Beyond Glycemic Control: Concurrent GLP-1 Receptor Agonist Use Is Associated with Reduced Urinary Adverse Events Following OnabotulinumtoxinA Treatment in Non-Diabetic Adults with Overactive Bladder
Abstract
1. Introduction
2. Results
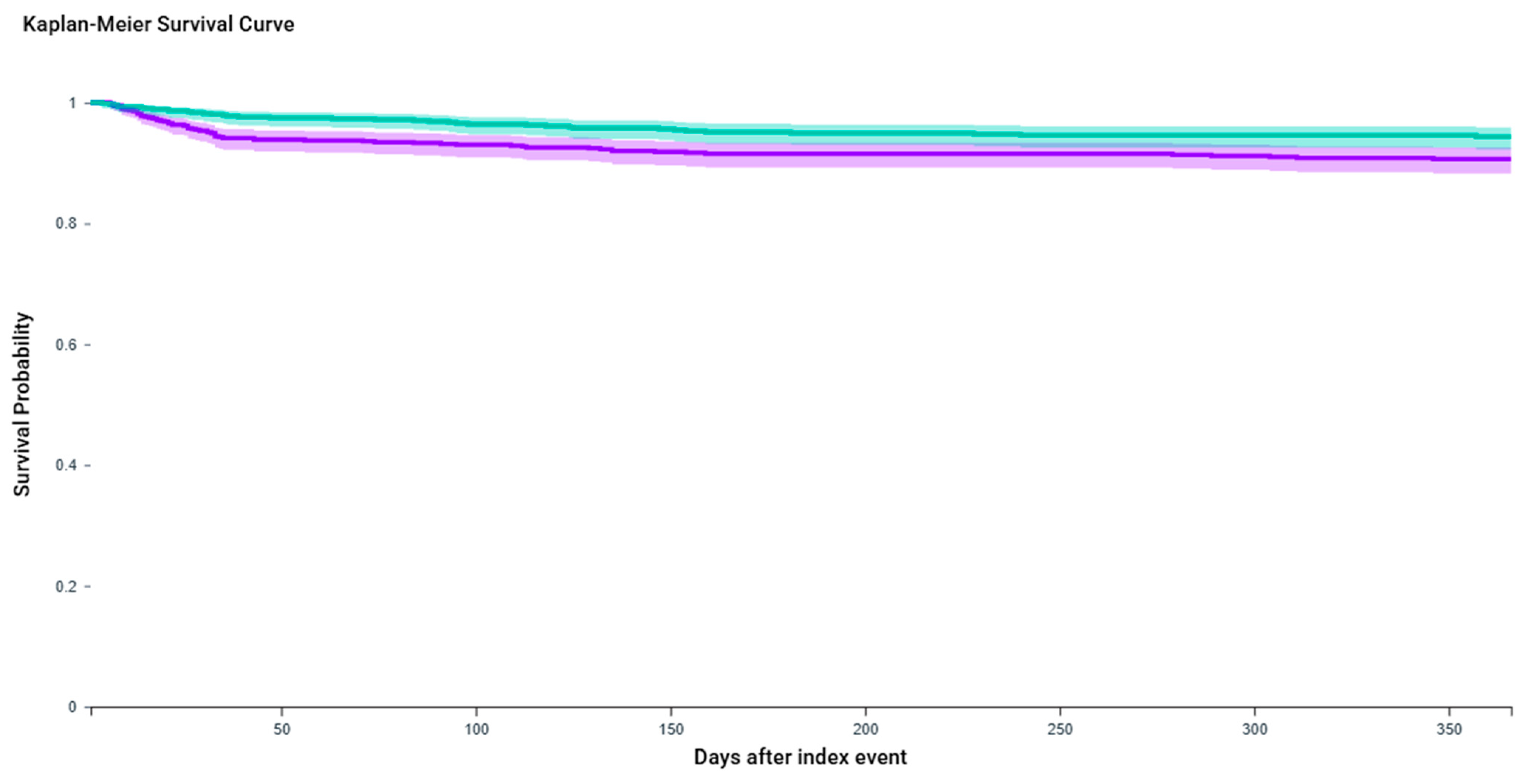
2.1. Urinary Retention
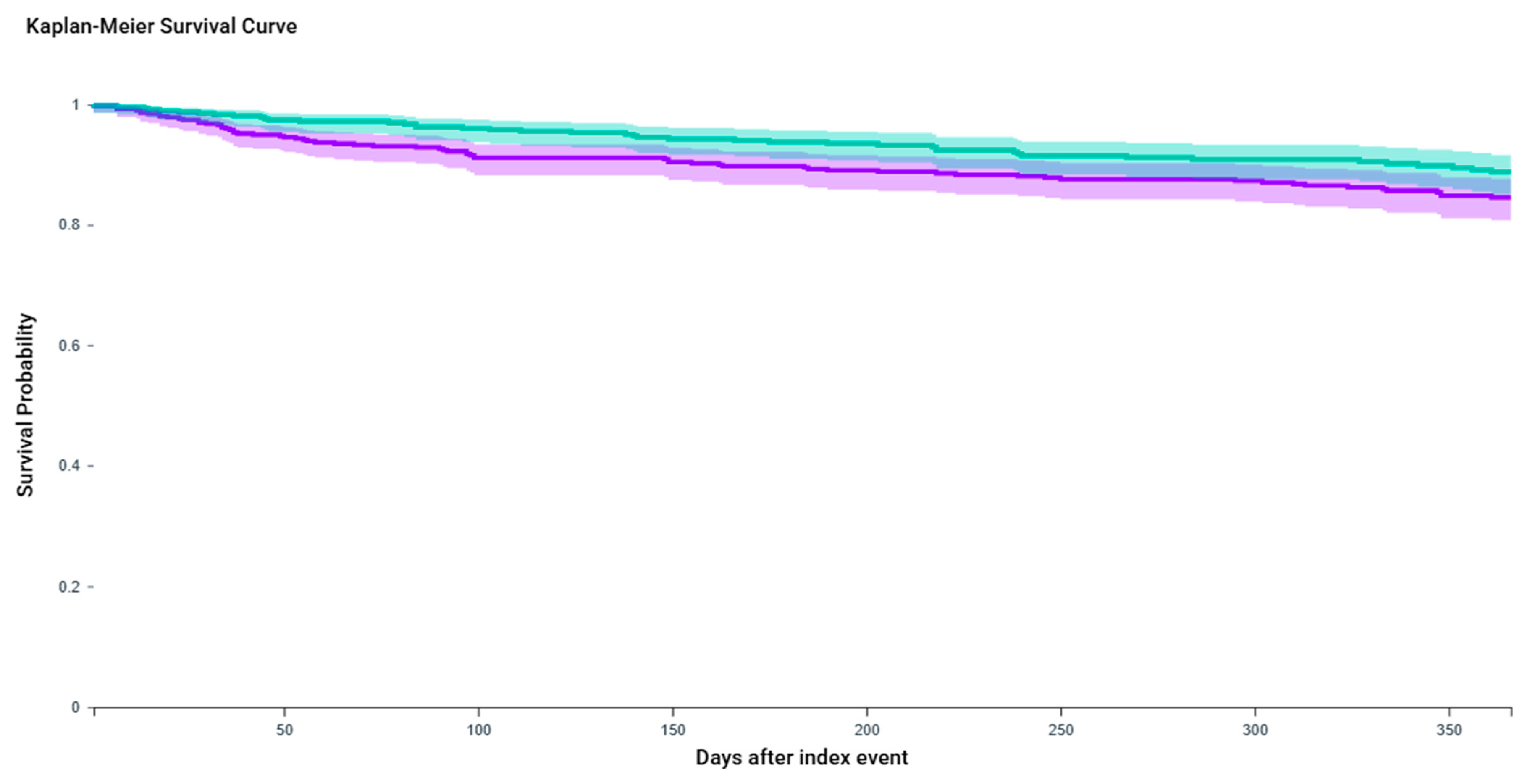
2.2. Urinary Tract Infection
2.3. Urinary Antispasmodic Use
3. Discussion
4. Conclusions
5. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OAB | Overactive bladder |
| UTI | Urinary Tract Infection |
| GLP-1 RA | Glucagon-Like Peptide-1 Receptor Agonist |
| BTX-A | OnabotulinumtoxinA |
| KM | Kaplan–Meier |
| BMI | Body Mass Index |
| ARC | Arcuate Nucleus |
| AP | Area Postrema |
| NTS | Nucleus Tractus Solitarius |
| POMC | Pro-Opiomelanocortin |
| NPY | Neuropeptide Y |
| AgRP | Agouti-Related Peptide |
| SMD | Standardized Mean Difference |
| HR | Hazard Ratio |
| FDA | U.S. Food and Drug Administration |
| PCOS | Polycystic Ovary Syndrome |
| AUA | American Urological Association |
| EAU | European Association of Urology |
| ICD-10-CM | International Classification of Diseases, 10th Revision, Clinical Modification |
| EHR | Electronic Health Record |
| IRB | Institutional Review Board |
| VA | Veterans Affairs (used in context of drug classes |
| IQR | Interquartile Range |
| CI | Confidence Interval |
| TriNetX | A federated real-world data research network/platform |
References
- Zhang, L.; Cai, N.; Mo, L.; Tian, X.; Liu, H.; Yu, B. Global prevalence of overactive bladder: A systematic review and meta-analysis. Int. Urogynecol. J. 2025, 36, 1547–1566. [Google Scholar] [CrossRef] [PubMed]
- UpToDate. Urgency Urinary Incontinence/Overactive Bladder (OAB) in Females: Treatment. Available online: https://www.uptodate.com/contents/urgency-urinary-incontinence-overactive-bladder-oab-in-females-treatment (accessed on 27 June 2025).
- Feloney, M.P.; Stauss, K.; Leslie, S.W. Sacral Neuromodulation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK567751/ (accessed on 27 June 2025).
- Orasanu, B.; Mahajan, S.T. The use of botulinum toxin for the treatment of overactive bladder syndrome. Indian J. Urol. 2013, 29, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.P.; Chung, D.E.; Dielubanza, E.J.; Enemchukwu, E.; Ginsberg, D.A.; Helfand, B.T.; Linder, B.J.; Reynolds, W.S.; Rovner, E.S.; Souter, L.; et al. The AUA/SUFU guideline on the diagnosis and treatment of idiopathic overactive bladder. J. Urol. 2024, 212, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Bonkat, G.; Kranz, J.; Cai, T.; Geerlings, S.E.; Köves, B.; Pilatz, A.; Medina-Polo, J.; Schneidewind, L.; Schubert, S.; Veeratterapillay, R.; et al. EAU Guidelines. In Proceedings of the EAU Annual Congress, Madrid, Spain, 21–24 March 2025; ISBN 978-94-92671-29-5. [Google Scholar]
- Chen, Y.H.; Kuo, J.H.; Huang, Y.T.; Lai, P.C.; Ou, Y.C.; Lin, Y.C. Evaluating the Efficacy and Safety of Botulinum Toxin in Treating Overactive Bladder in the Elderly: A Meta-Analysis with Trial Sequential Analysis of Randomized Controlled Trials. Toxins 2024, 16, 484. [Google Scholar] [CrossRef]
- Palm, K.M.; Abrams, M.K.; Sears, S.B.; Wherley, S.D.; Alfahmy, A.M.; Kamumbu, S.A.; Chakraborty, N.N.; Mahajan, S.T.; El-Nashar, S.A.; Henderson, J.W.; et al. The Response of the Urinary Microbiome to Botox. Int. Urogynecol. J. 2024, 35, 237–251. [Google Scholar] [CrossRef]
- Nitti, V.; Haag-Molkenteller, C.; Kennelly, M.; Chancellor, M.; Jenkins, B.; Schurch, B. Treatment of neurogenic detrusor overactivity and overactive bladder with Botox (onabotulinumtoxinA): Development, insights, and impact. Medicine 2023, 102 (Suppl. S1), e32377. [Google Scholar] [CrossRef]
- Lo, C.W.; Wu, M.Y.; Yang, S.S.; Jaw, F.S.; Chang, S.J. Comparing the Efficacy of OnabotulinumtoxinA, Sacral Neuromodulation, and Peripheral Tibial Nerve Stimulation as Third Line Treatment for the Management of Overactive Bladder Symptoms in Adults: Systematic Review and Network Meta-Analysis. Toxins 2020, 12, 128. [Google Scholar] [CrossRef]
- Hsieh, P.F.; Chiu, H.C.; Chen, K.C.; Chang, C.H.; Chou, E.C.L. Botulinum toxin A for the treatment of overactive bladder. Toxins 2016, 8, 59. [Google Scholar] [CrossRef]
- Semins, M.J.; Shore, A.D.; Makary, M.A.; Weiner, J.; Matlaga, B.R. The impact of obesity on urinary tract infection risk. Urology 2012, 79, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Bausch, K.; Stangl, F.P.; Prieto, J.; Bonkat, G.; Kranz, J. Urinary infection management in frail or comorbid older individuals. Eur. Urol. Focus. 2024, 10, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Rowe, T.A.; Juthani-Mehta, M. Urinary tract infection in older adults. Aging Health 2013, 9, 519–528. [Google Scholar] [CrossRef]
- Hagovska, M.; Švihra, J.; Buková, A.; Horbacz, A.; Dračková, D.; Lupták, J.; Švihra, J., Jr. The Relationship between Overweight and Overactive Bladder Symptoms. Obes. Facts. 2020, 13, 297–306. [Google Scholar] [CrossRef]
- Khullar, V.; Sexton, C.C.; Thompson, C.L.; Milsom, I.; Bitoun, C.E.; Coyne, K.S. The relationship between BMI and urinary incontinence subgroups: Results from EpiLUTS. Neurourol. Urodyn. 2014, 33, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Wei, C.; Huang, Y.; Fu, T.; Shen, W.; Xiao, W. Relationship between the weight-adjusted-waist index and urinary incontinence in women: A cross-sectional study of NHANES 2007 to 2020. Medicine 2025, 104, e42996. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, W.; Tang, Z.; Lin, X.; Wan, X.; Huang, S.; Luo, H.; Qian, Y.; He, Z.; Tang, F. The Association between Obesity and Wet Overactive Bladder: Results from 2005 to 2020 National Health and Nutrition Examination Survey. Obes. Facts. 2025, 18, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Pomian, A.; Lisik, W.; Kosieradzki, M.; Barcz, E. Obesity and Pelvic Floor Disorders: A Review of the Literature. Med. Sci. Monit. 2016, 22, 1880–1886. [Google Scholar] [CrossRef]
- Tala, M.R.Z.; Ficky, F.; Lubis, D.L.; Mirsya Warli, S. Efficacy of Body Weight Reduction in Improving Overactive Bladder Symptoms in Obese and Overweight Women: A Systematic Review. Nephro-Urol Mon. 2024, 17, e154345. [Google Scholar] [CrossRef]
- Collins, L.; Costello, R.A. Glucagon-Like Peptide-1 Receptor Agonists. In StatPearls; Bookshelf ID NBK551568; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Xu, D.; Nair, A.; Sigston, C.; Ho, C.; Li, J.; Yang, D.; Liao, X.; Chen, W.; Kuang, M.; Li, Y.; et al. Potential Roles of Glucagon-Like Peptide 1 Receptor Agonists (GLP-1 RAs) in Nondiabetic Populations. Cardiovasc Ther. 2022, 2022, 6820377. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Brown-Frandsen, K.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. SELECT Trial Investigators. Semaglutide and cardiovascular outcomes in obesity without diabetes. N. Engl. J. Med. 2023, 389, 2221–2232. [Google Scholar] [CrossRef]
- Heerspink, H.J.; Apperloo, E.; Davies, M.; Dicker, D.; Kandler, K.; Rosenstock, J.; Sørrig, R.; Lawson, J.; Zeuthen, N.; Cherney, D. Effects of Semaglutide on Albuminuria and Kidney Function in People with Overweight or Obesity with or Without Type 2 Diabetes: Exploratory Analysis From the STEP 1, 2, and 3 Trials. Diabetes Care 2023, 46, 801–810. [Google Scholar] [CrossRef]
- Cena, H.; Chiovato, L.; Nappi, R.E. Obesity, Polycystic Ovary Syndrome, and Infertility: A New Avenue for GLP-1 Receptor Agonists. J. Clin. Endocrinol. Metab. 2020, 105, e2695–e2709. [Google Scholar] [CrossRef]
- Jiang, Z.; Tan, J.; Yuan, Y.; Shen, J.; Chen, Y. Semaglutide ameliorates lipopolysaccharide-induced acute lung injury through inhibiting HDAC5-mediated activation of NF-κB signaling pathway. Hum. Exp. Toxicol. 2022, 41, 9603271221125931. [Google Scholar] [CrossRef] [PubMed]
- Shnaien, A.; Mohammad, A.; Hassan, E. Neuroprotective Effects of Semaglutide in Endotoxemia Mouse Model. Iran J. War Public Health 2023, 15, 199–205. [Google Scholar]
- Tan, S.A.; Tan, L. liraglutide and semaglutide attenuate inflammatory cytokines interferon-gamma, tumor necrosis factor-alpha, and interleukin-6: Possible mechanism of decreasing cardiovascular risk in diabetes mellitus. J. Am. Coll. Cardiol. 2019, 73 (Suppl. S1), 1866. [Google Scholar] [CrossRef]
- Mosenzon, O.; Capehorn, M.S.; De Remigis, A.; Rasmussen, S.; Weimers, P.; Rosenstock, J. Impact of semaglutide on high-sensitivity C-reactive protein: Exploratory patient-level analyses of SUSTAIN and PIONEER randomized clinical trials. Cardiovasc Diabetol. 2022, 21, 172. [Google Scholar] [CrossRef]
- Yazdany, T.; Jakus-Waldman, S.; Jeppson, P.C.; Schimpf, M.O.; Yurteri-Kaplan, L.A.; Ferzandi, T.R.; Weber-LeBrun, E.; Knoepp, L.; Mamik, M.; Viswanathan, M.; et al. American Urogynecologic Society Systematic Review: The Impact of Weight Loss Intervention on Lower Urinary Tract Symptoms and Urinary Incontinence in Overweight and Obese Women. Female Pelvic Med. Reconstr. Surg. 2020, 26, 16–29. [Google Scholar] [CrossRef]
- Subak, L.L.; Wing, R.; West, D.S.; Franklin, F.; Vittinghoff, E.; Creasman, J.M.; Richter, H.E.; Myers, D.; Burgio, K.L.; Gorin, A.A.; et al. Weight loss to treat urinary incontinence in overweight and obese women. N. Engl. J. Med. 2009, 360, 481–490. [Google Scholar] [CrossRef]
- Zacche, M.M.; Giarenis, I.; Thiagamoorthy, G.; Robinson, D.; Cardozo, L. Is there an association between aspects of the metabolic syndrome and overactive bladder? A prospective cohort study in women with lower urinary tract symptoms. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 217, 1–5. [Google Scholar] [CrossRef]
- Ljungberg, C.; Bredahl Kristensen, F.P.; Dalager-Pedersen, M.; Vandenbroucke-Grauls, C.; Sørensen, H.T.; Nørgaard, M.; Thomsen, R.W. Risk of Urogenital Infections in People with Type 2 Diabetes Initiating SGLT2is Versus GLP-1RAs in Routine Clinical Care: A Danish Cohort Study. Diabetes Care 2025, 48, 945–954. [Google Scholar] [CrossRef]
- Dave, C.V.; Schneeweiss, S.; Kim, D.; Fralick, M.; Tong, A.; Patorno, E. Sodium-Glucose Cotransporter-2 Inhibitors and the Risk for Severe Urinary Tract Infections: A Population-Based Cohort Study. Ann. Intern. Med. 2019, 171, 248–256. [Google Scholar] [CrossRef]
- Soogoor, A.R.; Agrawal, P.; Pupo, D.; Kohn, T.P.; Du Comb, W.; Alshak, M.N. MP45-11 Semaglutide Utilization in Weight Management and its Implications for Urinary Tract Infections and Urolithiasis. J. Urol. 2024, 211, e746. [Google Scholar] [CrossRef]
- Sandler, M.D.; Williams, A.D.; Wein, A.; Amin, K.; Syan, R. Effects of Glucagon like Peptide-1 agonists on patients with overactive bladder: A pilot study. Cont. Rep. 2025, 14, 100083. [Google Scholar] [CrossRef]
- Tan, H.C.; Dampil, O.A.; Marquez, M.M. Efficacy and Safety of Semaglutide for Weight Loss in Obesity Without Diabetes: A Systematic Review and Meta-Analysis. J. ASEAN Fed. Endocr. Soc. 2022, 37, 65–72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Doumouchtsis, S.K.; Loganathan, J.; Pergialiotis, V. The role of obesity on urinary incontinence and anal incontinence in women: A review. BJOG 2022, 129, 162–170. [Google Scholar] [CrossRef]
- Nitzan, O.; Elias, M.; Chazan, B.; Saliba, W. Urinary tract infections in patients with type 2 diabetes mellitus: Review of prevalence, diagnosis, and management. Diabetes Metab. Syndr. Obes. 2015, 8, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.E.; Abdelkarim, S.; Zenida, M.; Baiti, M.A.H.; Alhazmi, A.A.Y.; Alfaifi, B.A.H.; Majrabi, R.Q.M.; Khormi, N.Q.M.; Hakami, A.A.A.; Alqaari, R.A.M.; et al. Prevalence and Associated Risk Factors of Urinary Tract Infection among Diabetic Patients: A Cross-Sectional Study. Healthcare 2023, 11, 861. [Google Scholar] [CrossRef]
- Able, C.; Liao, B.; Saffati, G.; Maremanda, A.; Applewhite, J.; Nasrallah, A.A.; Sonstein, J.; Alzweri, L.; Kohn, T.P. Prescribing semaglutide for weight loss in non-diabetic, obese patients is associated with an increased risk of erectile dysfunction: A TriNetX database study. Int. J. Impot. Res. 2025, 37, 315–319. [Google Scholar] [CrossRef]
- Varnum, A.A.; Pozzi, E.; Deebel, N.A.; Evans, A.; Eid, N.; Sadeghi-Nejad, H.; Ramasamy, R. Impact of GLP-1 Agonists on Male Reproductive Health-A Narrative Review. Medicina 2023, 60, 50. [Google Scholar] [CrossRef]
- Alhajahjeh, A.; Al-Faouri, R.; Bahmad, H.F.; Bader, T.; Dobbs, R.W.; Abdulelah, A.A.; Abou-Kheir, W.; Davicioni, E.; Lee, D.I.; Shahait, M. From Diabetes to Oncology: Glucagon-like Peptide-1 (GLP-1) Receptor Agonist’s Dual Role in Prostate Cancer. Cancers 2024, 16, 1538. [Google Scholar] [CrossRef]
- Langroudi, A.P.; Chen, A.L.; Basran, S.; Sommer, E.R.; Stinson, J.; Cheng, Y.-S.; Del Giuduce, F.; Scott, M.; Eisenberg, M.L. Male sexual dysfunction associated with GLP-1 receptor agonists: A cross-sectional analysis of FAERS data. Int. J. Impot. Res 2025, 37, 661–667. [Google Scholar] [CrossRef]
- Fang, A.; Frigo, D.E.; Hahn, A.; Razouki, Z.; Hwang, J.; Koutroumpakis, E.; Lawen, T.; Smith, M.; Hamilton-Reeves, J.; DiGiovanni, J.; et al. GLP-1 Agonist Use Among Men with Localized Prostate Cancer: A Narrative Review and Rationale for Prospective Clinical Trials. Urology 2025, 201, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Salvio, G.; Ciarloni, A.; Ambo, N.; Bordoni, M.; Perrone, M.; Rossi, S.; Balercia, G. Effects of glucagon-like peptide 1 receptor agonists on testicular dysfunction: A systematic review and meta-analysis. Andrology 2025, 13, 1–13. [Google Scholar] [CrossRef]
- Nomiyama, T.; Kawanami, T.; Irie, S.; Hamaguchi, Y.; Terawaki, Y.; Murase, K.; Tsutsumi, Y.; Nagaishi, R.; Tanabe, M.; Morinaga, H.; et al. Exendin-4, a GLP-1 receptor agonist, attenuates prostate cancer growth. Diabetes 2014, 63, 3891–3905. [Google Scholar] [CrossRef]
- Zhou, L.; Dong, M.; Feng, G.; Zhang, Y.; Wang, J.; Kang, H.; Dong, Z.; Ning, J.; Zhao, Z.; Wang, C. Semaglutide mitigates testicular damage in diabetes by inhibiting ferroptosis. Biochem. Biophys. Res. Commun. 2024, 715, 149996. [Google Scholar] [CrossRef]
- Filippatos, T.D.; Panagiotopoulou, T.V.; Elisaf, M.S. Adverse Effects of GLP-1 Receptor Agonists. Rev. Diabet. Stud. 2014, 11, 202–230. [Google Scholar] [CrossRef]
- Chung, S.D.; Liu, H.T.; Lin, H.; Kuo, H.C. Elevation of serum c-reactive protein in patients with OAB and IC/BPS implies chronic inflammation in the urinary bladder. Neurourol. Urodyn. 2011, 30, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Jiang, Y.H.; Kuo, H.C. Increased serum adipokines implicate chronic inflammation in the pathogenesis of overactive bladder syndrome refractory to antimuscarinic therapy. PLoS ONE 2013, 8, e76706. [Google Scholar] [CrossRef]
- Pillalamarri, N.; Shalom, D.F.; Pilkinton, M.L.; Winkler, H.A.; Chatterjee, P.K.; Solanki, M.; Metz, C.N. Inflammatory Urinary Cytokine Expression and Quality of Life in Patients with Overactive Bladder. Female Pelvic Med. Reconstr. Surg. 2018, 24, 449–453. [Google Scholar] [CrossRef]
- Grundy, L.; Caldwell, A.; Brierley, S.M. Mechanisms Underlying Overactive Bladder and Interstitial Cystitis/Painful Bladder Syndrome. Front. Neurosci. 2018, 12, 931. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhou, J.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health Benefits and Molecular Mechanisms of Resveratrol: A Narrative Review. Foods 2020, 9, 340. [Google Scholar] [CrossRef]
- Singh, I.; Wang, L.; Xia, B.; Liu, J.; Tahiri, A.; El Ouaamari, A.; Wheeler, M.B.; Pang, Z.P. Activation of arcuate nucleus glucagon-like peptide-1 receptor-expressing neurons suppresses food intake. Cell Biosci. 2022, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Secher, A.; Jelsing, J.; Baquero, A.F.; Hecksher-Sørensen, J.; Cowley, M.A.; Dalbøge, L.S.; Hansen, G.; Grove, K.L.; Pyke, C.; Raun, K.; et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J. Clin. Investig. 2014, 124, 4473–4488. [Google Scholar] [CrossRef]
- Jais, A.; Brüning, J.C. Arcuate Nucleus-Dependent Regulation of Metabolism-Pathways to Obesity and Diabetes Mellitus. Endocr. Rev. 2022, 43, 314–328. [Google Scholar] [CrossRef]
- Beddows, C.A.; Shi, F.; Horton, A.L.; Dalal, S.; Zhang, P.; Ling, C.-C.; Yong, V.W.; Loh, K.; Cho, E.; Karagiannis, C.; et al. Pathogenic hypothalamic extracellular matrix promotes metabolic disease. Nature 2024, 633, 914–922. [Google Scholar] [CrossRef]
- Wright, E.E., Jr.; Aroda, V.R. Clinical review of the efficacy and safety of oral semaglutide in patients with type 2 diabetes considered for injectable GLP-1 receptor agonist therapy or currently on insulin therapy. Postgrad Med. 2020, 132 (Suppl. S2), 26–36. [Google Scholar] [CrossRef]
- Huang, K.-P.; Acosta, A.A.; Ghidewon, M.Y.; McKnight, A.D.; Almeida, M.S.; Nyema, N.T.; Hanchak, N.D.; Patel, N.; Gbenou, Y.S.K.; Adriaenssens, A.E.; et al. Dissociable hindbrain GLP1R circuits for satiety and aversion. Nature 2024, 632, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Amundsen, C.L.; Richter, H.E.; Menefee, S.A.; Komesu, Y.M.; Arya, L.A.; Gregory, W.T.; Myers, D.L.; Zyczynski, H.M.; Vasavada, S.; Nolen, T.L.; et al. OnabotulinumtoxinA vs. Sacral Neuromodulation on Refractory Urgency Urinary Incontinence in Women: A Randomized Clinical Trial. JAMA 2016, 316, 1366–1374. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Characteristic | OAB + BTX-A (Before Matching) | OAB + BTX-A + GLP1 RA (Before Matching) | p-Value (Before Matching) | OAB + BTX-A (After Matching) | OAB + BTX-A + GLP1 RA (After Matching) | p-Value (After Matching) |
|---|---|---|---|---|---|---|
| Age at Index (Mean ± SD) | 62.4 ± 17.6 | 59.4 ± 13.2 | <0.0001 | 59.3 ± 13.9 | 59.4 ± 13.2 | 0.8073 |
| Female | 15,971 (80.2%) | 904 (91.1%) | <0.0001 | 913 (92.0%) | 904 (91.1%) | 0.4668 |
| Male | 3563 (17.9%) | 63 (6.4%) | <0.0001 | 59 (5.9%) | 63 (6.4%) | 0.7085 |
| Not Hispanic or Latino | 15,513 (77.9%) | 800 (80.6%) | 0.0435 | 802 (80.8%) | 800 (80.6%) | 0.9093 |
| White | 15,858 (79.7%) | 752 (75.8%) | 0.0034 | 770 (77.6%) | 752 (75.8%) | 0.339 |
| Black or African American | 1706 (8.6%) | 127 (12.8%) | <0.0001 | 115 (11.6%) | 127 (12.8%) | 0.4104 |
| Hispanic or Latino | 1333 (6.7%) | 69 (7.0%) | 0.7498 | 71 (7.2%) | 69 (7.0%) | 0.8608 |
| Asian | 339 (1.7%) | 10 (1.0%) | 0.0955 | 10 (1.0%) | 10 (1.0%) | 1 |
| Overweight/Obesity | 5216 (26.2%) | 756 (76.2%) | <0.0001 | 755 (76.1%) | 756 (76.2%) | 0.958 |
| Hypertension | 8944 (44.9%) | 575 (58.0%) | <0.0001 | 564 (56.9%) | 575 (58.0%) | 0.6175 |
| Stress Incontinence | 5398 (27.1%) | 389 (39.2%) | <0.0001 | 391 (39.4%) | 389 (39.2%) | 0.9268 |
| Body Mass Index (BMI) 40–44.9 | 880 (4.4%) | 214 (21.6%) | <0.0001 | 219 (22.1%) | 214 (21.6%) | 0.7858 |
| BMI 45–49.9 | 431 (2.2%) | 110 (11.1%) | <0.0001 | 104 (10.5%) | 110 (11.1%) | 0.6641 |
| BMI 29–29.9 | 453 (2.3%) | 53 (5.3%) | <0.0001 | 48 (4.8%) | 53 (5.3%) | 0.6096 |
| BMI 28–28.9 | 457 (2.3%) | 40 (4.0%) | 0.0005 | 37 (3.7%) | 40 (4.0%) | 0.7273 |
| BMI 27–27.9 | 461 (2.3%) | 37 (3.7%) | 0.0044 | 38 (3.8%) | 37 (3.7%) | 0.9063 |
| Antimicrobials | 17,619 (88.5%) | 976 (98.4%) | <0.0001 | 928 (93.5%) | 976 (98.4%) | <0.0001 |
| Antiemetics | 12,021 (60.4%) | 832 (83.9%) | <0.0001 | 745 (75.1%) | 832 (83.9%) | <0.0001 |
| Laxatives | 11,381 (57.2%) | 740 (74.6%) | <0.0001 | 680 (68.5%) | 740 (74.6%) | 0.0028 |
| Anti-infectives, vaginal | 7163 (36.0%) | 585 (59.0%) | <0.0001 | 518 (52.2%) | 585 (59.0%) | 0.0025 |
| Outcome | Event Rate (BTX-A Group) | Event Rate (BTX-A + GLP-1 Group) | Risk Difference (95% CI) | KM Log-Rank p | HR (95% CI) |
|---|---|---|---|---|---|
| Urinary Retention | 8.60% | 4.90% | 3.66% (1.16–6.17%) | 0.0064 | 1.74 (1.16–2.59) |
| UTI | 13.30% | 8.80% | 4.54% (0.67–8.40%) | 0.042 | 1.48 (1.01–2.17) |
| Antispasmodic Use | 11.80% | 10.30% | 1.55% (−6.07 to 9.17%) | 0.7234 | 1.14 (0.55–2.39) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammad, M.A.M.; Quesada, S.G.; Belczyk, A.L.; Ghoniem, G.M. Beyond Glycemic Control: Concurrent GLP-1 Receptor Agonist Use Is Associated with Reduced Urinary Adverse Events Following OnabotulinumtoxinA Treatment in Non-Diabetic Adults with Overactive Bladder. Toxins 2025, 17, 542. https://doi.org/10.3390/toxins17110542
Hammad MAM, Quesada SG, Belczyk AL, Ghoniem GM. Beyond Glycemic Control: Concurrent GLP-1 Receptor Agonist Use Is Associated with Reduced Urinary Adverse Events Following OnabotulinumtoxinA Treatment in Non-Diabetic Adults with Overactive Bladder. Toxins. 2025; 17(11):542. https://doi.org/10.3390/toxins17110542
Chicago/Turabian StyleHammad, Muhammed A. M., Sophia G. Quesada, Aimee L. Belczyk, and Gamal M. Ghoniem. 2025. "Beyond Glycemic Control: Concurrent GLP-1 Receptor Agonist Use Is Associated with Reduced Urinary Adverse Events Following OnabotulinumtoxinA Treatment in Non-Diabetic Adults with Overactive Bladder" Toxins 17, no. 11: 542. https://doi.org/10.3390/toxins17110542
APA StyleHammad, M. A. M., Quesada, S. G., Belczyk, A. L., & Ghoniem, G. M. (2025). Beyond Glycemic Control: Concurrent GLP-1 Receptor Agonist Use Is Associated with Reduced Urinary Adverse Events Following OnabotulinumtoxinA Treatment in Non-Diabetic Adults with Overactive Bladder. Toxins, 17(11), 542. https://doi.org/10.3390/toxins17110542





