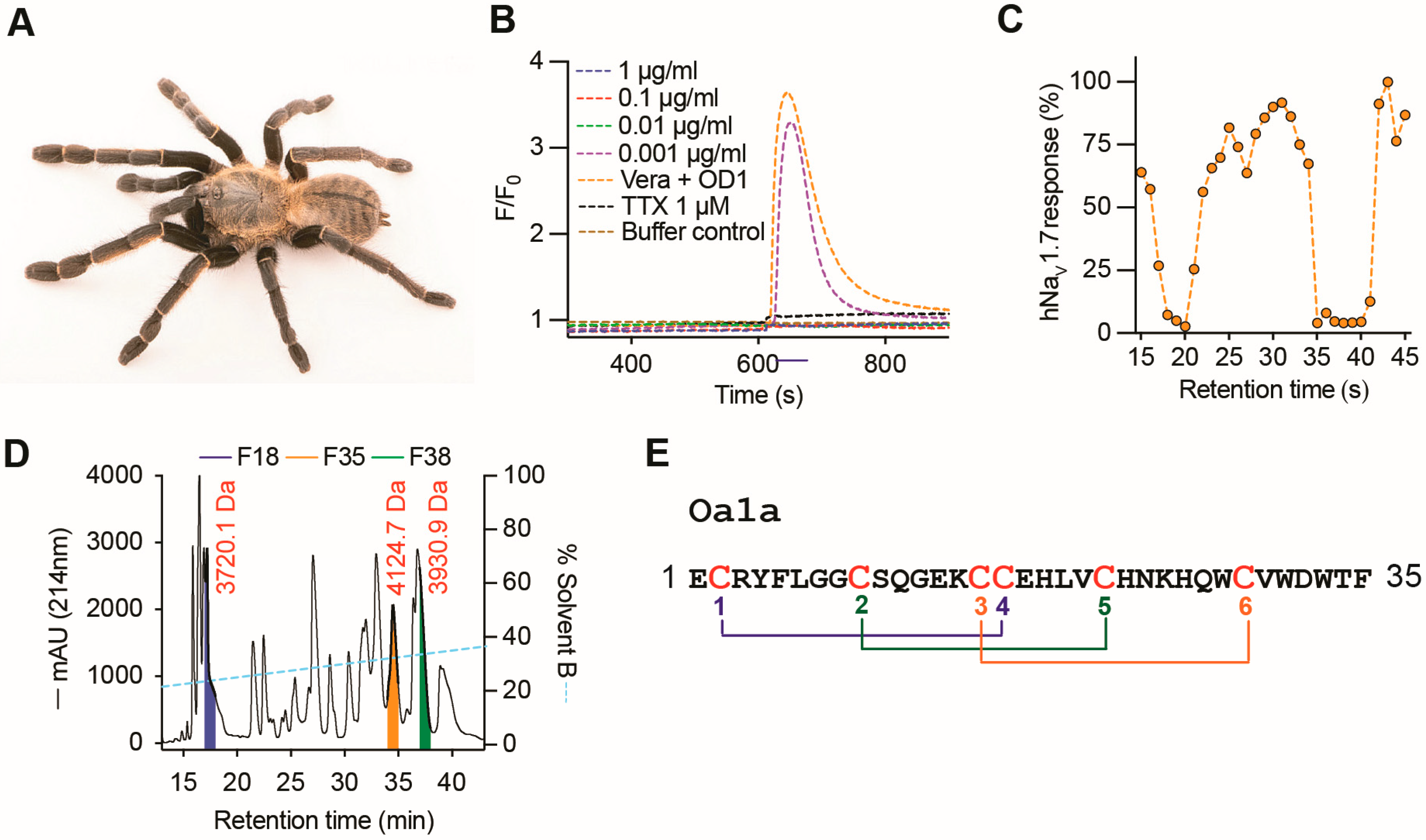
2.1. Discovery of Oa1a Peptide
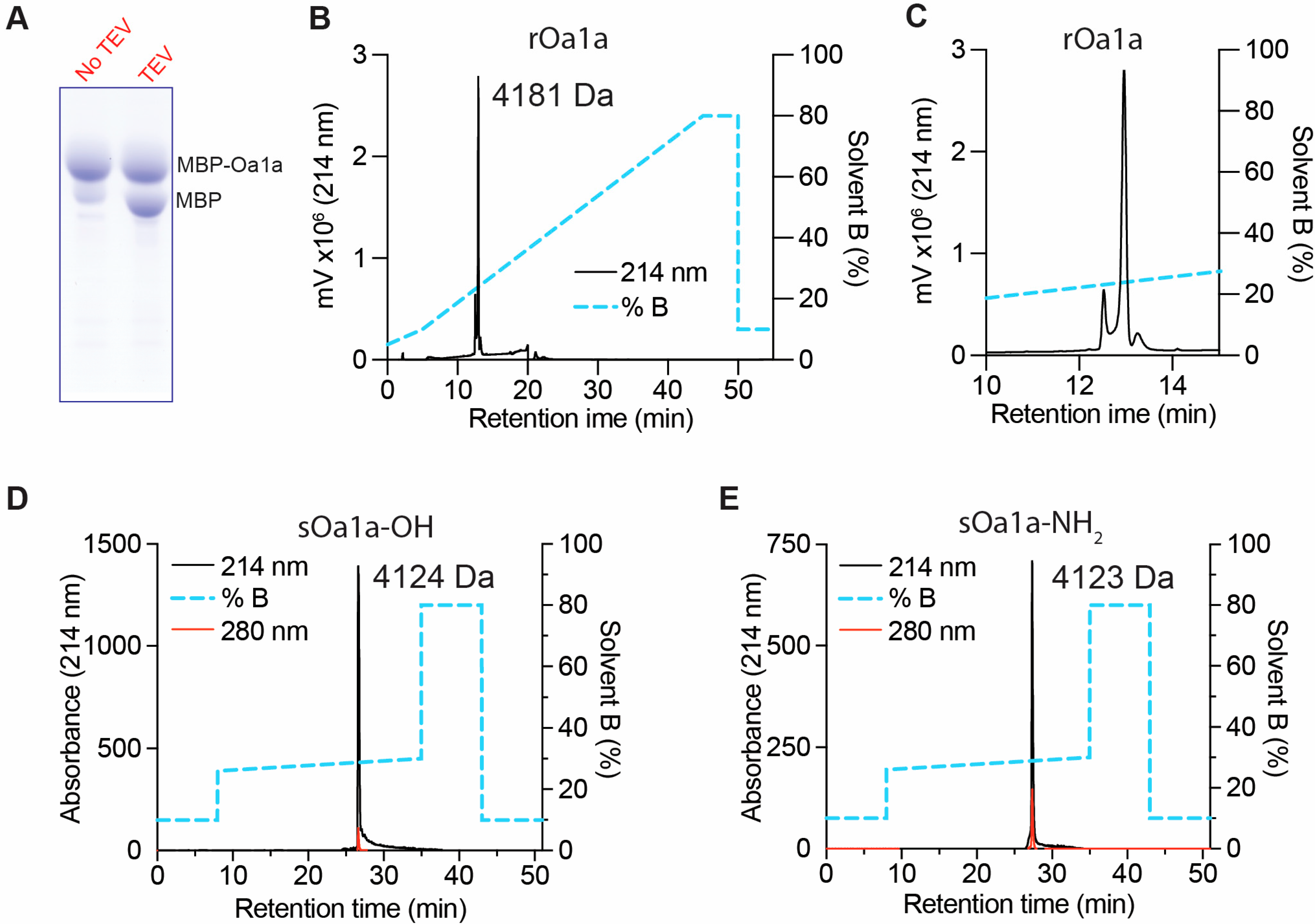
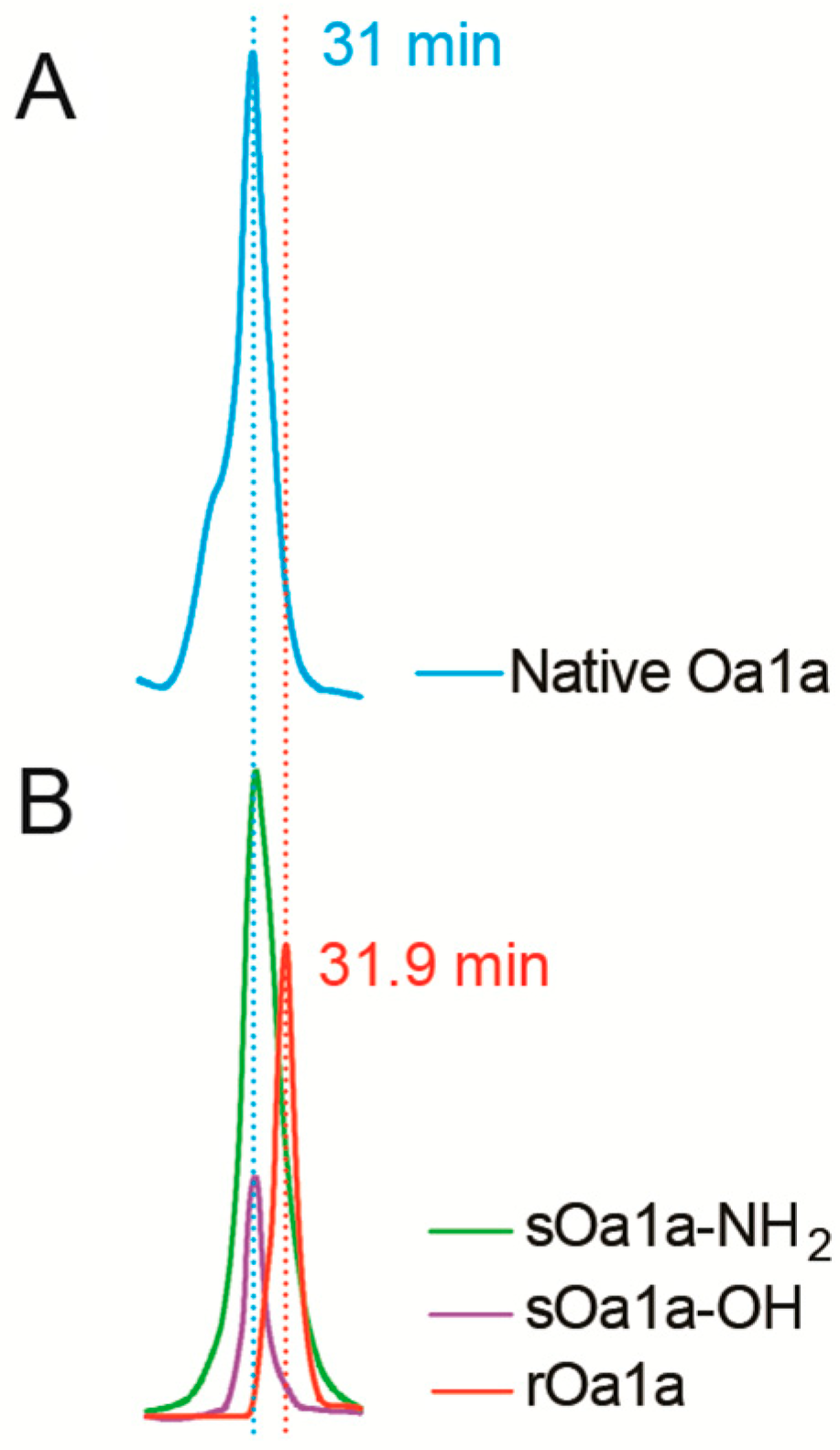
2.2. Production of Recombinant and Synthetic Oa1a
2.3. The Venom Peptide Oa1a Modulates NaV Channels
2.4. Mode of Action of Oa Peptides on NaV Channels
2.5. Structure–Function Relationships of Potency and Selectivity of Oa for NaVs
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Reagents
5.2. Cell Culture
5.3. Venom Fractionation
5.4. Calcium Influx Assay
5.5. Mass Spectrometry and Amino Acid Sequencing
5.6. Solid-Phase Peptide Synthesis and Oxidative Folding
5.7. Recombinant Expression
5.8. Whole-Cell Patch Clamp Electrophysiology
5.9. Molecular Modelling
5.10. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ICK | Inhibitory Cysteine Knot |
| NaV | Voltage-gated sodium channel |
| CaV | Voltage-gated calcium channel |
| KV | Voltage-gated potassium channel |
| HEK293 | Human Embryonic Kidney 293 cell line |
| TEV | Tobacco Etch Virus |
| RP-HPLC | Reverse Phase High Performance Liquid Chromatography |
References
- Ratibou, Z.; Inguimbert, N.; Dutertre, S. Predatory and Defensive Strategies in Cone Snails. Toxins 2024, 16, 94. [Google Scholar] [CrossRef]
- Kerkis, I.; de Brandao Prieto da Silva, A.R.; Pompeia, C.; Tytgat, J.; de Sa Junior, P.L. Toxin bioportides: Exploring toxin biological activity and multifunctionality. Cell. Mol. Life Sci. 2017, 74, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, P.R.; Peruzzo Neto, A.V.; John, E.B.O.; Pedebos, C.; Ligabue-Braun, R. From venoms to vanity: Exploring animal toxins as cosmeceuticals. Toxicon 2025, 266, 108518. [Google Scholar] [CrossRef]
- Billen, B.; Bosmans, F.; Tytgat, J. Animal peptides targeting voltage-activated sodium channels. Curr. Pharm. Des. 2008, 14, 2492–2502. [Google Scholar] [CrossRef]
- Muller, J.A.I.; Bourke, L.A.; Campbell, S.I.D.; Cardoso, F.C. Venom peptides regulating Ca2+ homeostasis: Neuroprotective potential. Trends Pharmacol. Sci. 2025, 46, 407–421. [Google Scholar] [CrossRef]
- Kozlov, S. Animal toxins for channelopathy treatment. Neuropharmacology 2018, 132, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.C.; Lewis, R.J. Structure-function and therapeutic potential of spider venom-derived cysteine knot peptides targeting sodium channels. Front. Pharmacol. 2019, 10, 366. [Google Scholar] [CrossRef]
- Gremski, L.H.; Matsubara, F.H.; Polli, N.L.C.; Antunes, B.C.; Schluga, P.H.C.; da Justa, H.C.; Minozzo, J.C.; Wille, A.C.M.; Senff-Ribeiro, A.; Veiga, S.S. Prospective Use of Brown Spider Venom Toxins as Therapeutic and Biotechnological Inputs. Front. Mol. Biosci. 2021, 8, 706704. [Google Scholar] [CrossRef]
- Peigneur, S.; de Lima, M.E.; Tytgat, J. Phoneutria nigriventer venom: A pharmacological treasure. Toxicon 2018, 151, 96–110. [Google Scholar] [CrossRef]
- Chen, R.; Liu, Y.; Qian, L.; Yi, M.; Yin, H.; Wang, S.; Xiang, B. Sodium channels as a new target for pain treatment. Front. Pharmacol. 2025, 16, 1573254. [Google Scholar] [CrossRef] [PubMed]
- Rusina, E.; Simonti, M.; Duprat, F.; Cestele, S.; Mantegazza, M. Voltage-gated sodium channels in genetic epilepsy: Up and down of excitability. J. Neurochem. 2024, 168, 3872–3890. [Google Scholar] [CrossRef] [PubMed]
- Banh, C.; Sic, A.; Knezevic, N.N. Sodium Channel Inhibitors in Clinical Development for Pain Management: A Focused Review. CNS Drugs 2025. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Lee, J.; Lee, S.Y.; Kwon, I.; Cho, H. Poly-dipeptides produced from C9orf72 hexanucleotide repeats cause selective motor neuron hyperexcitability in ALS. Proc. Natl. Acad. Sci. USA 2022, 119, e2113813119. [Google Scholar] [CrossRef]
- Kubat Oktem, E.; Mruk, K.; Chang, J.; Akin, A.; Kobertz, W.R.; Brown, R.H., Jr. Mutant SOD1 protein increases Nav1.3 channel excitability. J. Biol. Phys. 2016, 42, 351–370. [Google Scholar] [CrossRef]
- Saba, L.; Viscomi, M.T.; Martini, A.; Caioli, S.; Mercuri, N.B.; Guatteo, E.; Zona, C. Modified age-dependent expression of NaV1.6 in an ALS model correlates with motor cortex excitability alterations. Neurobiol. Dis. 2019, 130, 104532. [Google Scholar] [CrossRef]
- Cardoso, F.C.; Castro, J.; Grundy, L.; Schober, G.; Garcia-Caraballo, S.; Zhao, T.; Herzig, V.; King, G.F.; Brierley, S.M.; Lewis, R.J. A spider-venom peptide with multitarget activity on sodium and calcium channels alleviates chronic visceral pain in a model of irritable bowel syndrome. Pain 2021, 162, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Richards, K.L.; Milligan, C.J.; Richardson, R.J.; Jancovski, N.; Grunnet, M.; Jacobson, L.H.; Undheim, E.A.B.; Mobli, M.; Chow, C.Y.; Herzig, V.; et al. Selective NaV1.1 activation rescues Dravet syndrome mice from seizures and premature death. Proc. Natl. Acad. Sci. USA 2018, 115, E8077–E8085. [Google Scholar] [CrossRef]
- Stevens, M.; Peigneur, S.; Tytgat, J. Neurotoxins and their binding areas on voltage-gated sodium channels. Front. Pharmacol. 2011, 2, 71. [Google Scholar] [CrossRef]
- Hu, H.; Mawlawi, S.E.; Zhao, T.; Deuis, J.R.; Jami, S.; Vetter, I.; Lewis, R.J.; Cardoso, F.C. Engineering of a spider peptide via conserved structure-function traits optimizes sodium channel inhibition in vitro and anti-nociception in vivo. Front. Mol. Biosci. 2021, 8, 742457. [Google Scholar] [CrossRef]
- Deuis, J.R.; Ragnarsson, L.; Robinson, S.D.; Dekan, Z.; Chan, L.; Jin, A.H.; Tran, P.; McMahon, K.L.; Li, S.; Wood, J.N.; et al. The tarantula venom peptide Eo1a binds to the domain II S3-S4 extracellular loop of voltage-gated sodium channel NaV1.8 to enhance activation. Front. Pharmacol. 2021, 12, 789570. [Google Scholar] [CrossRef]
- Cardoso, F.C.; Dekan, Z.; Smith, J.J.; Deuis, J.R.; Vetter, I.; Herzig, V.; Alewood, P.F.; King, G.F.; Lewis, R.J. Modulatory features of the novel spider toxin μ-TRTX-Df1a isolated from the venom of the spider Davus fasciatus. Br. J. Pharmacol. 2017, 174, 2528–2544. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.C.; Dekan, Z.; Rosengren, K.J.; Erickson, A.; Vetter, I.; Deuis, J.; Herzig, V.; Alewood, P.; King, G.F.; Lewis, R.J. Identification and characterization of ProTx-III [μ-TRTX-Tp1a], a new voltage-gated sodium channel inhibitor from venom of the tarantula Thrixopelma Pruriens. Mol. Pharmacol. 2015, 88, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Wisedchaisri, G.; Tonggu, L.; Gamal El-Din, T.M.; McCord, E.; Zheng, N.; Catterall, W.A. Structural basis for high-affinity trapping of the NaV1.7 channel in Its resting state by tarantula toxin. Mol. Cell 2021, 81, 38–48.e4. [Google Scholar] [CrossRef] [PubMed]
- Priest, B.T.; Blumenthal, K.M.; Smith, J.J.; Warren, V.A.; Smith, M.M. ProTx-I and ProTx-II: Gating modifiers of voltage-gated sodium channels. Toxicon 2007, 49, 194–201. [Google Scholar] [CrossRef]
- Bladen, C.; Hamid, J.; Souza, I.A.; Zamponi, G.W. Block of T-type calcium channels by protoxins I and II. Mol. Brain 2014, 7, 36. [Google Scholar] [CrossRef]
- Shaikh, N.Y.; Sunagar, K. The deep-rooted origin of disulfide-rich spider venom toxins. eLife 2023, 12, e83761. [Google Scholar] [CrossRef]
- Luddecke, T.; Hurka, S.; Dresler, J.; Lubcke, T.; von Wirth, V.; Lochnit, G.; Timm, T.; Herzig, V.; Vilcinskas, A. Comparative venomics suggests an evolutionary adaption of spider venom from predation to defense. Commun. Biol. 2025, 8, 1496. [Google Scholar] [CrossRef]
- Corzo, G.; Escoubas, P. Pharmacologically active spider peptide toxins. Cell. Mol. Life Sci. 2003, 60, 2409–2426. [Google Scholar] [CrossRef]
- Bai, F.; Song, Y.; Cao, Y.; Ban, M.; Zhang, Z.; Sun, Y.; Feng, Y.; Li, C. Scorpion Neurotoxin Syb-prII-1 Exerts Analgesic Effect through Nav1.8 Channel and MAPKs Pathway. Int. J. Mol. Sci. 2022, 23, 65. [Google Scholar] [CrossRef]
- Zhao, R.; Qasim, A.; Sophanpanichkul, P.; Dai, H.; Nayak, M.; Sher, I.; Chill, J.; Goldstein, S.A.N. Selective block of human Kv1.1 channels and an epilepsy-associated gain-of-function mutation by AETX-K peptide. FASEB J. 2024, 38, e23381. [Google Scholar] [CrossRef]
- Tajti, G.; Wai, D.C.C.; Panyi, G.; Norton, R.S. The voltage-gated potassium channel KV1.3 as a therapeutic target for venom-derived peptides. Biochem. Pharmacol. 2020, 181, 114146. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.S.; Loyola, V.; Bicca, J.; Faro, L.; Vale, C.L.C.; Lotufo Denucci, B.; Mortari, M.R. Innovative treatments for epilepsy: Venom peptides, cannabinoids, and neurostimulation. J. Neurosci. Res. 2022, 100, 1969–1986. [Google Scholar] [CrossRef]
- Kim, E.; Hwang, D.H.; Mohan Prakash, R.L.; Asirvatham, R.D.; Lee, H.; Heo, Y.; Munawir, A.; Seyedian, R.; Kang, C. Animal Venom in Modern Medicine: A Review of Therapeutic Applications. Toxins 2025, 17, 371. [Google Scholar] [CrossRef]
- Li, L.; Hu, J.; Liu, Y.; Wang, Z.; Tang, H.; Zhou, D.; Su, H. Supramolecular crafting of peptides as novel antimicrobial materials. Mater. Horiz. 2025. [Google Scholar] [CrossRef]
- Herzig, V.; Hodgson, W.C. Intersexual variations in the pharmacological properties of Coremiocnemis tropix (Araneae, Theraphosidae) spider venom. Toxicon 2009, 53, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- PyMOL, version 2.0. The PyMOL Molecular Graphics System. Schrödinger, LLC.: New York, NY, USA, 2015.



| hNaV Subtype | rOa1a IC50 (nM) Mean ± SEM | sOa1a-NH2 IC50 (nM) Mean ± SEM | sOa1a-OH IC50 (nM) Mean ± SEM | Ratio OH/NH2 |
|---|---|---|---|---|
| NaV1.1 | 1494 ± 182 | 17 ± 1 | 274 ± 18 | 16 |
| NaV1.2 | 640 ± 85 | 154 ± 7 | >3000 | na |
| NaV1.3 | 899 ± 20 | 162 ± 8 | >3000 | na |
| NaV1.4 | 1031 ± 48 | 2289 ± 77 | >3000 | na |
| NaV1.5 | 1002 ± 31 | 192 ± 4 | 1266 ± 22 | 6.6 |
| NaV1.6 | 792 ± 19 | 223 ± 18 | 568 ± 38 | 2.6 |
| NaV1.7 | 432 ± 25 | 25 ± 0.4 | 410 ± 23 | 16 |
| hNaV Subtype | sOa1a-NH2 | sOa1a-OH | rOa1a |
|---|---|---|---|
| NaV1.1 | Off-rate 4.6 min (95% CI = 4.27 to 4.97 min) | Off-rate 3.56 min (95% CI = 3.35 to 3.80 min) | ND |
| NaV1.3 | Steady-state inactivation (mean ± SEM) Control V50 = −71 ± 0.71 mV Peptide V50 = −57 ± 1.4 mV ΔV50 = 14 mV Steady-state activation (mean ± SEM) Control V50 = −21 ± 0.4 mV Peptide V50 = −26 ± 1 mV Slowing fast inactivation (mean ± SEM) EC50 = 22.9 ± 1.8 nM | ND | Slowing fast inactivation (mean ± SEM) EC50 = 109.6 ± 7.2 nM |
| NaV1.7 | Steady-state inactivation (mean ± SEM) Control V50 = −66 ± 0.7 mV Peptide V50 = −69 ± 1.1 mV Steady-state activation (mean ± SEM) Control V50 = −28 ± 0.5 mV Peptide V50 = −30 ± 0.3 mV | Steady-state inactivation (mean ± SEM) Control V50 = −66 ± 0.3 mV Peptide V50 = −71 ± 0.8 mV Steady-state activation (mean ± SEM) Control V50 = −25 ± 0.7 mV Peptide V50 = −22 ± 1.7 mV | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nashikwala, A.S.; Kotapati, C.; Eagles, D.A.; Lewis, R.J.; Cardoso, F.C. Venom Peptides Across Asian and American Tarantulas Utilize Dual Pharmacology to Target Activation and Fast Inactivation of Voltage-Gated Sodium Channels. Toxins 2025, 17, 561. https://doi.org/10.3390/toxins17110561
Nashikwala AS, Kotapati C, Eagles DA, Lewis RJ, Cardoso FC. Venom Peptides Across Asian and American Tarantulas Utilize Dual Pharmacology to Target Activation and Fast Inactivation of Voltage-Gated Sodium Channels. Toxins. 2025; 17(11):561. https://doi.org/10.3390/toxins17110561
Chicago/Turabian StyleNashikwala, Amatulla S., Charan Kotapati, David A. Eagles, Richard J. Lewis, and Fernanda C. Cardoso. 2025. "Venom Peptides Across Asian and American Tarantulas Utilize Dual Pharmacology to Target Activation and Fast Inactivation of Voltage-Gated Sodium Channels" Toxins 17, no. 11: 561. https://doi.org/10.3390/toxins17110561
APA StyleNashikwala, A. S., Kotapati, C., Eagles, D. A., Lewis, R. J., & Cardoso, F. C. (2025). Venom Peptides Across Asian and American Tarantulas Utilize Dual Pharmacology to Target Activation and Fast Inactivation of Voltage-Gated Sodium Channels. Toxins, 17(11), 561. https://doi.org/10.3390/toxins17110561






