Ganglioside Binding Assay: A Complementary Approach for Enhanced Tetanus Toxoid Quality Control
Abstract
1. Introduction
2. Results
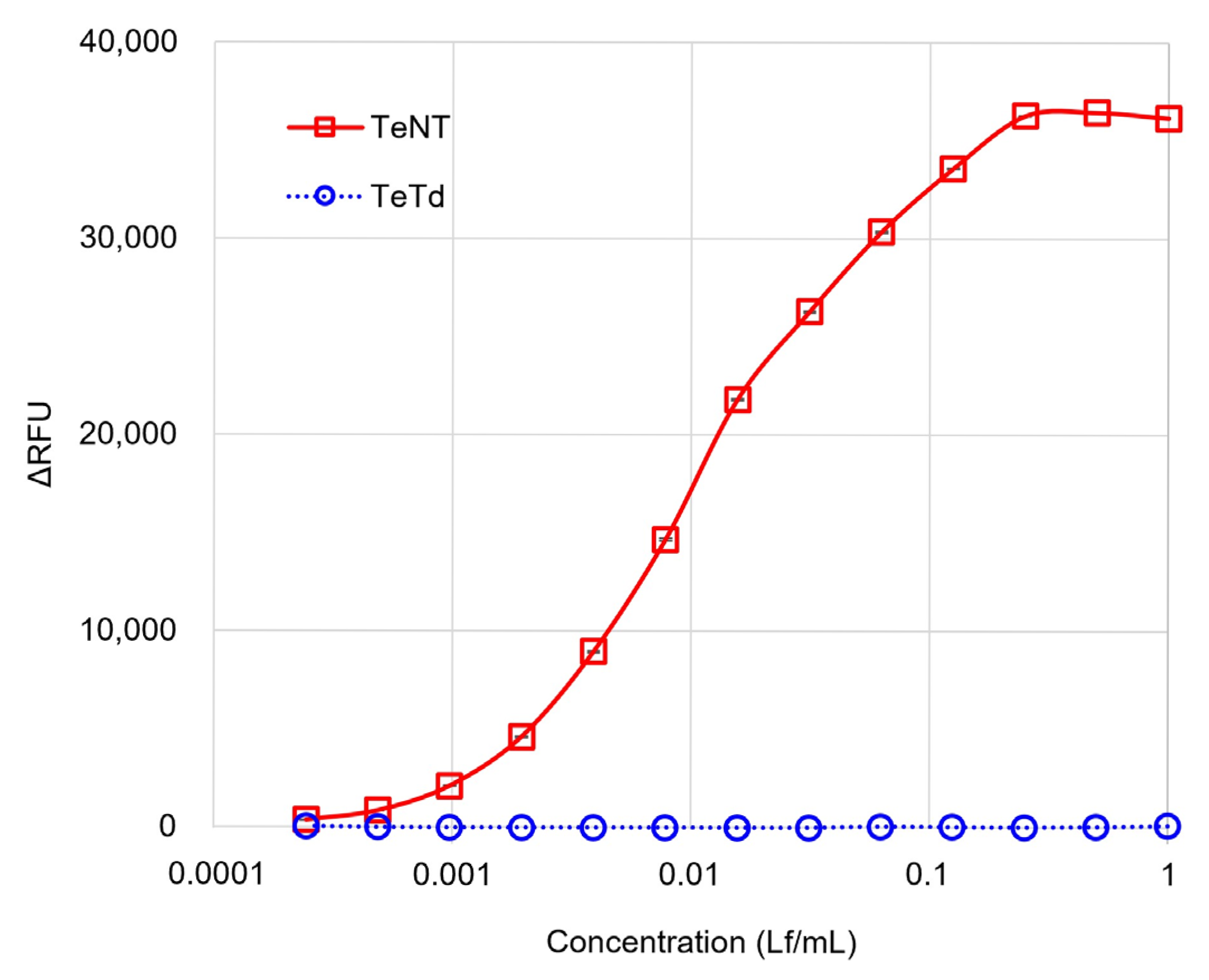
2.1. Dose–Response Curves of Toxins and Toxoids in the GB Assay
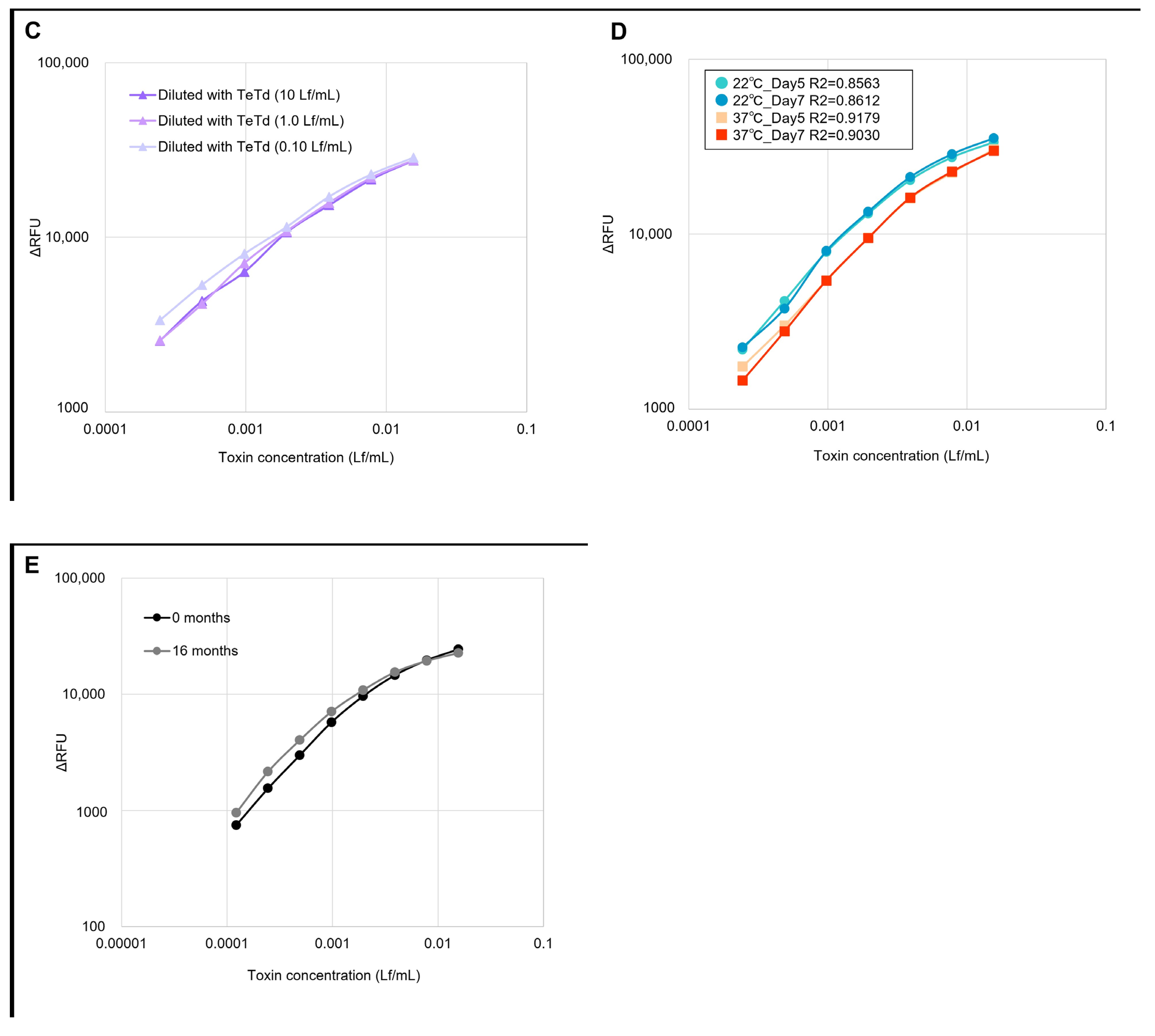
2.2. Linearity, Specificity, and Stability Tests of GB Assay
2.3. Detection of Toxicity Reversion in Time-Course Inactivation Samples Using GB Assay
2.4. Cross-Linking Status of Time-Course Inactivation Samples
2.5. Guinea Pig Tests Using Time-Course Inactivation Samples
3. Discussion
- -
- Phase 1 (Current study): Single-laboratory validation and correlation with existing methods;
- -
- Phase 2 (Proposed): Multi-center collaborative studies and regulatory consultation;
- -
- Phase 3 (Future): Regulatory acceptance and potential acceptance for routine implementation.
Study Limitations
- Single-laboratory validation environment: All validations were conducted within a controlled setting involving Kumamoto University of Health Sciences and KM Biologics Co., Ltd., representing Phase 1 validation that requires subsequent demonstration of transferability to independent laboratories.
- Cross-reactivity assessment pending: Evaluation against other clostridial neurotoxins (C. botulinum, C. novyi, and C. perfringens) is currently in progress, with toxin procurement and regulatory approvals underway.
- Inter-laboratory reproducibility had not yet been established: Although technology transfer to collaborating institutions has been initiated, formal multicenter validation studies are required to establish reproducibility parameters across different manufacturing processes and laboratory settings.
- Regulatory acceptance is contingent on future validation: Implementation as a routine testing method depends on the successful completion of multicenter validation and regulatory consultation phases.
4. Conclusions
5. Materials and Methods
5.1. Tetanus Toxin and Tetanus Toxoid
5.2. Measurement of Lf Value
5.3. Toxin Samples with Variable Inactivation Periods
5.4. Ganglioside Binding (GB) Assay
5.4.1. Ganglioside-Coated Plate
5.4.2. GB Assay
5.4.3. Method Validation
5.5. Animal Tests
5.6. SDS-PAGE Analysis
5.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, B.; More, V. Recent advances in drug discovery toxicology. Int. J. Toxicol. 2023, 42, 535–550. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- Balls, M. The three Rs: The way forward. ALTEX 2019, 36, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Stickings, P.; Rigsby, P.; Coombes, L.; Hockley, J.; Tierney, R.; Sesardic, D. Animal refinement and reduction: Alternative approaches for potency testing of diphtheria and tetanus vaccines. Procedia Vaccinol. 2011, 5, 200–212. [Google Scholar] [CrossRef][Green Version]
- Hendriksen, C.F.M. Replacement, reduction and refinement alternatives to animal use in vaccine potency measurement. Exp. Rev. Vaccines 2009, 8, 313–322. [Google Scholar] [CrossRef] [PubMed]
- NIID. Minimum Requirements for Biological Products; National Institute of Infectious Diseases: Tokyo, Japan, 2006. [Google Scholar]
- World Health Organization (WHO). Manual for Quality Control of Diphtheria, Tetanus and Pertussis Vaccines; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Behrensdorf-Nicol, H.A.; Bonifas, U.; Hanschmann, K.M.; Kramer, B.; Weisser, K. Binding and cleavage (BINACLE) assay for the functional in vitro detection of tetanus toxin: Applicability as alternative method for the safety testing of tetanus toxoids during vaccine production. Vaccine 2013, 31, 6247–6253. [Google Scholar] [CrossRef]
- Behrensdorf-Nicol, H.A.; Weisser, K.; Kramer, B. “BINACLE” assay for in vitro detection of active tetanus neurotoxin in toxoids. ALTEX 2015, 32, 137–142. [Google Scholar] [CrossRef][Green Version]
- Behrensdorf-Nicol, H.; Kramer, B.; Le Tallec, D.; Sinitskaya, N.; Behr-Gross, M.E. Collaborative study for the characterisation of the BINACLE assay for in vitro detection of tetanus toxicity in toxoids—Part 1. Pharmeur. Bio Sci. Notes 2024, 2024, 127–161. [Google Scholar][Green Version]
- Behrensdorf-Nicol, H.; Le Tallec, D.; Sinitskaya, N.; Behr-Gross, M.E.; Gongrich, C. Collaborative study for the characterisation of the BINACLE assay for in vitro detection of tetanus toxicity in toxoids—Part 2. Pharmeur. Bio Sci. Notes 2024, 2024, 162–192. [Google Scholar][Green Version]
- Sipione, S.; Monyror, J.; Galleguillos, D.; Steinberg, N.; Kadam, V. Gangliosides in the Brain: Physiology, Pathophysiology and Therapeutic Applications. Front. Neurosci. 2020, 14, 572965. [Google Scholar] [CrossRef]
- Chen, C.; Fu, Z.; Kim, J.J.; Barbieri, J.T.; Baldwin, M.R. Gangliosides as high affinity receptors for tetanus neurotoxin. J. Biol. Chem. 2009, 284, 26569–26577. [Google Scholar] [CrossRef]
- Kitamura, M.; Takamiya, K.; Aizawa, S.; Furukawa, K. Gangliosides are the binding substances in neural cells for tetanus and botulinum toxins in mice. Biochim. Biophys. Acta 1999, 1441, 1–3. [Google Scholar] [CrossRef]
- Kozaki, S.; Kamata, Y.; Watarai, S.; Nishiki, T.; Mochida, S. Ganglioside GT1b as a complementary receptor component for Clostridium botulinum neurotoxins. Microb. Pathog. 1998, 25, 91–99. [Google Scholar] [CrossRef]
- Schiavo, G.; Benfenati, F.; Poulain, B.; Rossetto, O.; Polverino de Laureto, P.; DasGupta, B.R.; Montecucco, C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature 1992, 359, 832–835. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, C.; van der Gun, J.W.; Kreeftenberg, J.G. Development of an ELISA for measuring the activity of tetanus toxoid in vaccines and comparison with the toxin neutralization test in mice. J. Immunol. Methods 1994, 168, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, C.; Winsnes, R. Serological methods for potency testing of tetanus toxoid vaccines for human use. Dev. Biol. 2002, 111, 131–140. [Google Scholar]
- Rummel, A.; Hafner, K.; Mahrhold, S.; Darashchonak, N.; Holt, M.; Jahn, R.; Beermann, S.; Karnath, T.; Bigalke, H.; Binz, T. Botulinum neurotoxins C, E and F bind gangliosides via a conserved binding site prior to stimulation-dependent uptake with botulinum neurotoxin F utilising the three isoforms of SV2 as second receptor. J. Neurochem. 2009, 110, 1942–1954. [Google Scholar] [CrossRef]
- Yowler, B.C.; Schengrund, C.L. Botulinum neurotoxin A changes conformation upon binding to ganglioside GT1b. Biochemistry 2004, 43, 9725–9731. [Google Scholar] [CrossRef]
- Ekong, T.A.; Feavers, I.M.; Sesardic, D. Recombinant SNAP-25 is an effective substrate for Clostridium botulinum type A toxin endopeptidase activity in vitro. Microbiology 1997, 143, 3337–3347. [Google Scholar] [CrossRef]
- Coombes, L.; Tierney, R.; Rigsby, P.; Sesardic, D.; Stickings, P. In vitro antigen ELISA for quality control of tetanus vaccines. Biologicals 2012, 40, 466–472. [Google Scholar] [CrossRef]
- Behrensdorf-Nicol, H.A.; Kegel, B.; Bonifas, U.; Silberbach, K.; Klimek, J.; Weiber, K.; Krämer, B.; Weisser, K. Residual enzymatic activity of the tetanus toxin light chain present in tetanus toxoid batches used for vaccine production. Vaccine 2008, 26, 3835–3841. [Google Scholar] [CrossRef]
- Hendriksen, C.F.M. Refinement, reduction, and replacement of animal use for regulatory testing. ILAR J. 2009, 50, 31–42. [Google Scholar]
- Sesardic, D.; Gaines Das, R. Alternatives to animals for testing the safety and potency of biological products. Altern. Lab. Anim. 2000, 28, 347–363. [Google Scholar] [CrossRef]
- Metz, B.; Kersten, G.F.; Hoogerhout, P.; Brugghe, H.F.; Timmermans, H.A.M.; de Jong, A.; Meiring, H.; Hove, J.T.; Hennink, W.E.; Crommelin, D.J.A.; et al. Identification of formaldehyde-induced modifications in proteins. J. Biol. Chem. 2004, 279, 6235–6243. [Google Scholar] [CrossRef] [PubMed]
- Fraenkel-Conrat, H.; Olcott, H.S. The reaction of formaldehyde with proteins. J. Am. Chem. Soc. 1948, 70, 2673–2674. [Google Scholar] [CrossRef] [PubMed]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins. BioTechniques 2004, 37, 790–802. [Google Scholar] [CrossRef]
- Behrensdorf-Nicol, H.A.; Krämer, B. Is the test for irreversibility of tetanus toxoids still relevant? Vaccine 2019, 37, 1721–1724. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Cui, J.; Liu, D.; Zhang, W.; Xue, C.; Li, C.; Wang, J.; Zhou, Y. Preparation and characterization of a neutralizing murine monoclonal antibody against tetanus toxin. J. Immunol. Methods 2023, 513, 113427. [Google Scholar] [CrossRef]
- Lei, D.; Schmidt, H.; Knezevic, I.; Zhou, T.; Kang, H.; Kopp, S. Removal of the innocuity test from The International Pharmacopoeia and WHO recommendations for vaccines and biological products. Biologicals 2020, 66, 17–20. [Google Scholar] [CrossRef]
- De Mattia, F.; Chapsal, J.M.; Descamps, J.; Halder, M.; Jarrett, N.; Kross, I.; Mortiaux, F.; Ponsar, C.; Redhead, K.; McKelvie, J.; et al. The consistency approach for quality control of vaccines—A strategy to improve quality control and implement 3Rs. Biologicals 2011, 39, 59–65. [Google Scholar] [CrossRef]
- Minor, P.D. Vaccine quality control and the evolution of viral vaccines. Vaccine 2015, 33, 5532–5539. [Google Scholar] [CrossRef]
- ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures: Text and Methodology Q2(R1). International Conference on Harmonisation: Geneva, Switzerland, 2005; pp. 1–13. [Google Scholar]
- Hassall, L.; Yara, D.A.; Riches-Duit, R.; Rigsby, P.; Dobly, A.; Vermeulen, M.; Francotte, A.; Faber, B.; Stickings, P. Development of a monoclonal antibody sandwich ELISA for the quality control of human and animal tetanus vaccines. ALTEX 2024, 41, 588–604. [Google Scholar]
- Karkhaneh, F.; Sadr, Z.K.; Rad, A.M.; Divsalar, A. Detection of tetanus toxoid with iron magnetic nanobioprobe. Biomed. Phys. Eng. Express 2024, 10, 055026. [Google Scholar] [CrossRef]
- Hubbard, K.; Beske, P.; Lyman, M.; McNutt, P. Functional evaluation of biological neurotoxins in networked cultures of stem cell-derived central nervous system neurons. J. Vis. Exp. 2015, 96, e52361. [Google Scholar] [CrossRef]
- Zhuang, Q.Q.; Chen, R.T.; Zheng, Y.J.; Huang, K.Y.; Peng, H.P.; Lin, Z.; Chen, W.; Chen, G. Detection of tetanus toxoid with fluorescent tetanus human IgG-AuNC-based immunochromatography test strip. Biosens. Bioelectron. 2021, 177, 112977. [Google Scholar] [CrossRef]
- Lilley, E.; Coppens, E.; Das, P.; Galaway, F.; Isbrucker, R.; Sheridan, S.; Stickings, P.; Holmes, A. Integrating 3Rs approaches in WHO guidelines for the batch release testing of biologicals: Responses from a survey of vaccines and biological therapeutics manufacturers. Biologicals 2023, 81, 101660. [Google Scholar] [CrossRef]



| Heat Treatment of Samples | Preparation Volume | Injection Dose | Observations | Samples: Days After Inactivation Initiation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (Lf/mL) | (mL) | (Lf/Head) | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 8 | |||
| - | 100 | 5 | 500 | Time to onset | Paralysis | ND | 6 | 6 | ND | ND | ND |
| Death | 3 | 6 | 12 | ND | ND | ND | |||||
| Survival/Number of Guinea Pig | 0/4 | 0/4 | 0/4 | 4/4 | 4/4 | 4/4 | |||||
| 37 °C 20 days | 8 | 5 | 40 | Time to onset | Paralysis | 1 | 2 | 6 | ND | ND | ND |
| Death | 2 | 2 | 8 | ND | ND | ND | |||||
| Survival/Number of Guinea Pig | 0/4 | 0/4 | 0/4 | 4/4 | 4/4 | 4/4 | |||||
| Samples | In Vitro Reversion Test | Injection Dose (Lf/Head) | Animal No. | Days Post Challenge | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | ||||
| Day 1 | - | 500 | 1 | - | - | d | ||||||||||
| 2 | - | - | d | |||||||||||||
| 3 | - | - | d | |||||||||||||
| 4 | - | - | d | |||||||||||||
| + | 40 | 5 | +++ | d | ||||||||||||
| 6 | +++ | d | ||||||||||||||
| 7 | +++ | d | ||||||||||||||
| 8 | +++ | d | ||||||||||||||
| Day 2 | - | 500 | 9 | - | - | - | - | - | d | |||||||
| 10 | - | - | - | - | - | d | ||||||||||
| 11 | - | - | - | - | - | ++ | d | |||||||||
| 12 | - | - | - | - | - | + | d | |||||||||
| + | 40 | 13 | - | + | d | |||||||||||
| 14 | - | - | d | |||||||||||||
| 15 | - | + | +++ | d | ||||||||||||
| 16 | - | - | d | |||||||||||||
| Day 3 | - | 500 | 17 | - | - | - | - | - | + | + | + | + | + | +++ | d | |
| 18 | - | - | - | - | - | + | - | + | + | + | +++ | d | ||||
| 19 | - | - | - | - | - | - | - | + | + | + | +++ | +++ | d | |||
| 20 | - | - | - | - | - | - | - | + | + | + | +++ | d | ||||
| + | 40 | 21 | - | - | - | - | - | - | + | ++++ | d | |||||
| 22 | - | - | - | - | - | - | + | ++++ | d | |||||||
| 23 | - | - | - | - | - | + | + | d | ||||||||
| 24 | - | - | - | - | - | + | + | ++++ | d | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanoue, Y.; Shitada, C.; Nakamichi, M.; Nakamichi, N.; Sakamoto, C.; Kim, H.; Kumeda, K.; Ochiai, M.; Yamaori, S.; Senoh, M.; et al. Ganglioside Binding Assay: A Complementary Approach for Enhanced Tetanus Toxoid Quality Control. Toxins 2025, 17, 500. https://doi.org/10.3390/toxins17100500
Tanoue Y, Shitada C, Nakamichi M, Nakamichi N, Sakamoto C, Kim H, Kumeda K, Ochiai M, Yamaori S, Senoh M, et al. Ganglioside Binding Assay: A Complementary Approach for Enhanced Tetanus Toxoid Quality Control. Toxins. 2025; 17(10):500. https://doi.org/10.3390/toxins17100500
Chicago/Turabian StyleTanoue, Yuki, Chie Shitada, Mariko Nakamichi, Naomi Nakamichi, Chiyomi Sakamoto, Hyun Kim, Kohsuke Kumeda, Masaki Ochiai, Susumu Yamaori, Mitsutoshi Senoh, and et al. 2025. "Ganglioside Binding Assay: A Complementary Approach for Enhanced Tetanus Toxoid Quality Control" Toxins 17, no. 10: 500. https://doi.org/10.3390/toxins17100500
APA StyleTanoue, Y., Shitada, C., Nakamichi, M., Nakamichi, N., Sakamoto, C., Kim, H., Kumeda, K., Ochiai, M., Yamaori, S., Senoh, M., & Takahashi, M. (2025). Ganglioside Binding Assay: A Complementary Approach for Enhanced Tetanus Toxoid Quality Control. Toxins, 17(10), 500. https://doi.org/10.3390/toxins17100500





