Comparison of Different Treatment Outcomes for Refractory Overactive Bladder: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
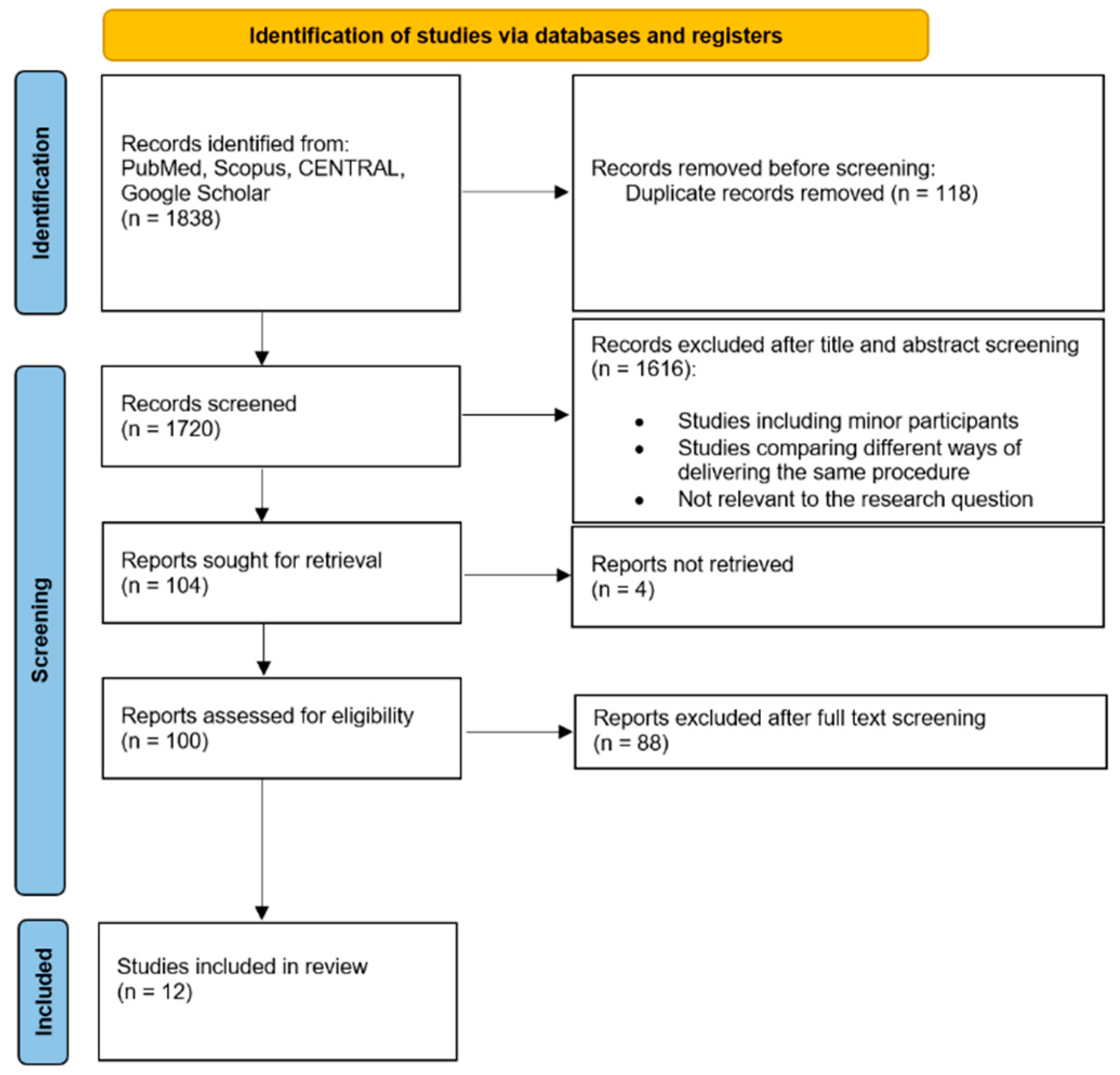
2. Results
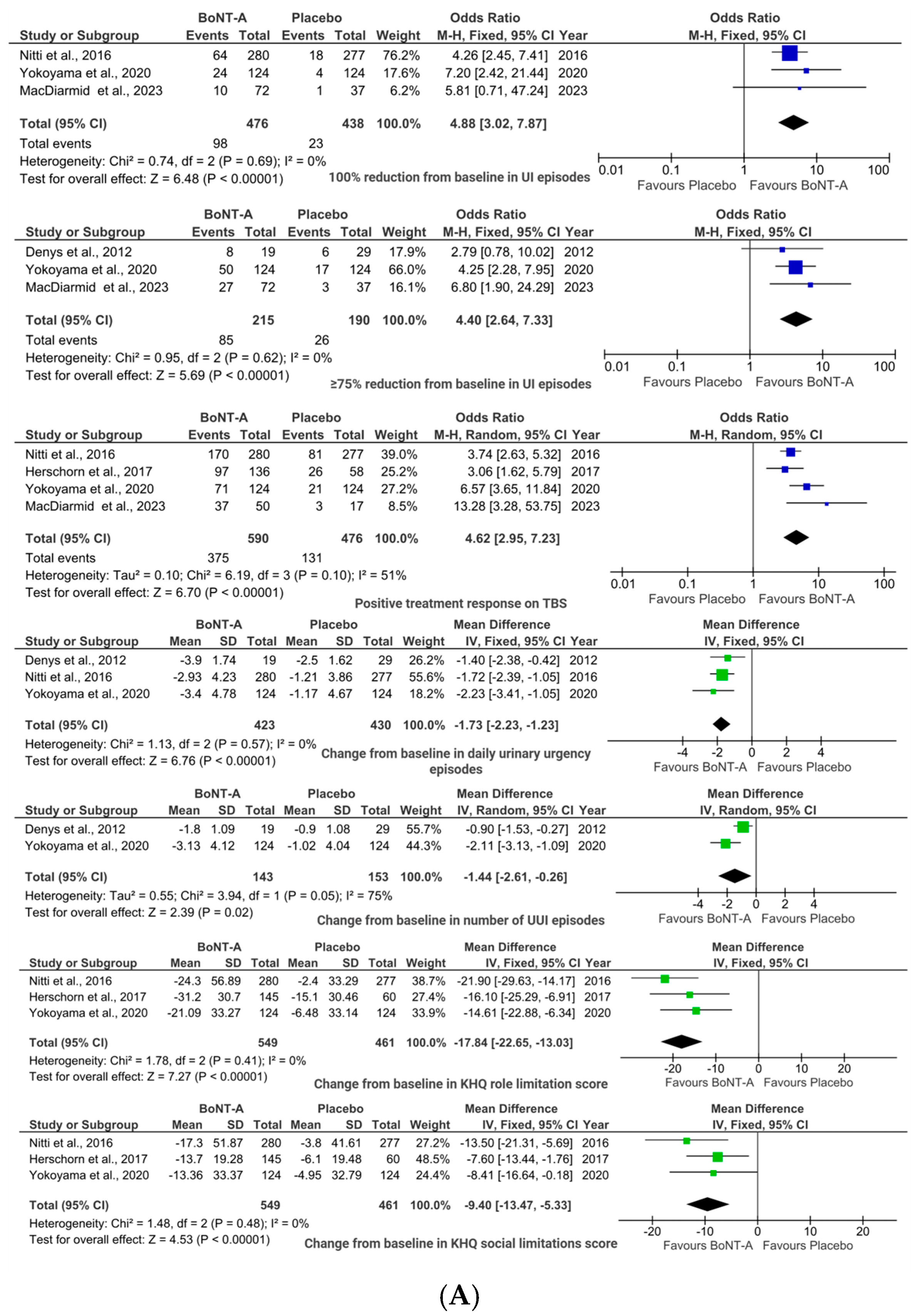
2.1. BoNT-A vs. Placebo
2.1.1. Efficacy Outcomes
2.1.2. Safety Outcomes
2.1.3. Sensitivity Analyses
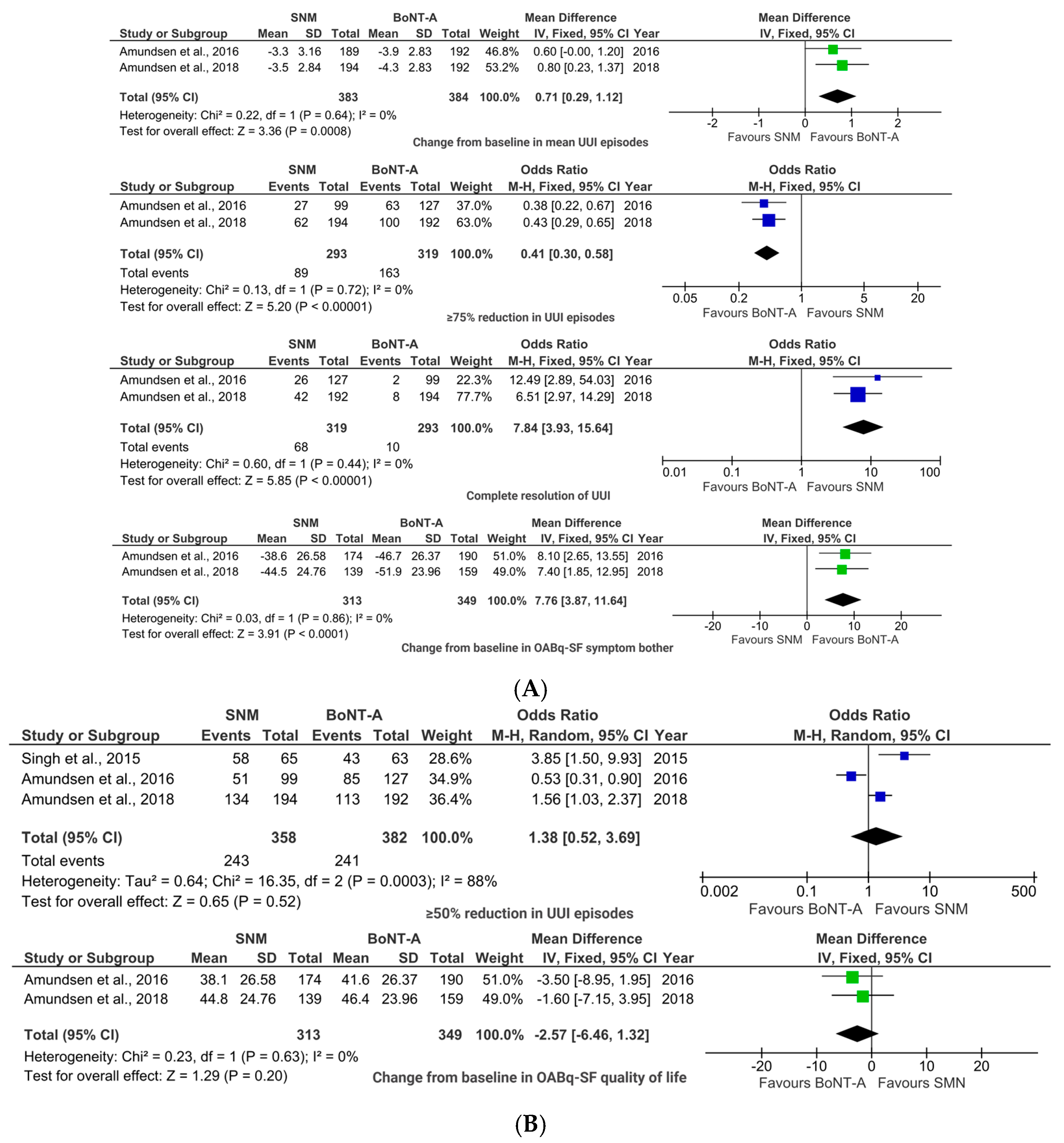
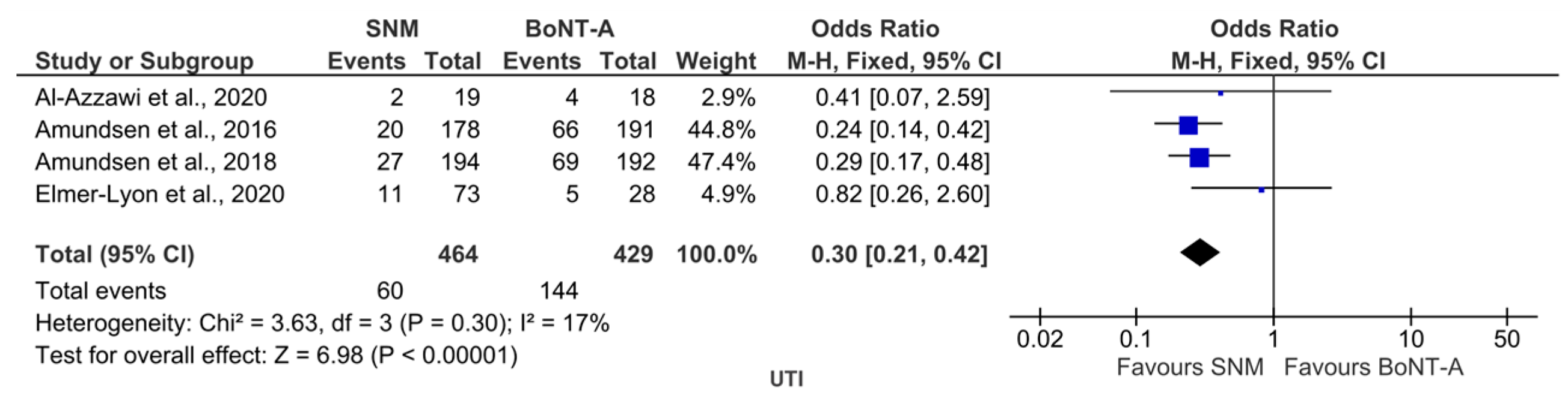
2.2. BoNT-A vs. SNM
2.2.1. Efficacy Outcomes
2.2.2. Safety Outcome
2.2.3. Sensitivity Analyses
2.3. Risk of Bias Assessment
2.3.1. The Critical Appraisal Skills Programme (CASP) Assessment of RCTs [34]
2.3.2. CASP Cohort Assessment [35]
3. Discussion
3.1. Main Findings
3.1.1. BoNT-A vs. Placebo
3.1.2. BoNT-A vs. SNM
3.1.3. Sensitivity Analyses
3.2. Strengths and Limitations
3.3. Implications for Practice
4. Conclusions
5. Materials and Methods
5.1. Registration and Data Sources
5.2. Inclusion and Exclusion Criteria
5.3. Comparators
5.4. Data Extraction
5.5. Study Quality and Risk of Bias Assessment
5.6. Data Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OAB | Overactive bladder |
| UUI | Urgency urinary incontinence |
| UI | Urinary incontinence |
| UTI | Urinary tract infections |
| QoL | Quality of life |
| SNM | Sacral neuromodulation |
| BoNT-A | OnabotulinumtoxinA |
| PROs | Patient-reported outcomes |
| PROMs | Patient-reported outcome measures |
| RCT(s) | Randomized controlled trial(s) |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| CENTRAL | Cochrane Central Register of Controlled Trials |
| BSUG | British Society of Urogynaecology |
| NICE | National Institute for Health and Care Excellence |
| AUA | American Urological Association |
| CASP | Critical Appraisal Skills Programme |
| TBS | Treatment Benefit Scale |
| KHQ | King’s Health Questionnaire |
References
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; Van Kerrebroeck, P.; Victor, A.; Wein, A. The standardisation of terminology in lower urinary tract function: Report from the standardisation sub-committee of the International Continence Society. Urology 2003, 61, 37–49. [Google Scholar] [CrossRef]
- Haylen, B.T.; De Ridder, D.; Freeman, R.M.; Swift, S.E.; Berghmans, B.; Lee, J.; Monga, A.; Petri, E.; Rizk, D.E.; Sand, P.K.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol. Urodyn. 2010, 29, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, K.J.; Chen, Z.; Moore, K.H.; Grundy, L. Urinary Tract Infection in Overactive Bladder: An Update on Pathophysiological Mechanisms. Front. Physiol. 2022, 13, 886782. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Kim, D.Y.; Cho, K.J.; Hashimoto, M.; Matsuoka, K.; Kamijo, T.; Wang, Z.; Karnup, S.; Robertson, A.M.; Tyagi, P.; et al. Pathophysiology of Overactive Bladder and Pharmacologic Treatments Including β3-Adrenoceptor Agonists -Basic Research Perspectives. Int. Neurourol. J. 2024, 28 (Suppl. 1), 12–33. [Google Scholar] [CrossRef] [PubMed]
- Chapple, C. Chapter 2: Pathophysiology of neurogenic detrusor overactivity and the symptom complex of “overactive bladder”. Neurourol. Urodyn. 2014, 33 (Suppl. S3), S6–S13. [Google Scholar] [CrossRef]
- Abdel-Fattah, M.; Chapple, C.; Cooper, D.; Breeman, S.; Bell-Gorrod, H.; Kuppanda, P.; Guerrero, K.; Dixon, S.; Cotterill, N.; Ward, K.; et al. Invasive urodynamic investigations in the management of women with refractory overactive bladder symptoms (FUTURE) in the UK: A multicentre, superiority, parallel, open-label, randomised controlled trial. Lancet 2025, 405, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.C.; Liu, S.P.; Lee, K.S.; Liao, L.; Wang, J.; Yoo, T.K.; Chu, R.; Sumarsono, B. Prevalence of overactive bladder in C hina, T aiwan and S outh K orea: R esults from a cross-sectional, population-based study. LUTS Low. Urin. Tract Symptoms 2019, 11, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.F.; Huang, C.Y.; Wang, S.Y.; Chang, S.R. Prevalence of and Associated Factors for Overactive Bladder Subtypes in Middle-Aged Women: A Cross-Sectional Study. Medicina 2022, 58, 383. [Google Scholar] [CrossRef] [PubMed]
- Funada, S.; Watanabe, N.; Goto, T.; Negoro, H.; Akamatsu, S.; Ueno, K.; Uozumi, R.; Ichioka, K.; Segawa, T.; Akechi, T.; et al. Cognitive behavioral therapy for overactive bladder in women: Study protocol for a randomized controlled trial. BMC Urol. 2020, 20, 129. [Google Scholar] [CrossRef]
- Al-Dossari, R.; Kalra, M.; Adkison, J.; Nguyen, B.-M. Non-surgical management of urinary incontinence. J. Am. Board Fam. Med. 2024, 37, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Dmochowski, R.R.; Newman, D.K.; Rovner, E.S.; Zillioux, J.; Malik, R.D.; Ackerman, A.L. Patient and Clinician Challenges with Anticholinergic Step Therapy in the Treatment of Overactive Bladder: A Narrative Review. Adv. Ther. 2023, 40, 4741–4757. [Google Scholar] [CrossRef] [PubMed]
- Al-Azzawi, I.S.; Al-Hindawi, H.T. A comparative study between sacral neuromodulation and intravesical botulinum toxin injection for patients with refractory overactive bladder. Arab. J. Urol. 2020, 18, 88–93. [Google Scholar] [CrossRef]
- He, Q.; Li, B.; Zhang, C.; Zhang, J.; Luo, D.; Wang, K. Treatment for refractory overactive bladder: A systematic review and meta-analysis of sacral neuromodulation and onabotulinumtoxinA. Int. Urogynecol. J. 2020, 32, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, Y.; Shi, B.; Zhang, Q.; Guo, H. Comparison of different types of therapy for overactive bladder: A systematic review and network meta-analysis. Front. Med. 2022, 9, 1014291. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.-W.; Wu, M.-Y.; Yang, S.S.-D.; Jaw, F.-S.; Chang, S.-J. Comparing the efficacy of onabotulinumtoxinA, sacral neuromodulation, and peripheral tibial nerve stimulation as third line treatment for the management of overactive bladder symptoms in adults: Systematic review and network meta-analysis. Toxins 2020, 12, 128. [Google Scholar] [CrossRef]
- Yang, G.; Xu, Y.; Qu, G.; Zhang, Y. Refractory overactive bladder patients who chose sacral neuromodulation therapy after failed OnabotulinumtoxinA treatment: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0230355. [Google Scholar] [CrossRef] [PubMed]
- Barreto, J.A.C.M.B.; Simões, M.I.T.; Engenheiro, G.G.; Matos, J.I.F.; Leal, J.A.R. The role of botulinum toxin in the management of nonneurogenic overactive bladder in children: Highlights for clinical practice. A systematic review. Curr. Urol. 2024, 18, 1–6. [Google Scholar] [CrossRef]
- Lachkar, S.; Ibrahimi, A.; Boualaoui, I.; El Sayegh, H.; Nouini, Y. Botulinum toxin A in idiopathic overactive bladder: A narrative review of 5410 cases. Can. J. Urol. 2025, 32, 145–165. [Google Scholar] [CrossRef] [PubMed]
- Abrar, M.; Pindoria, N.; Malde, S.; Chancellor, M.; DeRidder, D.; Sahai, A. Predictors of poor response and adverse events following botulinum toxin A for refractory idiopathic overactive bladder: A systematic review. Eur. Urol. Focus 2021, 7, 1448–1467. [Google Scholar] [CrossRef]
- Liang, P.; Yu, L.; Xia, B.; Zhang, D. Comparative efficacy and safety of mirabegron and vibegron in female patients with overactive bladder: A systematic review and meta-analysis of randomized controlled trials. Urology 2025, 199, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Amundsen, C.L.; Richter, H.E.; Menefee, S.A.; Komesu, Y.M.; Arya, L.A.; Gregory, W.T.; Myers, D.L.; Zyczynski, H.M.; Vasavada, S.; Nolen, T.L.; et al. OnabotulinumtoxinA vs Sacral Neuromodulation on Refractory Urgency Urinary Incontinence in Women: A Randomized Clinical Trial. JAMA 2016, 316, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Amundsen, C.L.; Komesu, Y.M.; Chermansky, C.; Gregory, W.T.; Myers, D.L.; Honeycutt, E.F.; Vasavada, S.P.; Nguyen, J.N.; Wilson, T.S.; Harvie, H.S.; et al. Two-Year Outcomes of Sacral Neuromodulation Versus OnabotulinumtoxinA for Refractory Urgency Urinary Incontinence: A Randomized Trial. Eur. Urol. 2018, 74, 66–73. [Google Scholar] [CrossRef]
- Chermansky, C.J.; Richter, H.E.; Jacoby, K.; Titanji, W.; Jenkins, B.; Geib, T.; Brucker, B.M. Intravesical Instillation of OnabotulinumtoxinA in the Treatment of Refractory Overactive Bladder in Participants with Urinary Incontinence. J. Urol. 2022, 208, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Denys, P.; Le Normand, L.; Ghout, I.; Costa, P.; Chartier-Kastler, E.; Grise, P.; Hermieu, J.F.; Amarenco, G.; Karsenty, G.; Saussine, C.; et al. Efficacy and safety of low doses of onabotulinumtoxinA for the treatment of refractory idiopathic overactive bladder: A multicentre, double-blind, randomised, placebo-controlled dose-ranging study. Eur. Urol. 2012, 61, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Dmochowski, R.; Chapple, C.; Nitti, V.W.; Chancellor, M.; Everaert, K.; Thompson, C.; Daniell, G.; Zhou, J.; Haag-Molkenteller, C. Efficacy and safety of onabotulinumtoxinA for idiopathic overactive bladder: A double-blind, placebo controlled, randomized, dose ranging trial. J. Urol. 2010, 184, 2416–2422. [Google Scholar] [CrossRef]
- Elmer-Lyon, C.G.; Streit, J.A.; Takacs, E.B.; Ten Eyck, P.P.; Bradley, C.S. Urinary tract infection and drug-resistant urinary tract infection after intradetrusor onabotulinumtoxinA injection versus sacral neuromodulation. Int. Urogynecol. J. 2020, 31, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; El Nashar, S.A.; Trabuco, E.C.; Klingele, C.J.; Gebhart, J.B.; Occhino, J.A. Comparison of Short Term Outcomes of Sacral Nerve Stimulation and Intradetrusor Injection of OnabotulinumtoxinA (Botox) in Women with Refractory Overactive Bladder. Female Pelvic Med. Reconstr. Surg. 2015, 21, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, O.; Honda, M.; Yamanishi, T.; Sekiguchi, Y.; Fujii, K.; Nakayama, T.; Mogi, T. OnabotulinumtoxinA (botulinum toxin type A) for the treatment of Japanese patients with overactive bladder and urinary incontinence: Results of single-dose treatment from a phase III, randomized, double-blind, placebo-controlled trial (interim analysis). Int. J. Urol. 2020, 27, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Herschorn, S.; Kohan, A.; Aliotta, P.; McCammon, K.; Sriram, R.; Abrams, S.; Lam, W.; Everaert, K. The Efficacy and Safety of OnabotulinumtoxinA or Solifenacin Compared with Placebo in Solifenacin Naive Patients with Refractory Overactive Bladder: Results from a Multicenter, Randomized, Double-Blind Phase 3b Trial. J. Urol. 2017, 198, 167–175. [Google Scholar] [CrossRef]
- MacDiarmid, S.; Glazier, D.B.; McCrery, R.J.; Kennelly, M.J.; Nelson, M.; Ifantides, K.B.; McCammon, K.A. Efficacy and safety of an alternative onabotulinumtoxinA injection paradigm for refractory overactive bladder. Neurourol. Urodyn. 2024, 43, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Nitti, V.W.; Dmochowski, R.; Herschorn, S.; Sand, P.; Thompson, C.; Nardo, C.; Yan, X.; Haag-Molkenteller, C.; Group, E.S. OnabotulinumtoxinA for the Treatment of Patients with Overactive Bladder and Urinary Incontinence: Results of a Phase 3, Randomized, Placebo Controlled Trial. J. Urol. 2017, 197, S216–S223. [Google Scholar] [CrossRef]
- Niu, H.-L.; Ma, Y.-H.; Zhang, C.-J. Comparison of OnabotulinumtoxinA versus sacral neuromodulation for refractory urinary urge incontinence: A systematic review and meta-analysis of randomized controlled trials. Int. J. Surg. 2018, 60, 141–148. [Google Scholar] [CrossRef]
- Oxford Centre for Triple Value Healthcare. CASP Checklist: CASP Randomised Controlled Trial Checklist. 2024. Available online: https://casp-uk.net/casp-tools-checklists/randomised-controlled-trial-rct-checklist/ (accessed on 22 June 2025).
- Oxford Centre for Triple Value Healthcare. CASP Checklist: CASP Cohort Study Checklist. 2024. Available online: https://casp-uk.net/casp-tools-checklists/cohort-study-checklist/ (accessed on 22 June 2025).
- Goodman, M.; Pedersen, C. Should the Food and Drug Administration Limit Placebo-Controlled Trials? Voices Bioeth. 2022, 8. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Kuo, J.-H.; Huang, Y.-T.; Lai, P.-C.; Ou, Y.-C.; Lin, Y.-C. Evaluating the efficacy and safety of botulinum toxin in treating overactive bladder in the elderly: A meta-analysis with trial sequential analysis of randomized controlled trials. Toxins 2024, 16, 484. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.Q.; Xu, Y.Q.; Xu, J.; Ding, X.Y.; Guo, C. Meta-Analysis of Randomized Controlled Trials Using Botulinum Toxin A at Different Dosages for Urinary Incontinence in Patients With Overactive Bladder. Front. Pharmacol. 2019, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.-C.; Kao, Y.-L.; Ho, Y.-H.; Wu, K.-Y.; Kuo, H.-C. Intravesical Injection of Botulinum Toxin Type A in Patients with Refractory Overactive Bladder—Results between Young and Elderly Populations, and Factors Associated with Unfavorable Outcomes. Toxins 2023, 15, 95. [Google Scholar] [CrossRef] [PubMed]
- Kavcic, N.; Avsenak, A.; Zmazek, J.; Serdinsek, T.; But, I. Efficacy and safety of intradetrusor abobotulinumtoxinA and incobotulinumtoxinA in women with overactive bladder and the value of local anesthesia: A randomized clinical study. Wien. Klin. Wochenschr. 2025, 137, 470–478. [Google Scholar] [CrossRef]
- Mohamed-Ahmed, R.; Tomlinson, C.; Taithongchai, A.; Rantell, A.; Araklitis, G.; Robinson, D.; Cardozo, L. Long term safety outcomes and continuation rates of repeated Intravesical Botulinum Toxin A injections for Detrusor Overactivity: 16 year’s experience of a Tertiary Centre in the UK. Continence 2024, 9, 101066. [Google Scholar] [CrossRef]
- Henriet, B.; Roumeguere, T. Botulinum toxin injection for refractory non-neurogenic overactive bladder. Systematic review. Rev. Medicale Brux. 2015, 36, 29–37. [Google Scholar]
- Chui, W.; Bazo, A.; Yao, H.H.; Kealey, J.; Pepdjonovic, L.; O’Connell, H.E.; Gani, J.; Parkinson, R. The use of intradetrusor botulinum toxin in the geriatric population. BJUI Compass 2025, 6, e70048. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; O’Kane, M.; Cardozo, L. Adherence to Overactive Bladder Syndrome Treatments Recent Developments and Future Perspectives. Int. J. Womens Health 2023, 15, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Abd Wahab, H.H.b.; O’Callaghan, M. Seminal papers in urology: Two-year outcomes of Sacral Neuromodulation Versus OnabotulinumtoxinA for refractory urgency urinary incontinence: A Randomized Trial. BMC Urol. 2024, 24, 16. [Google Scholar] [CrossRef]
- Long, H.A.; French, D.P.; Brooks, J.M. Optimising the value of the critical appraisal skills programme (CASP) tool for quality appraisal in qualitative evidence synthesis. Res. Methods Med. Health Sci. 2020, 1, 31–42. [Google Scholar] [CrossRef]
- Noyes, J.; Booth, A.; Flemming, K.; Garside, R.; Harden, A.; Lewin, S.; Pantoja, T.; Hannes, K.; Cargo, M.; Thomas, J. Cochrane Qualitative and Implementation Methods Group guidance series—Paper 3: Methods for assessing methodological limitations, data extraction and synthesis, and confidence in synthesized qualitative findings. J. Clin. Epidemiol. 2018, 97, 49–58. [Google Scholar] [CrossRef]
- Hannes, K.; Macaitis, K. A move to more systematic and transparent approaches in qualitative evidence synthesis: Update on a review of published papers. Qual. Res. 2012, 12, 402–442. [Google Scholar] [CrossRef]
- National Guideline Alliance (UK). Botulinum toxin type A–treatment dose for OAB management. In Evidence Reviews for the Management of Overactive Bladder: Urinary Incontinence and Pelvic Organ Prolapse in Women: Management: Evidence Review D; National Institute for Health and Care Excellence (NICE): London, UK, 2019. [Google Scholar]
- Chapple, C.; Sievert, K.D.; MacDiarmid, S.; Khullar, V.; Radziszewski, P.; Nardo, C.; Thompson, C.; Zhou, J.; Haag-Molkenteller, C. OnabotulinumtoxinA 100 U significantly improves all idiopathic overactive bladder symptoms and quality of life in patients with overactive bladder and urinary incontinence: A randomised, double-blind, placebo-controlled trial. Eur. Urol. 2013, 64, 249–256. [Google Scholar] [CrossRef]
- Orasanu, B.; Mahajan, S.T. The use of botulinum toxin for the treatment of overactive bladder syndrome. Indian J. Urol. 2013, 29, 2–11. [Google Scholar] [CrossRef]
- Centre for Reviews and Dissemination (CRD). International Prospective Register of Systematic Reviews (PROSPERO). Available online: https://www.crd.york.ac.uk/prospero/ (accessed on 24 February 2025).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, C.; Pan, H.; Song, C. Ultrasound guided transabdominal botulinum toxin injection for refractory overactive bladder treatment. Sci. Rep. 2025, 15, 18162. [Google Scholar] [CrossRef] [PubMed]
- White, W.M.; Pickens, R.B.; Doggweiler, R.; Klein, F.A. Short-term efficacy of botulinum toxin a for refractory overactive bladder in the elderly population. J. Urol. 2008, 180, 2522–2526. [Google Scholar] [CrossRef] [PubMed]
- Chartier-Kastler, E.; Normand, L.L.; Ruffion, A.; Saussine, C.; Braguet, R.; Rabut, B.; Ragni, E.; Perrouin-Verbe, M.-A.; Pierrevelcin, J.; Rousseau, T.; et al. Sacral Neuromodulation with the InterStim System for Overactive Bladder: 3-Year Results from the French Prospective, Multicenter, Observational SOUNDS Study. Eur. Urol. Focus 2022, 8, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- British Society of Urogynaecology. Botox Injections to Treat Overactive Bladder. 2019. Available online: https://bsug.org.uk/budcms/includes/kcfinder/upload/files/Botox-BSUG-Dec-2019.pdf (accessed on 9 July 2025).
- Cameron, A.P.; Chung, D.E.; Dielubanza, E.J.; Enemchukwu, E.; Ginsberg, D.A.; Helfand, B.T.; Linder, B.J.; Reynolds, W.S.; Rovner, E.S.; Souter, L.; et al. The AUA/SUFU guideline on the diagnosis and treatment of idiopathic overactive bladder. J. Urol. 2024, 212, 11–20. [Google Scholar] [CrossRef] [PubMed]
- NICE. Urinary Incontinence and Pelvic Organ Prolapse in Women: Management; NICE Guideline NG123; NICE: London, UK, 2019. [Google Scholar]
- ReviewManager. RevMan, version 5.4.1; The Cochrane Collaboration, 2020. Available online: https://www.cochrane.org/ (accessed on 9 July 2025).


| Study, Publication Year | Study Design | Interventions | Sample Size | Proportion of Female Participants (%) | Cost-Effectiveness Data Reported (Yes/No) | Funding Source |
|---|---|---|---|---|---|---|
| Al-Azzawi et al. [12], 2020 | Prospective chort | BoNT-A (200 IU) vs. SNM | 37 | 100% | Yes | None declared |
| Amundsen et al. [22], 2016 | RCT | BoNT-A (200 IU) vs. SNM | 381 | 100% | No | The Eunice Kennedy Shriver National Institute of Child Health and Human Development. The NIH Office of Research on Women’s Health at National Institutes of Health. |
| Amundsen et al. [23], 2018 | RCT | BoNT-A (200 IU) vs. SNM | 386 | 100% | No | The Eunice Kennedy Shriver National Institute of Child Health and Human Development. The NIH Office of Research on Women’s Health at National Institutes of Health. |
| Chermansky et al. [24], 2022 | RCT | BoNT-A (100 IU) vs. Placebo * | 115 | 90% | No | AbbVie |
| Denys et al. [25], 2012 | RCT | BoNT-A (100 IU) vs. Placebo | 54 | 87% | No | “Assistance Publique—Hôpitaux de Paris” Department of Clinical Research and Development), Grant of the French Ministry of Health (“Programme Hospitalier de Recherche Clinique”) |
| Dmochowski et al. [26], 2010 | RCT | BoNT-A (100 IU) vs. Placebo | 313 | 92% | No | AbbVie |
| Elmer-Lyon et al. [27], 2020 | Retrospective cohort | BoNT-A (100-300 IU) vs. SNM | 101 | 100% | No | None declared |
| Singh et al. [28], 2015 | Retrospective cohort | BoNT-A (200 IU) vs. SNM | 128 | 100% | No | None declared |
| Yokoyama et al. [29], 2020 | RCT | BoNT-A (100 IU) vs. Placebo | 248 | 75% | No | GlaxoSmithKline |
| Herschorn et al. [30], 2017 | RCT | BoNT-A (100 IU) vs. Placebo | 205 | 84% | No | Abb Vie |
| MacDiarmid et al. [31], 2023 | RCT | BoNT-A (100 IU) vs. Placebo | 120 | 93% | No | AbbVie |
| Nitti et al. [32], 2016 | RCT | BoNT-A (100 IU) vs. Placebo | 557 | 88% | No | None declared |
| CASP Criteria/Study | Amundsen et al., 2016 [22] | Amundsen et al., 2018 [23] | Chermansky et al., 2022 [24] | Denys et al., 2012 [25] | Dmochowski et al., 2010 [26] | Yokoyama et al., 2020 [29] | Herschorn et al., 2017 [30] | MacDiarmid et al., 2023 [31] | Nitti et al., 2016 [32] |
|---|---|---|---|---|---|---|---|---|---|
| 1. Did the study address a clearly formulated research question? | y | y | y | y | y | y | y | y | y |
| 2. Was the assignment of participants to interventions randomized? | y | y | y | y | y | y | y | y | y |
| 3. Were all participants who entered the study accounted for at its conclusion? | y | y | y | n | y | y | y | y | y |
| 4. (a) Were the participants ‘blind’ to intervention they were given? | n | n | y | y | y | y | y | y | y |
| 4. (b) Were the investigators ‘blind’ to the intervention they were giving to participants? | n | n | y | y | y | y | y | y | y |
| 4. (c) Were the people assessing/analyzing outcome/s ‘blinded’? | ct | ct | y | y | y | ct | ct | y | y |
| 5. Were the study groups similar at the start of the randomized controlled trial? | y | y | y | y | y | y | y | y | y |
| 6. Apart from the experimental intervention, did each study group receive the same level of care (that is, were they treated equally)? | y | y | y | y | y | y | y | y | y |
| 7. Were the effects of intervention reported comprehensively? | y | y | y | y | y | y | y | y | y |
| 8. Was the precision of the estimate of the intervention or treatment effect reported? | y | y | y | n | n | y | y | y | y |
| 9. Do the benefits of the experimental intervention outweigh the harms and costs? | ct | ct | ct | ct | ct | ct | ct | ct | ct |
| 10. Can the results be applied to your local population/in your context? | y | y | y | y | y | y | y | y | y |
| 11. Would the experimental intervention provide greater value to the people in your care than any of the existing interventions? | ct | ct | n | y | y | y | y | y | y |
| CASP Criteria/Study | Al-Azzawi et al., 2020 [12] | Elmer-Lyon et al., 2020 [27] | Singh et al., 2015 [28] |
|---|---|---|---|
| 1. Did the study address a clearly focused issue? | y | y | y |
| 2. Was the cohort recruited in an acceptable way? | y | n | y |
| 3. Was the exposure accurately measured to minimize bias? | y | y | y |
| 4. Was the outcome accurately measured to minimize bias? | n | y | n |
| 5. (a) Have the authors identified all important confounding factors? | n | n | y |
| 5. (b) Have they taken account of the confounding factors in the design and/or analysis? | y | n | y |
| 6. (a) Was the follow up of subjects complete enough? | y | y | y |
| 6. (b) Was the follow up of subjects long enough? | n | y | n |
| 7. What are the results of this study? | The procedures compared had similar safety and efficiency profiles. | UTI rates were similar in patients undergoing BoNT-A injections and SNM. | Treatment failure rates at six months were lower in the SNM group vs. BoNT-A group. |
| 8. How precise are the results? | y | y | y |
| 9. Do you believe the results? | y | y | y |
| 10. Can the results be applied to the local population? | y | y | y |
| 11. Do the results of this study fit with other available evidence? | n | y | y |
| 12. What are the implications of this study for practice? | Patient preference, availability, cost, and surgical expertise should guide treatment choice, as both interventions have comparable efficacy but differ in invasiveness, cost/resource requirements, and durability of treatment. | Clinicians can reassure patients that UTI risk is comparable between BoNT-A and SNM. Recurrent UTI or prior prolapse repair increases UTI risk after either procedure. | SNM may be preferred over BoNT-A for short-term efficacy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roman, M.P.; Ciortea, R.; Doumouchtsis, S.K.; Măluțan, A.M.; Bucuri, C.E.; Ormindean, C.M.; Suciu, V.E.; Nati, I.D.; Căilean, A.; Mihu, D. Comparison of Different Treatment Outcomes for Refractory Overactive Bladder: A Systematic Review and Meta-Analysis. Toxins 2025, 17, 479. https://doi.org/10.3390/toxins17100479
Roman MP, Ciortea R, Doumouchtsis SK, Măluțan AM, Bucuri CE, Ormindean CM, Suciu VE, Nati ID, Căilean A, Mihu D. Comparison of Different Treatment Outcomes for Refractory Overactive Bladder: A Systematic Review and Meta-Analysis. Toxins. 2025; 17(10):479. https://doi.org/10.3390/toxins17100479
Chicago/Turabian StyleRoman, Maria Patricia, Răzvan Ciortea, Stergios K. Doumouchtsis, Andrei Mihai Măluțan, Carmen Elena Bucuri, Cristina Mihaela Ormindean, Viorela Elena Suciu, Ionel Daniel Nati, Andreea Căilean, and Dan Mihu. 2025. "Comparison of Different Treatment Outcomes for Refractory Overactive Bladder: A Systematic Review and Meta-Analysis" Toxins 17, no. 10: 479. https://doi.org/10.3390/toxins17100479
APA StyleRoman, M. P., Ciortea, R., Doumouchtsis, S. K., Măluțan, A. M., Bucuri, C. E., Ormindean, C. M., Suciu, V. E., Nati, I. D., Căilean, A., & Mihu, D. (2025). Comparison of Different Treatment Outcomes for Refractory Overactive Bladder: A Systematic Review and Meta-Analysis. Toxins, 17(10), 479. https://doi.org/10.3390/toxins17100479





