Functional Effects of BoNT-A Application in Masseter Muscle in Patients with Symptoms of Bruxism
Abstract
1. Introduction
2. Results
- Tertile I included patients whose baseline values were below the 33rd percentile, representing the lowest levels of masseter muscle tension and stiffness.
- Tertile II consisted of patients with baseline values between the 33rd and 67th percentiles, representing intermediate levels.
- Tertile III included patients above the 67th percentile, reflecting the highest baseline values with elevated muscle tension and stiffness.
- BoNT-A reduces muscle tension and stiffness under tension but does not significantly affect muscles at rest in most patients.
- The effects are most pronounced after three weeks and partially diminish by three months.
- The most significant and persistent changes were observed in patients with the highest muscle tension before the injection procedure (Tertile III).
- The greatest changes were observed in measurements taken under tension, which is consistent with findings from studies on healthy individuals conducted using the same device by other researchers [45].
- Patients with the lowest baseline masseter muscle tension and stiffness.
- Tertile II grouped individuals with average parameters.
- Tertile III included patients with the highest baseline values, reflecting elevated muscle tension and stiffness.
3. Discussion
4. Conclusions
5. Limitations
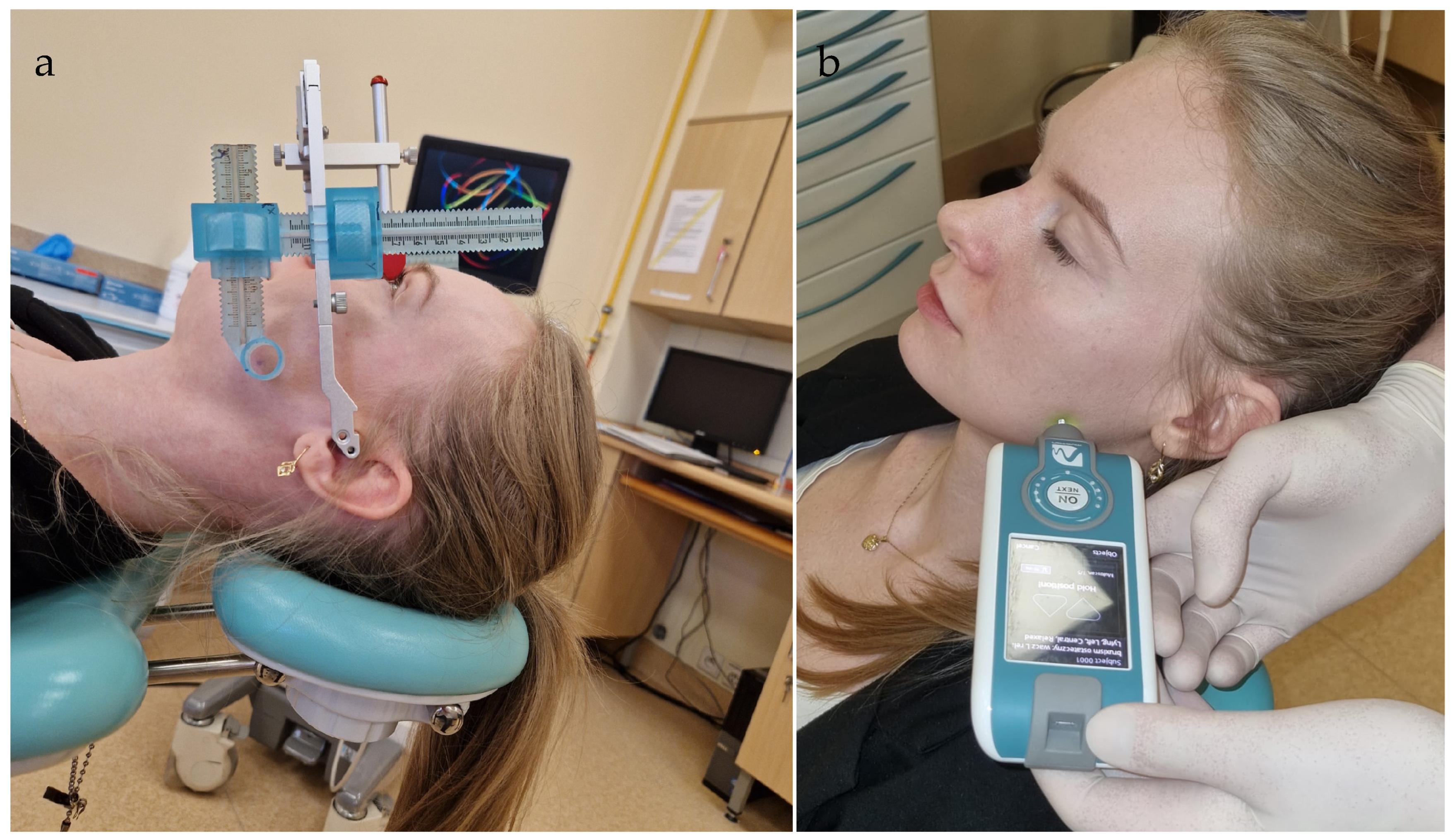
6. Materials and Methods
- Age 18–40 years
- Occurrence of 60% or more bruxism criteria based on the BruxScreen form (interview and clinical examination), combined with clinically significant masticatory muscle symptoms (pain, tension, fatigue) that interfere with daily function. The qualification threshold of 60% bruxism symptoms was established pragmatically, serving as a compromise between sensitivity and specificity in the absence of diagnostic criteria. While this cut-off lacks formal empirical validation, it was selected to maintain clinical relevance. Recent literature [61,62,63] underscores the methodological challenges in defining bruxism severity and highlights the need for more refined assessment tools. Future studies should incorporate standardized approaches, such as the STAB protocol, and combine validated questionnaires with instrumental or ambulatory monitoring methods where feasible. Each participant was asked to complete the Oral Behavior Checklist (OBC) [64,65]. Based on its results, individuals reporting characteristic bruxism behaviors occurring more than twice per week were selected for further screening. These participants subsequently completed the BruxScreen questionnaire, which, at the time, was recommended by a group of international experts for non-instrumental bruxism assessment [66]. Patients meeting the criteria based on this screening were evaluated further. While the STAB (Standardized Tool for the Assessment of Bruxism) protocol [6] represents the current gold standard for bruxism assessment, it was not yet fully available at the time of study initiation. Therefore, this study acknowledges the limitations of the assessment protocol used and the need for future studies to incorporate STAB methodology.
- tooth loss resulting in lack of one or more support zones
- recent maxillofacial trauma
- BMI > 30
- muscle relaxant use
- pregnancy
- allergy to albumins
- patients taking medications favoring interaction with the botulinum toxin, such as quinidine, calcium channel blockers
- parallel temporomandibular disorder (TMD) treatment according to INfORM/IADR guidelines
- significant psychological disorders requiring active treatment (as these may influence muscle tension patterns independently)
- The first injection (15 U) was administered in the deep belly of the muscle, near the mandibular angle 1.5 cm from mandibular ramus and 1.5 cm from mandibular body.
- The second injection (15 U) was administered in the superficial belly of the muscle, approximately 0.5 cm below its point of greatest prominence.
- The third injection (10 U) was administered in the superficial belly, 1.5 cm above muscle greatest prominence.
- Tone is characterized by Oscillation frequency [Hz] (muscle stiffness) of biological soft tissues on the cellular level. Oscillation Frequency characterizes the tone of superficial skeletal muscles in their passive or resting state without voluntary contraction (EMG signal silent). The Oscillation Frequency of a muscle in its contracted state characterizes state of tension.
- Dynamic Stiffness [N/m] characterizes the resistance of biological soft tissues to a force of deformation. The term Dynamic Stiffness originates from the dynamic measurement method applied in Myoton technology. The inverse of stiffness is compliance.
- Logarithmic Decrement [arb] characterizes the dampening of tissue oscillation. The faster the tissue oscillation fades, the higher the dissipation of mechanical energy induced by the measurement impulse. The decrement in tissues’ natural oscillation inversely describes elasticity. Elasticity is the biomechanical property of soft tissues that characterizes the ability to recover its initial shape from being deformed. The higher the decrement, the lower the elasticity. In theory, a decrement of zero (0) represents absolute elasticity (absence of dampening). The inverse of elasticity is plasticity.
- Mechanical Stress Relaxation Time [ms] characterizes tissue’s recovery time from displacement. The higher a tissue’s tension or stiffness, the faster a tissue recovers its shape, meaning the lower the value.
- Ratio of Relaxation and Deformation time [arb] characterizes creep, the gradual elongation of tissue over time when placed under constant tensile stress. The higher a tissue’s tension, structural integrity, or stiffness, the higher its resistance to creep, meaning the lower the value.
- Left masseter muscle examination at rest (5 measurements).
- Right masseter muscle examination at rest (5 measurements).
- Left masseter muscle examination at maximum tension (5 measurements).
- Right masseter muscle examination at maximum tension (5 measurements).
- Visit 1 (V1)—examination before BoNT-A administration (baseline).
- Visit 2 (V2)—examination 3 weeks after BoNT-A administration.
- Visit 3 (V3)—examination 3 months after BoNT-A administration.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zieliński, G.; Pająk, A.; Wójcicki, M. Global Prevalence of Sleep Bruxism and Awake Bruxism in Pediatric and Adult Populations: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 4259. [Google Scholar] [CrossRef]
- van Selms, M.K.A.; Visscher, C.M.; Naeije, M.; Lobbezoo, F. Bruxism and associated factors among Dutch adolescents. Community Dent. Oral. Epidemiol. 2013, 41, 353–363. [Google Scholar] [CrossRef]
- Briones, M.A.; Basantes, R.E.; Escobar, D.O. Prevalencia de bruxismo y estrés en estudiantes de odontología de la Universidad Regional Autónoma de los Andes. Gac Médica Estud. 2023, 4, e274. [Google Scholar]
- Lobbezoo, F.; Ahlberg, J.; Raphael, K.G.; Wetselaar, P.; Glaros, A.G.; Kato, T.; Santiago, V.; Winocur, E.; De Laat, A.; De Leeuw, R.; et al. International consensus on the assessment of bruxism: Report of a work in progress. J. Oral. Rehabil. 2018, 45, 837–844. [Google Scholar] [CrossRef]
- Verhoeff, M.C.; Lobbezoo, F.; Ahlberg, J.; Bender, S.; Bracci, A.; Colonna, A.; Dal Fabbro, C.; Durham, J.; Glaros, A.G.; Häggman-Henrikson, B.; et al. Updating the Bruxism Definitions: Report of an International Consensus Meeting. J. Oral. Rehabil. 2025, 52, 1335–1342. [Google Scholar] [CrossRef]
- Manfredini, D.; Ahlberg, J.; Aarab, G.; Bender, S.; Bracci, A.; Cistulli, P.A.; Conti, P.C.; De Leeuw, R.; Durham, J.; Emodi-Perlman, A.; et al. Standardised Tool for the Assessment of Bruxism. J. Oral. Rehabil. 2024, 51, 29–58. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.S.; Quadri, S.A.; Mosaddad, S.A.; Heboyan, A. The relationship between psychological factors and temporomandibular disorders: A systematic review and meta-analysis. Head Face Med. 2025, 21, 46. [Google Scholar] [CrossRef] [PubMed]
- Heyat, M.B.B.; Akhtar, F.; Khan, M.H.; Ullah, N.; Gul, I.; Khan, H.; Lai, D. Detection, Treatment Planning, and Genetic Predisposition of Bruxism: A Systematic Mapping Process and Network Visualization Technique. CNS Neurol. Disord. Drug Targets 2021, 20, 755–775. [Google Scholar] [CrossRef] [PubMed]
- Caivano, T.; Felipe-Spada, N.; Roldán-Cubero, J.; Tomàs-Aliberas, J. Influence of genetics and biopsychosocial aspects as etiologic factors of bruxism. CRANIO® 2021, 39, 183–185. [Google Scholar] [CrossRef]
- Rintakoski, K.; Hublin, C.; Lobbezoo, F.; Rose, R.J.; Kaprio, J. Genetic Factors Account for Half of the Phenotypic Variance in Liability to Sleep-Related Bruxism in Young Adults: A Nationwide Finnish Twin Cohort Study. Twin Res. Hum. Genet. 2012, 15, 714–719. [Google Scholar] [CrossRef]
- Przystańska, A.; Jasielska, A.; Ziarko, M.; Pobudek-Radzikowska, M.; Maciejewska-Szaniec, Z.; Prylińska-Czyżewska, A.; Wierzbik-Strońska, M.; Gorajska, M.; Czajka-Jakubowska, A. Psychosocial Predictors of Bruxism. BioMed Res. Int. 2019, 2019, 2069716. [Google Scholar] [CrossRef]
- Pavlou, I.A.; Spandidos, D.A.; Zoumpourlis, V.; Papakosta, V.K. Neurobiology of bruxism: The impact of stress (Review). Biomed. Rep. 2024, 20, 59. [Google Scholar] [CrossRef]
- De La Torre Canales, G.; Câmara-Souza, M.B.; Muñoz Lora, V.R.M.; Guarda-Nardini, L.; Conti, P.C.R.; Rodrigues Garcia, R.M.; Del Bel Cury, A.A.; Manfredini, D. Prevalence of psychosocial impairment in temporomandibular disorder patients: A systematic review. J. Oral. Rehabil. 2018, 45, 881–889. [Google Scholar] [CrossRef]
- Manfredini, D.; Lobbezoo, F. Relationship between bruxism and temporomandibular disorders: A systematic review of literature from 1998 to 2008. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2010, 109, e26–e50. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Leite, C.M.; Stuginski-Barbosa, J.; Conti, P.C.R. How psychosocial and economic impacts of COVID-19 pandemic can interfere on bruxism and temporomandibular disorders? J. Appl. Oral Sci. FOB 2020, 28, e20200263. [Google Scholar] [CrossRef] [PubMed]
- Kardeş, E.; Kardeş, S. Google searches for bruxism, teeth grinding, and teeth clenching during the COVID-19 pandemic. J. Orofac. Orthop. Fortschritte Kieferorthopadie 2021, 83, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Quadri, M.F.A.; Mahnashi, A.; Al Almutahhir, A.; Tubayqi, H.; Hakami, A.; Arishi, M.; Alamir, A. Association of Awake Bruxism with Khat, Coffee, Tobacco, and Stress among Jazan University Students. Int. J. Dent. 2015, 2015, e842096. [Google Scholar] [CrossRef]
- Wieckiewicz, M.; Paradowska-Stolarz, A.; Wieckiewicz, W. Psychosocial Aspects of Bruxism: The Most Paramount Factor Influencing Teeth Grinding. BioMed Res. Int. 2014, 2014, 469187. [Google Scholar] [CrossRef]
- Greene, C.S.; Manfredini, D. Overtreatment “Successes”—What Are the Negative Consequences for Patients, Dentists, and the Profession? J. Oral Facial Pain Headache Spring 2023, 37, 81–90. [Google Scholar] [CrossRef]
- Lobbezoo, F.; Verhoeff, M.C.; Ahlberg, J.; Manfredini, D.; Aarab, G.; Koutris, M.; Svensson, P.; Thymi, M.; Visscher, C.M.; Lavigne, G.J. A century of bruxism research in top-ranking medical journals. Cephalalgia Rep. 2024, 7, 1–9. [Google Scholar] [CrossRef]
- Matusz, K.; Maciejewska-Szaniec, Z.; Gredes, T.; Pobudek-Radzikowska, M.; Glapiński, M.; Górna, N.; Przystańska, A. Common therapeutic approaches in sleep and awake bruxism—An overview. Neurol. Neurochir. Pol. 2022, 56, 455–463. [Google Scholar]
- Barth, S.W.; Lehner, M.D.; Dietz, G.P.H.; Schulze, H. Pharmacologic treatments in preclinical tinnitus models with special focus on Ginkgo biloba leaf extract EGb 761®. Mol. Cell Neurosci. 2021, 116, 103669. [Google Scholar]
- Demirkol, N.; Sari, F.; Bulbul, M.; Demirkol, M.; Simsek, I.; Usumez, A. Effectiveness of occlusal splints and low-level laser therapy on myofascial pain. Lasers Med. Sci. 2015, 30, 1007–1012. [Google Scholar] [CrossRef]
- Awan, K.H.; Patil, S.; Alamir, A.W.H.; Maddur, N.; Arakeri, G.; Carrozzo, M.; Brennan, P.A. Botulinum toxin in the management of myofascial pain associated with temporomandibular dysfunction. J. Oral Pathol. Med. 2019, 48, 192–200. [Google Scholar] [CrossRef]
- Alajbeg, I.Z.; Boric Brakus, R.; Brakus, I. Comparison of amitriptyline with stabilization splint and placebo in chronic TMD patients: A pilot study. Acta Stomatol. Croat. 2018, 52, 114–122. [Google Scholar] [CrossRef]
- Dudzic, M.; Pieczyńska, A.; Drużdż, A.; Rajewska, A.; Hojan, K. Is There a “Non-Motor Effect” of Botulinum Toxin Treatment in Cervical Dystonia in Addition to Its Effects on Motor Symptoms? Toxins 2025, 17, 396. [Google Scholar] [CrossRef] [PubMed]
- Val, M.; Delcanho, R.; Ferrari, M.; Guarda Nardini, L.; Manfredini, D. Is Botulinum Toxin Effective in Treating Orofacial Neuropathic Pain Disorders? A Systematic Review. Toxins 2023, 15, 541. [Google Scholar] [CrossRef]
- da Silva Ramalho, J.A.; Palma, L.F.; Ramalho, K.M.; Tedesco, T.K.; Morimoto, S. Effect of botulinum toxin A on pain, bite force, and satisfaction of patients with bruxism: A randomized single-blind clinical trial comparing two protocols. Saudi Dent. J. 2023, 35, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Navarro, A.B.; Romero-de Ávila, M.; Tarraga-Marcos, L.; Madrona-Marcos, F.; Tarraga-López, P.J. Valoración del tratamiento del bruxismo mediante toxina botulínica. J. Negat. No Posit. Results 2022, 7, 4–17. [Google Scholar]
- Alcolea, J.M.; Mkhitaryan, L.; Erazo, P. Tratamiento del bruxismo con toxina botulínica tipo A. Estudio clínico prospectivo. Cir. Plast. Ibero-Latinoam. 2019, 45, 435–448. [Google Scholar] [CrossRef][Green Version]
- Rami, S. The uses of BTX-A in maxillofacial surgery. PMFA 2016, 3. [Google Scholar]
- Lang, A.M. A preliminary comparison of the efficacy and tolerability of botulinum toxin serotypes A and B in the treatment of myofascial pain syndrome: A retrospective, open-label chart review. Clin. Ther. 2003, 25, 2268–2278. [Google Scholar] [CrossRef]
- Hallett, M. Mechanism of action of botulinum neurotoxin: Unexpected consequences. Toxicon 2018, 147, 73–76. [Google Scholar] [CrossRef]
- Ernberg, M.; Hedenberg-Magnusson, B.; List, T.; Svensson, P. Efficacy of botulinum toxin type A for treatment of persistent myofascial TMD pain: A randomized, controlled, double-blind multicenter study. Pain 2011, 152, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, H.J. Botulinum Toxin for the Treatment of Neuropathic Pain. Toxins 2017, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, A.; Carruthers, J. History of Botulinum Toxin for Medical and Aesthetic Use. In W: Botulinum Toxins; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 1–12. [Google Scholar]
- Scott, A.B. Botulinum Toxin Injection into Extraocular Muscles as an Alternative to Strabismus Surgery. Ophthalmology 1980, 87, 1044–1049. [Google Scholar] [CrossRef]
- Clark, R.P. The First Cosmetic Use of Botulinum Toxin. Plast. Reconstr. Surg. 2019, 144, 723e. [Google Scholar] [CrossRef]
- Tan, E.K.; Jankovic, J.; Ondo, W. Bruxism in Huntington’s disease. Mov. Disord. 2000, 15, 171–173. [Google Scholar] [CrossRef]
- Sendra, L.A.; Montez, C.; Vianna, K.C.; Barboza, E.P. Clinical outcomes of botulinum toxin type A injections in the management of primary bruxism in adults: A systematic review. J. Prosthet. Dent. 2021, 126, 33–40. [Google Scholar] [CrossRef]
- Ågren, M.; Sahin, C.; Pettersson, M. The effect of botulinum toxin injections on bruxism: A systematic review. J. Oral Rehabil. 2020, 47, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Jewell, M. Book Review: Pictorial Atlas of Botulinum Toxin Injection: Dosage, Localization, Application. Aesthetic Surg. J. 2011, 31, 990. [Google Scholar] [CrossRef]
- Gałczyńska-Rusin, M.; Pobudek-Radzikowska, M.; Gawriołek, K.; Czajka-Jakubowska, A. Gender-Related Biomechanical Properties of Masseter Muscle among Patients with Self-Assessment of Bruxism: A Comparative Study. J. Clin. Med. 2022, 11, 845. [Google Scholar] [CrossRef]
- Mezey, S.E.; Müller-Gerbl, M.; Toranelli, M.; Türp, J.C. The human masseter muscle revisited: First description of its coronoid part. Ann. Anat.—Anat. Anz. 2022, 240, 151879. [Google Scholar] [CrossRef]
- Gałczyńska-Rusin, M.; Pobudek-Radzikowska, M.; Maciejewska-Szaniec, Z.; Przystańska, A.; Czajka-Jakubowska, A. Evaluating the reliability of myotonometry for assessing masseter muscle hypertrophy in healthy subjects. J. Oral Facial Pain Headache 2025, 39, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.S.; Almoyad, M.A.A.; Binduhayyim, R.I.H.; Quadri, S.A.; Gurumurthy, V.; Bavabeedu, S.S.; Kuruniyan, M.S.; Naseef, P.P.; Mosaddad, S.A.; Heboyan, A. The effectiveness of botulinum toxin for temporomandibular disorders: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0300157. [Google Scholar] [CrossRef]
- Walczyńska-Dragon, K.; Grzybowska-Ganszczyk, D.; Hadzik, P.; Fiegler-Rudol, J.; Dubiel-Holecko, I.K.; Nitecka-Buchta, A.; Baron, S. Bruxism as a Biopsychosocial Disorder: An Interdisciplinary Cross-Sectional Study. J. Clin. Med. 2025, 14, 6803. [Google Scholar] [CrossRef] [PubMed]
- De la Torre Canales, G.; Poluha, R.L.; Bonjardim, L.R.; Ernberg, M.; Conti, P.C.R. Botulinum toxin-A effects on pain, somatosensory and psychosocial features of patients with refractory masticatory myofascial pain: A randomized double-blind clinical trial. Sci. Rep. 2024, 14, 4201. [Google Scholar] [CrossRef] [PubMed]
- Montaser, M.M.S.; Elsokkary, N.H.; Shararah, A.E.A.I. Effect of botulinum toxin type A on masticatory function and musculoskeletal structure in rabbits. Sci. Rep. 2025, 15, 15323. [Google Scholar] [CrossRef]
- Ågren, M.; Nanchaipruek, Y.; Phumariyapong, P.; Apinuntham, C.; Rakchart, S.; Pettersson, M.; Wanitphakdeedecha, R. Duration of bite force reduction following a single injection of botulinum toxin in the masseter muscle bilaterally: A one-year non-randomized trial. J. Oral Rehabil. 2023, 50, 343–350. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Lin, H.-Y.; Chu, C.-A.; Wu, W.-T.; Chen, L.-R.; Özçakar, L.; Chang, K.-V. Assessing thickness and stiffness of superficial/deep masticatory muscles in orofacial pain: An ultrasound and shear wave elastography study. Ann. Med. 2023, 55, 2261116. [Google Scholar] [CrossRef]
- Yu, L.J.; Yan, X.; Kim, T.H. Effects of combined jaw and cervicoscapular exercises on mouth opening and muscle properties in cervical extension type. Sci. Rep. 2025, 15, 19049. [Google Scholar] [CrossRef]
- Wendt, M.; Waszak, M. Assessment of the stiffness of the upper trapezius muscle in a group of asymptomatic people with cervical spine rotation asymmetry. PLoS ONE 2024, 19, e0298544. [Google Scholar]
- Buvinic, S.; Balanta-Melo, J.; Kupczik, K.; Vásquez, W.; Beato, C.; Toro-Ibacache, V. Muscle-Bone Crosstalk in the Masticatory System: From Biomechanical to Molecular Interactions. Front. Endocrinol. 2021, 11, 606947. [Google Scholar] [CrossRef] [PubMed]
- Jost, W.H.; Bäumer, T.; Bevot, A.; Birkmann, U.; Buhmann, C.; Grosheva, M.; Guntinas-Lichius, O.; Laskawi, R.; Paus, S.; Pflug, C.; et al. Botulinum neurotoxin type A in the interdisciplinary treatment of sialorrhea in adults and children—Update and practice recommendations. Front. Neurol. 2023, 14, 1275807. [Google Scholar] [CrossRef]
- Bussadori, S.K.; Motta, L.J.; Horliana, A.C.R.T.; Santos, E.M.; Martimbianco, A.L.C. The Current Trend in Management of Bruxism and Chronic Pain: An Overview of Systematic Reviews. J. Pain Res. 2020, 13, 2413–2421. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, D.; Winocur, E.; Guarda-Nardini, L.; Paesani, D.; Lobbezoo, F. Epidemiology of bruxism in adults: A systematic review of the literature. J. Orofac. Pain 2013, 27, 99–110. [Google Scholar] [CrossRef]
- de Almeida Hoff, E.; Grossi, R.K.; Bozzetti Pigozzi, L.; Bueno, C.H.; Pattussi, M.P.; Rossi, T.; Irigaray, T.Q.; Weber, J.B.B.; Grossi, M.L. Depression and the risk of developing temporomandibular disorders in different diagnostic groups: A systematic review with meta-analysis. J. Craniomandib. Pract. 2025, 43, 730–742. [Google Scholar] [CrossRef]
- Stanisic, N.; Saracutu, O.I.; Colonna, A.; Wu, W.; Manfredini, D.; Häggman-henrikson, B. Awake bruxism prevalence across populations: A systematic review and meta-analysis. J. Évid-Based Dent. Pr. 2025, 25, 102171. [Google Scholar] [CrossRef]
- Jost, W.H.; Drużdż, A.; Pandey, S.; Biering-Sørensen, B.; Kreisler, A.; Tatu, L.; Altmann, C.F.; Sławek, J. Dose per muscle in cervical dystonia: Pooled data from seven movement disorder centres. Neurol. Neurochir. Pol. 2021, 55, 174–178. [Google Scholar] [CrossRef]
- Lavigne, G.J.; Rompré, P.H.; Guitard, F.; Sessle, B.J.; Kato, T.; Montplaisir, J.Y. Lower number of K-complexes and K-alphas in sleep bruxism: A controlled quantitative study. Clin. Neurophysiol. 2002, 113, 686–693. [Google Scholar] [CrossRef]
- Bracci, A.; Lobbezoo, F.; Colonna, A.; Bender, S.; Conti, P.C.R.; Emodi-Perlman, A.; Häggman-Henrikson, B.; Klasser, G.D.; Michelotti, A.; Lavigne, G.J.; et al. Research routes on awake bruxism metrics: Implications of the updated bruxism definition and evaluation strategies. J. Oral Rehabil. 2024, 51, 150–161. [Google Scholar]
- Thymi, M.; Lobbezoo, F.; Aarab, G.; Ahlberg, J.; Baba, K.; Carra, M.C.; Gallo, L.M.; De Laat, A.; Manfredini, D.; Lavigne, G.; et al. Signal acquisition and analysis of ambulatory electromyographic recordings for the assessment of sleep bruxism: A scoping review. J. Oral Rehabil. 2021, 48, 846–871. [Google Scholar] [CrossRef]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J. Oral Facial Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz, M.R.; Ohrbach, R.; McCall, W.D. Oral Behaviors Checklist: Reliability of Performance in Targeted Waking-State Behaviors. J. Orofac. Pain 2006, 20, 306–316. [Google Scholar]
- Lobbezoo, F.; Ahlberg, J.; Verhoeff, M.C.; Aarab, G.; Bracci, A.; Koutris, M.; Nykänen, L.; Thymi, M.; Wetselaar, P.; Manfredini, D. The bruxism screener (BruxScreen): Development, pilot testing and face validity. J. Oral Rehabil. 2024, 51, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Summary of Product Characteristics Botox 100 Allergan Units Powder for Solution for Injection. Available online: https://www.medicines.org.uk/emc/product/859/smpc (accessed on 21 August 2025).
- De la Torre Canales, G.; Câmara-Souza, M.B.; do Amaral, C.F.; Garcia, R.C.M.R.; Manfredini, D. Is there enough evidence to use botulinum toxin injections for bruxism management? A systematic literature review. Clin. Oral Investig. 2017, 21, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Buzatu, R.; Luca, M.M.; Castiglione, L.; Sinescu, C. Efficacy and Safety of Botulinum Toxin in the Management of Temporomandibular Symptoms Associated with Sleep Bruxism: A Systematic Review. Dent. J. 2024, 12, 156. [Google Scholar] [CrossRef]
- Nigam, P.K.; Nigam, A. Botulinum toxin. Indian J. Dermatol. 2010, 55, 8. [Google Scholar] [CrossRef]
- Siprut, J. An Evaluation of a MYOTON Device as a Means for Spaceflight Monitoring of Muscular Biomarkers. Master’s Thesis, University of California San Diego, San Diego, CA, USA, 2021. [Google Scholar]
- Ianieri, G.; Saggini, R.; Marvulli, R.; Tondi, G.; Aprile, A.; Ranieri, M.; Benedetto, G.; Altini, S.; Lancioni, G.; Goffredo, L.; et al. New Approach in the Assessment of the Tone, Elasticity and the Muscular Resistance: Nominal Scales Vs Myoton. Int. J. Immunopathol. Pharmacol. 2009, 22 (Suppl. S3), 21–24. [Google Scholar] [CrossRef]
- Yu, J.F.; Chang, T.T.; Zhang, Z.J. The Reliability of MyotonPRO in Assessing Masseter Muscle Stiffness and the Effect of Muscle Contraction. Med. Sci. Monit. 2020, 26, e926578. [Google Scholar] [CrossRef]
- Dietsch, A.M.; Clark, H.M.; Steiner, J.N.; Solomon, N.P. Effects of Age, Sex, and Body Position on Orofacial Muscle Tone in Healthy Adults. J. Speech Lang. Hear. Res. JSLHR 2015, 58, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Technology. Myoton. Available online: https://myoton.com/technology/ (accessed on 21 August 2025).
- Maden, T.; Usgu, G.; Tuncer, A. Myotonometric comparison of sternocleidomastoideus and masseter muscles in multiple sclerosis patients with swallowing problem and healthy individuals. Mult. Scler. Relat. Disord. 2022, 57, 103387. [Google Scholar] [CrossRef]
- Ramazanoglu, E.; Turhan, B.; Usgu, S. Evaluation of the tone and viscoelastic properties of the masseter muscle in the supine position, and its relation to age and gender. Dent. Med. Probl. 2021, 58, 155–161. [Google Scholar] [CrossRef]
- Montes-Carmona, J.F.; Gonzalez-Perez, L.M.; Infante-Cossio, P. Treatment of Localized and Referred Masticatory Myofascial Pain with Botulinum Toxin Injection. Toxins 2021, 13, 6. [Google Scholar] [CrossRef]
- Evidente, V.G.H.; Pappert, E.J. Botulinum Toxin Therapy for Cervical Dystonia: The Science of Dosing. Tremor Other Hyperkinetic Mov. 2014, 4, 273. [Google Scholar] [CrossRef]
- Li, K.; Tan, K.; Yacovelli, A.; Bi, W.G. Effect of botulinum toxin type A on muscular temporomandibular disorder: A systematic review and meta-analysis of randomized controlled trials. J. Oral Rehabil. 2024, 51, 886–897. [Google Scholar] [CrossRef] [PubMed]

| Site | Muscle State | Variable |
Interaction
Tertile × Time (p < 0.05) | Tertile I (n = 19) | Tertile II (n = 19) | Tertile III (n = 19) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V1 vs. V2 | V1 vs. V3 | V2 vs. V3 | V1 vs. V2 | V1 vs. V3 | V2 vs. V3 | V1 vs. V2 | V1 vs. V3 | V2 vs. V3 | ||||
| Left masseter muscle | Relaxed | Tone | F(4.108) = 3.80 p = 0.007 | # F(2,36) = 1.98 p = 0.153 | # F(2,36) = 1.40 p = 0.260 | # F(2,36) = 11.30 p < 0.001 | ||||||
| <0.001 * | 0.019 * | 0.161 * | ||||||||||
| 1 > (2 = 3) | ||||||||||||
| Stiffness | F(4.108) = 2.66 p = 0.036 | # F(2,36) = 8.37 p = 0.001 | # F(2,36) = 5.73 p = 0.0067 | # F(2,36) = 1.36 p = 0.270 | ||||||||
| 0.003 * | 0.003 * | 1.000 * | 0.608 * | 0.006 * | 0.063 * | |||||||
| 1 < (2 = 3) | 1 < 3 | |||||||||||
| Elasticity | F(4.108) = 1.35 p = 0.255 | # F(2,36) = 2.61 p = 0.087 | # F(2,36) = 0.81 p = 0.453 | # F(2,36) = 4.13 p = 0.024 | ||||||||
| 0.904 * | 0.077 * | 0.029 * | ||||||||||
| 2 > 3 | ||||||||||||
| Relaxation | F(4.108) = 1.43 p = 0.229 | # F(2,36) = 2.39 p = 0.106 | # F(2,36) = 3.65 p = 0.036 | # F(2,36) = 10.77 p < 0.001 | ||||||||
| 0.355 * | 0.027 * | 0.398 * | 0.048 * | <0.001 * | 0.092 * | |||||||
| 1 < 3 | 1 > (2 = 3) | |||||||||||
| Creep | F(4.108) = 1.52 p = 0.202 | # F(2,36) = 2.60 p = 0.216 | # F(2,36) = 7.07 p = 0.003 | # F(2,36) = 9.98 p < 0.001 | ||||||||
| 0.520 * | 0.002 * | 0.039 * | 0.796 * | 0.001 * | 0.003 * | |||||||
| (1 = 2) > 3 | (1 = 2) > 3 | |||||||||||
| Contracted | Tone | F(4.108) = 19.98 p < 0.001 | # F(2,36) = 24.07 p < 0.001 | # F(2,36) = 67.57 p < 0.001 | # F(2,36) = 259.57 p < 0.001 | |||||||
| <0.001 * | 0.226 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | ||||
| 2 < (1 = 3) | 2 < 3 < 1 | 2 < 3 < 1 | ||||||||||
| Stiffness | F(4.108) = 24.07 p < 0.001 | # F(2,36) = 19.97 p < 0.001 | # F(2,36) = 61.64 p < 0.001 | # F(2,36) = 235.62 p < 0.001 | ||||||||
| <0.001 * | 0.043 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | ||||
| 2 < 1 < 3 | 2 < 3 < 1 | 2 < 3 < 1 | ||||||||||
| Elasticity | F(4.108) = 6.64 p < 0.001 | # F(2,36) = 66.20 p < 0.001 | # F(2,36) = 64.76 p < 0.001 | # F(2,36) = 19.68 p < 0.001 | ||||||||
| <0.001 * | 0.001 * | <0.001 * | <0.001 * | 0.332 * | <0.001 * | 0.001 * | 0.073 * | <0.001 * | ||||
| 1 < 3 < 2 | (1 = 3) < 2 | (1 = 3) < 2 | ||||||||||
| Relaxation | F(4.108) = 142.27 p < 0.001 | # F(2,36) = 142.27 p < 0.001 | # F(2,36) = 41.05 p < 0.001 | # F(2,36) = 21.43 p < 0.001 | ||||||||
| <0.001 * | <0.001 * | <0.001 * | <0.001 * | 0.463 * | <0.001 * | <0.001 * | 0.512 * | <0.001 * | ||||
| 1 < 3 < 2 | (1 = 3) < 2 | (1 = 3) < 2 | ||||||||||
| Creep | F(4.108) = 4.02 p = 0.004 | # F(2,36) = 172.49 p < 0.001 | # F(2,36) = 37.12 p < 0.001 | # F(2,36) = 32.91 p < 0.001 | ||||||||
| <0.001 * | <0.001 * | <0.001 * | <0.001 * | 0.238 * | <0.001 * | <0.001 * | 0.494 * | <0.001 * | ||||
| 1 < 3 < 2 | (1 = 3) < 2 | (1 = 3) < 2 | ||||||||||
| Right masseter muscle | Relaxed | Tone | F(4.108) = 4.55 p = 0.002 | # F(2,36) = 1.19 p = 0.316 | # F(2,36) = 0.38 p = 0.685 | # F(2,36) = 14.40 p < 0.001 | ||||||
| <0.001 * | 0.001 * | 0.581 * | ||||||||||
| (2 = 3) < 1 | ||||||||||||
| Stiffness | F(4.108) = 0.84 p = 0.504 | # F(2,36) = 2.17 p = 0.129 | # F(2,36) = 1.58 p = 0.221 | # F(2,36) = 1.74 p = 0.190 | ||||||||
| Elasticity | F(4.108) = 2.05 p = 0.092 | # F(2,36) = 0.25 p = 0.778 | # F(2,36) = 3.05 p = 0.061 | # F(2,36) = 3.86 p = 0.030 | ||||||||
| 0.932 * | 0.038 * | 0.083 * | ||||||||||
| 3 < 1 | ||||||||||||
| Relaxation | F(4.108) = 1.95 p = 0.107 | # F(2,36) = 0.45 p = 0.642 | # F(2,36) = 3.58 p = 0.040 | # F(2,36) = 7.01 p = 0.003 | ||||||||
| 0.994 * | 0.076 * | 0.061 * | 0.559 * | 0.002 * | 0.035 * | |||||||
| - | (1 = 2) > 3 | |||||||||||
| Creep | F(4.108) = 1.16 p = 0.331 | # F(2,36) = 0.80 p = 0.458 | # F(2,36) = 2.60 p = 0.088 | # F(2,36) = 5.91 p = 0.006 | ||||||||
| 0.346 * | 0.004 * | 0.125 * | ||||||||||
| 1 > 3 | ||||||||||||
| Contracted | Tone | F(4.108) = 25.23 p < 0.001 | # F(2,36) = 49.82 p < 0.001 | # F(2,36) = 111.97 p < 0.001 | # F(2,36) = 150.94 p < 0.001 | |||||||
| <0.001 * | 0.935 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | ||||
| 2 < (1 = 3) | 2 < 3 < 1 | 2 < 3 < 1 | ||||||||||
| Stiffness | F(4.108) = 19.11 p < 0.001 | # F(2,36) = 29.17 p < 0.001 | # F(2,36) = 75.94 p < 0.001 | # F(2,36) = 120.47 p < 0.001 | ||||||||
| <0.001 * | 0.637 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | ||||
| 2 < (1 = 3) | 2 < 3 < 1 | 2 < 3 < 1 | ||||||||||
| Elasticity | F(4.108) = 8.37 p < 0.001 | # F(2,36) = 88.55 p < 0.001 | # F(2,36) = 17.55 p < 0.001 | # F(2,36) = 14.27 p < 0.001 | ||||||||
| <0.001 * | 0.001 * | <0.001 * | <0.001 * | 0.399 * | <0.001 * | 0.287 * | 0.002 * | <0.001 * | ||||
| 1 < 3 < 2 | (1 = 3) < 2 | 3 < (1 = 2) | ||||||||||
| Relaxation | F(4.108) = 9.06 p < 0.001 | # F(2,36) = 109.85 p < 0.001 | # F(2,36) = 94.39 p < 0.001 | # F(2,36) = 37.51 p < 0.001 | ||||||||
| <0.001 * | <0.001 * | <0.001 * | <0.001 * | 0.023 * | <0.001 * | <0.001 * | 0.176 * | <0.001 * | ||||
| 1 < 3 < 2 | 1 < 3 < 2 | (1 = 3) < 2 | ||||||||||
| Creep | F(4.108) = 9.68 p < 0.001 | # F(2,36) = 118.70 p < 0.001 | # F(2,36) = 70.22 p < 0.001 | # F(2,36) = 63.12 p < 0.001 | ||||||||
| <0.001 * | <0.001 * | <0.001 * | <0.001 * | 0.238 * | <0.001 * | <0.001 * | 0.036 * | <0.001 * | ||||
| 1 < 3 < 2 | 1 < 3 < 2 | 3 < 1 < 2 | ||||||||||
| Site | Muscle State | Variable | Tertile I (n = 19) | Tertile II (n = 19) | Tertile III (n = 19) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Value | Mean Value | Mean Value | |||||||||
| ±Standard Deviation | ±Standard Deviation | ±Standard Deviation | |||||||||
| V1 | V2 | V3 | V1 | V2 | V3 | V1 | V2 | V3 | |||
| left masseter muscle | relaxed | Tone | 13.4 | 13.6 | 13.9 | 15.0 | 15.2 | 15.7 | 17.0 | 15.8 | 16.3 |
| ±0.9 | ±1.4 | ±1.6 | ±0.3 | ±1.1 | ±2.2 | ±1.6 | ±1.2 | ±1.5 | |||
| Stiffness | 239 | 275 | 275 | 288 | 304 | 343 | 348 | 340 | 357 | ||
| ±17 | ±46 | ±47 | ±9 | ±42 | ±83 | ±42 | ±42 | ±45 | |||
| Elasticity | 1.64 | 1.72 | 1.69 | 1.82 | 1.87 | 1.80 | 2.10 | 2.12 | 2.00 | ||
| ±0.07 | ±0.13 | ±0.21 | ±0.05 | ±0.16 | ±0.26 | ±0.18 | ±0.23 | ±0.20 | |||
| Relaxation | 16.3 | 16.6 | 15.1 | 19.0 | 18.4 | 17.8 | 22.0 | 20.5 | 19.3 | ||
| ±1.9 | ±2.0 | ±3.1 | ±0.4 | ±1.7 | ±2.4 | ±2.6 | ±3.3 | ±2.9 | |||
| Creep | 1.02 | 1.15 | 1.01 | 1.17 | 1.12 | 1.01 | 1.37 | 1.34 | 1.18 | ||
| ±0.09 | ±0.50 | ±0.22 | ±0.03 | ±0.11 | ±0.19 | ±0.19 | ±0.19 | ±0.20 | |||
| contracted | Tone | 19.4 | 16.1 | 20.7 | 23.1 | 16.1 | 20.3 | 26.7 | 16.8 | 21.3 | |
| ±1.5 | ±1.8 | ±3.3 | ±0.9 | ±1.9 | ±2.8 | ±1.5 | ±1.3 | ±2.1 | |||
| Stiffness | 463 | 331 | 539 | 622 | 348 | 512 | 786 | 346 | 570 | ||
| ±61 | ±56 | ±157 | ±57 | ±76 | ±137 | ±50 | ±44 | ±104 | |||
| Elasticity | 0.83 | 1.50 | 1.08 | 1.07 | 1.58 | 1.14 | 1.37 | 1.68 | 1.19 | ||
| ±0.10 | ±0.16 | ±0.23 | ±0.07 | ±0.21 | ±0.20 | ±0.15 | ±0.30 | ±0.23 | |||
| Relaxation | 6.5 | 14.1 | 8.8 | 8.9 | 15.8 | 9.9 | 11.4 | 15.6 | 10.4 | ||
| ±0.6 | ±2.2 | ±1.4 | ±0.7 | ±3.7 | ±2.6 | ±1.5 | ±2.9 | ±3.1 | |||
| Creep | 0.44 | 0.90 | 0.58 | 0.57 | 0.91 | 0.64 | 0.70 | 0.98 | 0.65 | ||
| ±0.03 | ±0.11 | ±0.11 | ±0.03 | ±0.20 | ±0.13 | ±0.09 | ±0.13 | ±0.18 | |||
| right masseter muscle | relaxed | Tone | 13.2 | 13.3 | 13.7 | 14.9 | 14.7 | 14.7 | 16.6 | 15.2 | 15.5 |
| ±0.8 | ±1.1 | ±1.9 | ±0.3 | ±0.8 | ±1.6 | ±1.4 | ±1.2 | ±1.6 | |||
| Stiffness | 248 | 255 | 268 | 283 | 292 | 307 | 339 | 323 | 342 | ||
| ±18 | ±34 | ±47 | ±9 | ±37 | ±64 | ±38 | ±38 | ±61 | |||
| Elasticity | 1.69 | 1.70 | 1.73 | 1.86 | 1.87 | 1.76 | 2.10 | 2.08 | 1.94 | ||
| ±0.09 | ±0.18 | ±0.29 | ±0.03 | ±0.17 | ±0.20 | ±0.15 | ±0.23 | ±0.30 | |||
| Relaxation | 17.1 | 17.7 | 17.3 | 19.2 | 19.2 | 17.7 | 22.2 | 21.6 | 20.1 | ||
| ±1.5 | ±2.5 | ±3.0 | ±0.5 | ±1.5 | ±2.9 | ±2.0 | ±2.9 | ±3.0 | |||
| Creep | 1.06 | 1.10 | 1.05 | 1.17 | 1.18 | 1.09 | 1.36 | 1.31 | 1.25 | ||
| ±0.09 | ±0.16 | ±0.17 | ±0.02 | ±0.10 | ±0.18 | ±0.14 | ±0.17 | ±0.14 | |||
| contracted | Tone | 19.7 | 15.5 | 19.8 | 23.6 | 16.2 | 19.8 | 27.5 | 16.9 | 21.0 | |
| ±2.0 | ±1.6 | ±2.5 | ±0.7 | ±1.4 | ±2.2 | ±2.3 | ±1.6 | ±2.4 | |||
| Stiffness | 467 | 313 | 490 | 638 | 348 | 514 | 791 | 350 | 552 | ||
| ±93 | ±48 | ±117 | ±23 | ±59 | ±116 | ±78 | ±63 | ±119 | |||
| Elasticity | 0.82 | 1.44 | 1.06 | 1.08 | 1.58 | 1.20 | 1.71 | 1.89 | 1.27 | ||
| ±0.09 | ±0.20 | ±0.11 | ±0.12 | ±0.35 | ±0.27 | ±0.50 | ±0.28 | ±0.20 | |||
| Relaxation | 6.5 | 15.4 | 9.5 | 8.3 | 15.2 | 9.8 | 11.8 | 16.1 | 10.6 | ||
| ±0.8 | ±2.7 | ±2.2 | ±0.5 | ±2.0 | ±2.0 | ±1.9 | ±3.7 | ±2.1 | |||
| Creep | 0.44 | 0.93 | 0.61 | 0.54 | 0.91 | 0.63 | 0.73 | 1.02 | 0.64 | ||
| ±0.04 | ±0.14 | ±0.16 | ±0.03 | ±0.12 | ±0.13 | ±0.10 | ±0.17 | ±0.14 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matusz, K.; Drużdż, A.; Górna, N.; Glapiński, M.; Gałczyńska-Rusin, M.; Czajka-Jakubowska, A.; Michalak, M.; Przystańska, A. Functional Effects of BoNT-A Application in Masseter Muscle in Patients with Symptoms of Bruxism. Toxins 2025, 17, 540. https://doi.org/10.3390/toxins17110540
Matusz K, Drużdż A, Górna N, Glapiński M, Gałczyńska-Rusin M, Czajka-Jakubowska A, Michalak M, Przystańska A. Functional Effects of BoNT-A Application in Masseter Muscle in Patients with Symptoms of Bruxism. Toxins. 2025; 17(11):540. https://doi.org/10.3390/toxins17110540
Chicago/Turabian StyleMatusz, Krystian, Artur Drużdż, Natalie Górna, Mariusz Glapiński, Małgorzata Gałczyńska-Rusin, Agata Czajka-Jakubowska, Michał Michalak, and Agnieszka Przystańska. 2025. "Functional Effects of BoNT-A Application in Masseter Muscle in Patients with Symptoms of Bruxism" Toxins 17, no. 11: 540. https://doi.org/10.3390/toxins17110540
APA StyleMatusz, K., Drużdż, A., Górna, N., Glapiński, M., Gałczyńska-Rusin, M., Czajka-Jakubowska, A., Michalak, M., & Przystańska, A. (2025). Functional Effects of BoNT-A Application in Masseter Muscle in Patients with Symptoms of Bruxism. Toxins, 17(11), 540. https://doi.org/10.3390/toxins17110540





