Abstract
Prorocentrum, a dinoflagellate responsible for producing diarrhetic shellfish poisoning (DSP) toxins, poses significant threats to marine ecosystems, aquaculture industries, and human health. DSP toxins, including okadaic acid (OA), dinophysis toxin (DTX), and their diverse derivatives, continue to be identified and characterized. In this study, we report the isolation of four new diol esters of OA/DTX-1 from large-scale cultures of Prorocentrum lima. Their chemical structures were elucidated through comprehensive NMR and MS analyses, along with structural comparisons with the well-known OA. Notably, compound 1 featured an additional ester group within the diol unit, while compound 2 was revealed to be a C11 diol ester. The cytotoxicity of these newly isolated derivatives was evaluated against three cell lines: Neuro2a (mouse), HCT116 (human), and HepG2 (human). All diol esters exhibited cytotoxic effects, with compound 3 displaying toxicity comparable to OA. These results expand our understanding of DSP toxin diversity and provide valuable insight into the structural variations and biological activity of diol esters of OA/DTX-1.
Key Contribution:
This study presents the isolation and structural characterization of four new diol esters from P. lima cultures, accompanied by an assessment of their cytotoxicity.
1. Introduction
Dinoflagellates of the genus Prorocentrum are well-known producers of diarrhetic shellfish poisoning (DSP) toxins, including okadaic acid (OA) and dinophysistoxins (DTXs), similar to Dinophysis species [1,2,3]. These toxins, particularly OA and DTX-1 and -2, are potent inhibitors of protein phosphates PP1 and PPA2 [4]. Consumption of shellfish contaminated with these toxins caused gastrointestinal issues such as diarrhea, nausea, and vomiting, as well as more significant health problems [5]. Over the past few decades, extensive research has focused on DSP toxins and their derivatives produced by these harmful marine algae.
Studies have identified a wide variety of derivatives of OA and DTXs in cultured Prorocentrum species, expanding our understanding of their chemical diversity [6,7,8,9,10,11]. Representative derivatives include water-soluble sulfated diesters and diol esters with carbon chains ranging from C6 to C10. Sulfated diesters have been isolated using rapid extraction techniques or cellular boiling, leading to the identification of nine sulfated diesters (DTX-4a, 4b, 5a, 5b, and 5c from OA and DTX-7a, 7b, 7c, and 7d from DTX-1) [12,13,14,15]. In contrast, diol esters have been isolated through mild solvent extraction of cellular biomass (Table S1) [6,8,9,10,11,15,16,17,18,19,20], with new variants continually being discovered [16].
These derivatives are believed to serve as a self-protection mechanism for toxin-producing organisms [12,15]. Sulfated diesters are hypothesized to act as precursors, hydrolyzing into diol esters and subsequently converting into the toxic free forms of OA and DTXs when cells are ruptured or damaged. Recent research supported this proposed mechanism [15]. In the context of OA and DTX transformations, the toxicity of intermediate diol esters can be posed as a question. While some studies have demonstrated the toxicity of diol esters in experimental systems, such as the diatom Thalassiosira weissflogii and in mouse models [17,21], data on their toxicity remain limited.
Interestingly, diol esters are often detected in higher quantities than free OA and DTXs [21,22]. Their composition appears to vary among across Prorocentrum strains from different geographic regions [23,24], suggesting ecological significance and potential physiological functions. This variability offers an opportunity to estimate strain-specific differences in Prorocentrum species by analyzing toxin profiles that include free OA, DTXs, and diverse diol esters.
During our investigation into toxin profile, we isolate three new OA diols and one DTX-1 diol ester from large-scale laboratory cultures of Prorocentrum lima. Here, the chemical structures of these compounds will be elucidated using NMR and MS techniques to reveal a remarkable structural diversity among diol esters. Furthermore, cytotoxicity tests across three cell lines will be conducted to assess the relative toxicity of the isolated diol esters compared to OA.
2. Results
The P. lima culture was harvested and extracted with MeOH. The extracts were then subjected to a series of chromatographic separations, resulting in the isolation of four new diol derivatives of OA/DTX-1, as shown in Figure 1.
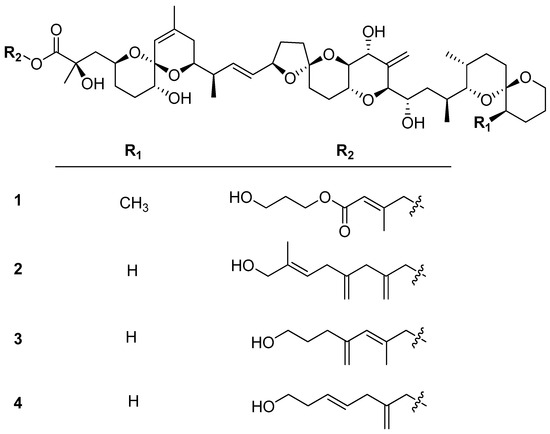
Figure 1.
Four new diol derivatives of OA/DTX-1 from the cultures P. lima.
2.1. Structure Determination of Compounds 1–4
The molecular formula of 1 was isolated as a colorless solid, with a molecular formula of C53H82O16, as determined by its ammonium-adducted ion peak ([M + NH4]+, m/z = 992.5941, Δ = 0.3 ppm) in the HR-ESI MS spectrum, and the observed carbon signals in the 13C NMR spectrum. The 1H and 13C NMR spectra of 1 exhibited strong similarities to those of dinophysistoxin-1 (DTX-1) isolated from P. lima, suggesting that compound 1 is a derivative of DTX-1. Detailed analysis using 1D and 2D NMR spectra (Figures S2–S5) allowed for the assignment of chemical shifts in the DTX-1 framework of compound 1, which closely matched the shifts observed for DTX-1, except for carbons corresponding to C-1 to C-4 and C-39 (Table S2). The additional moiety, inferred from the molecular formula to be C8H13O3, was characterized by one methyl (δC 16.0), two olefinic (δC 116.1 and 154.0), one methylene (δC 32.8), three oxymethylene (δC 59.4, 62.1, and 68.6), and one carbonyl carbon (δC 167.8), as analyzed based on the 13C and HSQC spectra (Table 1). The three deshielded carbon signals (δC 154.0, 116.1, and 167.8) suggested the existence of an α, β-unsaturated carbonyl functional group, which was supported by the UV absorption band at 214 nm in the IR spectrum. The structure of the additional moiety was elucidated using COSY and HMBC correlations, as illustrated in Figure 2a. The HMBC correlation between H-5′ and C-4′ established the linkage between the α, β-unsaturated carbonyl group and a 1,3-propandiol unit. The ester linkage between C-1′ and the DTX-1 core was confirmed by the HMBC correlation between H-1′ and carbonyl carbon within DTX-1. Lastly, the geometry of the double bond at C-2′ was determined to be in the E configuration based on the DP4+ analysis, which predicted 100% probability. The conformational conformers for E and Z isomers of the diol fragment were optimized by the DFT method at the B3LYP/6-31G(d,p) level, and the NMR shielding tensors for optimized conformers within 4 KJ/mol were calculated using DFT method at the MPW1PW81/6-311G(d,p) with the PCM model in MeOH. DP4+ analysis of the calculated and experimental 1H and 13C chemical shifts was conducted using the Excel-based program provided by Sarotti (Figure S6) [25].

Table 1.
13C NMR data for the diol moiety of compounds 1–4 (CD3OD).
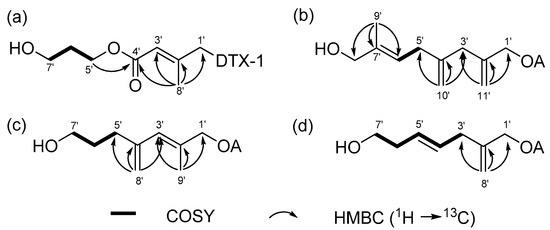
Figure 2.
COSY (bold lines), key HMBC (arrows) correlations in the diol moiety of (a) compounds 1, (b) 2, (c) 3, and (d) 4.
Compound 2 was identified with the molecular formula C55H84O14, as confirmed by its ammonium-adducted ion peak ([M + NH4]+ = 986.6167) (Figure S7) and the 13C NMR spectrum. The 1H NMR spectrum of 2 was closely similar to that of 1, though it displayed notable differences, particularly at 0.95 ppm and within the deshielded range. Analysis of 1D and 2D NMR spectra (Figures S8–S12) revealed that compound 2 is a derivative of okadaic acid (OA). The 13C NMR and HSQC spectra indicated that the structural fragment attached to OA consisted of one methyl (δC 13.8), one methine (δC 123.6), two methylene (δC 34.5 and 41.9), two oxymethylene (δC 67.5 and 68.7), two exomethylene (δC 112.9 and 114.5), and three non-protonated carbons (δC 137.9, 143.0, and 146.7). The connectivity of these carbons was determined using COSY and HMBC correlations, as shown in Figure 2b. The geometry of Δ6′ was determined to be in E form by the ROE correlation between H-5′ and H3-9′. Thus, 2 was named as (E)-8′-hydroxy-7′-methyl-2′,4′-dimethyleneoct-6′-enyl okadate, classified as an OA C11 diol.
Compound 3 was characterized with a molecular formula of C53H82O14, supported by its ammonium-adducted ion [M + NH4]+ = 960.6043 (Figure S13) and the 13C NMR spectrum. The 1H NMR spectrum of 3 showed a close resemblance to that of 2, although the 13C NMR spectrum revealed a different number of carbons. Compound 3 was identified as a derivative of OA, belonging to the C9-diol class, based on COSY and HMBC correlations (Figures S14–S17) illustrated in Figure 2c. The geometry of the olefinic group in the fragment was assigned to the E configuration by comparing the carbon chemical shift in C-8′ with that observed in 1 (Table 2). This assignment was further validated by DP4+ probability calculation, as performed for 1 (Figure S18). Thus, 3 was determined to be (E)-7′-hydroxy-2′-methyl-4′-methylenehept-2′-enyl okadate, and was classified as an OA C9 diol.

Table 2.
1H NMR data for the diol moiety compounds 1‒4 (CD3OD).
Compound 4 was determined to have a molecular formula of C52H80O14, as indicated by its ammonium-adducted ion [M + NH4]+ = 946.5886 (Figure S19) and the 13C NMR spectrum. The 1H NMR spectra of 3 and 4 were very similar, except for a difference at around 5.9 ppm. Compound 4, also a derivative of OA, was structurally shorter than 3, with fewer carbons (Figures S20–S24). Notably, 4 lacked a methyl signal in the C7 chain. The structural features of the fragment were shown in Figure 2d. The geometry of the double bond was assigned as E form by the ROE correlation between H-3′ and H-5′, and H-4′ and H-6′. Consequently, 4 was (E)-7′-hydroxy-2′-methylenehept-4′-enyl okadate, classified as an OA C8-diol.
2.2. Cytotoxicity Assessment of Compounds 1–4
The cytotoxicity of the diol derivatives (1–4), isolated from P. lima, was tested against three cell lines: HCT116 (human colon cancer cells), Neuro2a (mouse brain neuroblastoma cells), and HepG2 (human liver carcinoma cells) and a comparison was performed, with OA serving as the positive control. Each cell line was exposed to the compounds at concentrations of 0.1, 1, and 10 µM for 24 h (Figure 3). Among the cell lines, Neuro2a showed the highest sensitivity to the compounds. Based on IC50 values, compound 3 exhibited the strongest cytotoxicity, with effects similar to those of the positive control, OA (Table 3). The IC50 values for 3 were 0.07, 0.17, and 0.17 μM for Neuro2a, HCT116, and HepG2 cells, respectively, while those for OA were 0.07, 0.14, and 0.14 μM. Compounds 2 and 4 exhibited mild cytotoxicity across all three cell lines, with IC50 values ranging from 4.30 to 5.78 μM. Compound 1 showed relatively high cytotoxicity in Neuro2a and HCT116 cells, with IC50 values of 0.10 and 1.54, respectively, but was less toxic to HepG2 cells, where the IC50 exceeded 10 μM. To assess whether the cytotoxicity was due to apoptosis, flow cytometric analysis using Annexin V-FITC/PI staining was performed to detect apoptotic and necrotic cell populations (Figure S25).
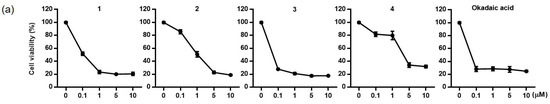
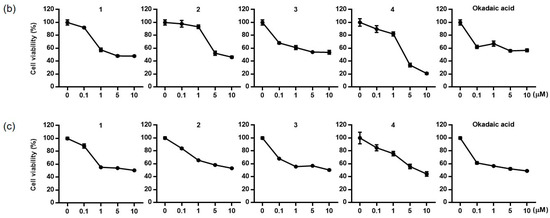
Figure 3.
Cytotoxic effects of compounds 1–4 and okadaic acid on Neuro-2a (a), HCT116 (b), and HepG2 (c) cells.

Table 3.
IC50 values of compounds 1–4 and okadaic acid against Neuro-2a, HCT116 and HepG2 cells.
3. Discussion
Three new OA diol esters and one new DTX-1 diol ester were isolated from the nonpolar cytotoxic fraction of laboratory-cultured P. lima. The structures of all compounds were elucidated through detailed analysis of 1D and 2D NMR spectra, supported by MS data. Compound 1, a DTX-1 derivative, was notable due to the presence of an additional ester group within the diol unit, marking the first discovery of a diester diol derivative of DTX-1. This unique structure represents a new framework distinct from previously reported OA or DTX-1 diol esters. This functional group likely forms through initial oxidation at C-4′ followed by esterification with 1,3-propandiol. Compound 2 is the first OA derivative identified with a C11-diol structure. Previously reported OA and DTX-1 diol or triol derivatives typically feature esterification with C6 to C10 carbon unit and an exomethylene or an olefinic methyl group at C-2′. Some also exhibited additional methyl or exomethylene branch along the carbon chain (Table S1). In contrast, compound 2 exhibited three branched carbons along a linear carbon chain, diverging from the conventional OA and DTX-1 diol ester structures. New structural characterizations were also determined for compound 3 (OA C9-diol) and compound 4 (OA C8- diol), expanding components of OA diol toxins relevant for toxin monitoring. The discovery of compounds 1 and 2 substantially broadens the spectrum of OA/DTX diol toxins, offering valuable insights into the structural diversity of these toxin derivatives. While LC-MS/MS is widely employed for detecting new OA/DTX-1 derivatives, isolation and structural characterization of these compounds require detailed and comprehensive analyses to refine and expand the existing toxin profiles.
In this study, the cytotoxicity of newly identified diol derivatives was evaluated by determining IC50 values and comparing them to OA using widely employed cell lines for cytotoxicity assessment, namely Neuro2a, HCT116, and HepG2. Among the isolated diol derivatives, compound 3 demonstrated the highest cytotoxicity, with levels comparable to OA across all three cell lines. The cytotoxic effects of the four diol derivatives varied among the three cell lines, showing similar or weaker toxicity than OA. Previous research by Wu et al. examined the toxicity in mice with hydrolyzed and unhydrolyzed P. lima cells [21]. Unhydrolyzed cells, which contain diol esters, demonstrated higher toxicity, indicating that these diol esters contribute to the overall toxic effects of P. lima. Our findings indicate that the toxicity of Prorocentrum species may vary among strains, likely reflecting differences in their specific diol ester compositions. To further investigate this relationship, future studies should explore the correlation between the toxicities of different P. lima strains and their toxin profiles.
4. Materials and Methods
4.1. Instrumentation
Optical rotation was measured on a JASCO P-1010 polarimeter (Jasco, Tokyo, Japan) using a 200 µL cell. The UV spectrum was recorded on a Varian Cary 50 spectrophotometer (Varian, Palo Alto, CA, USA), and the IR spectrum was obtained using a JASCO FT/IR 4100 spectrometer (Jasco, Tokyo, Japan). NMR spectra were measured in MeOH-d4 (with residual solvent peaks at δH 3.30 and δC 49.0) using a Bruker Avance II 900 MHz (Bruker BioSpin, Rheinstetten, Germany) at the Korea Basic Science Institute (KBSI) and a Varian VNMRS 500 MHz spectrometer (Varian, Palo Alto, CA, USA). HRESIMS data were collected using a SCIEX X500R instrument (SCIEX, Framingham, MA, USA). HPLC was performed with an Agilent 1200 system (Agilent, Santa Clara, CA, USA) equipped with an RI detector.
4.2. Cultures of P. lima
The same P. lima strain used in a previous study was utilized for this investigation [7]. To obtain minor components, an additional 1000 L culture was grown using the same cultivation method and harvested in the late-stationary phase of cell growth. This process yielded approximately 350 g of dinoflagellate material, which was then used for the isolation of derivatives of OA/DTX-1.
4.3. Extraction and Isolation of OA and DTX-1 Derivatives
The harvested cells were centrifuged, and the resulting pellet was extracted with 100% MeOH at 25 °C for 24 h. After lyophilization, the cells were extracted with 100% MeOH and then the extract (~20 g) was partitioned between H2O and CH2Cl2. The aqueous fraction was further partitioned with H2O and butanol, while the organic phase was subjected to partitioning with 85% aqueous MeOH and hexane. The butanol layer and 85% aqueous MeOH layer were then combined and subjected on fractionation using open-column chromatography on a reversed-phase column. A stepwise gradient elution was performed, starting from 50% H2O: 50% MeOH (MR1) and gradually increasing to 100% MeOH (MR6) in 10% MeOH increments, resulting in six fractions labeled MR1 to MR6. Compounds 1–4 were isolated from the bioactive MR5 fraction. This fraction underwent reversed-phase silica HPLC, producing eight subfractions (rp1–rp8). For this separation, a semipreparative C8 column was utilized at a flow rate of 2 mL/min with UV detection at 210 nm. The mobile phase consisted of H2O (A) and acetonitrile (B), with the volume of B increased from 40% to 100% over 45 min. Subsequently, compound 1 (0.7 mg) was purified at a retention time of 28 min, and the compound 3 (2.2 mg) was purified at 32 min from subfraction rp5 using reversed-phase HPLC on a semipreparative C18 column using 85% aqueous MeOH as the eluting solvent, detected by an RI detector. Similarly, compound 2 (0.7 mg) was purified from rp6, and compound 4 (2.6 mg) was purified from rp4. The quantities of the four isolated compounds were lower than those of OA (3.0 mg) and DTX-1 (5.2 mg).
1: colorless solid, [α]25D + 85.0 (c 0.06, MeOH); UV (MeOH) λmax (log ε) 214 (3.84) nm; IR νmax 3431, 2926, 1724, 1596, and 1080 cm−1; 1H and 13C NMR data, Table 1 and Table 2; HRESIMS m/z 992.5938 [M + NH4]+ (calcd for C53H82O16, 992.5941, Δ = 0.3).
2: colorless solid, [α]25D + 97.0 (c 0.08, MeOH); IR νmax 3411, 2926, 1739, 1591, and 1080 cm−1; 1H and 13C NMR data, Table 1 and Table 2; HRESIMS m/z 986.6167 [M + NH4]+ (calcd for C55H84O14, 986.6199, Δ = 2.7).
4.4. DF4+ Probability Calculation for the Diol Moiety of Compounds 1 and 3
A conformational search for the two isomers (E and Z) was conducted using the MMFF module in the Spartan 20 program. For each isomer, hundreds of accessible conformers generated from the search were filtered to those within a 10 kJ/mol energy range, according to a previously reported protocol. The selected conformers were further optimized using DFT at the B3LYP/6-31G(d,p) level in the Gaussian 16 program. The electronic and thermal free energies of each conformer were calculated, and conformers with a 4 kJ/mol energy threshold were chosen. For these low-energy conformers, 1H and 13C NMR shielding tensors were calculated using the DFT method at the MPW1PW81/6-311G(d,p) level with the PCM model in MeOH. The NMR shielding tensors of each isomer were then averaged with the weights of the low-energy conformers using Boltzmann distribution. Based on these averaged NMR shielding tensors, a DP4+ probability calculation was performed using a previously reported Excel spreadsheet [24].
4.5. Cell Cultures
HCT116 cells (human colon cancer cells) and HepG2 cells (human liver carcinoma cells) were obtained from the Korea Cell Line Bank (Seoul, Republic of Korea), while Neuro2a cells (mouse brain neuroblastoma cells) were purchased from ATCC. The cells were maintained in DMEM supplemented with 10% FBS, penicillin (100 IU/mL), and streptomycin (10 mg/mL), at 37 °C in a humidified atmosphere containing 5% CO2 and 95% relative humidity.
4.6. Cytotoxicity Assessment
The test compounds were dissolved in DMSO (final concentration of 0.1%) and diluted in serum-free culture medium. Prior to the assay, cells were seeded at the following densities in 96-well plates (100 μL per well) and incubated for 24 h: HCT-116 at 1 × 105 cells/mL, Neuro2a cells: 2 × 105 cells/mL, and HepG2 cell: 5 × 104 cells/mL. Cells were then treated with the vehicle control or test compounds at the specified concentrations for 24 h. The inhibitory effect on cell proliferation was evaluated using the CCK-8 assay. After treatment, 10 μL of CCK-8 solution was added to each well, and the cells were incubated for 2 h. Absorbance was measured at 450 nm using a microplate reader.
4.7. Flow Cytometry for Apoptosis Analysis
HCT-116, HepG2, and Neuro2a cells were seeded in 6-well plates and treated with the test compounds at the specified concentrations for 24 h. To analyze apoptosis, cells were detached using a plastic cell scraper, harvested, and resuspended with DMEM containing 1% FBS (dilution buffer) at a concentration of 5 × 105 cells/mL. A mixture of 100 μL Annexin V/dead cell reagent and 100 μL of the cell suspension was prepared and incubated in the dark for 20 min at 25 °C. Apoptosis was then quantified using a MUSE cell analyzer (Merck Millipore, Germany).
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/toxins17010028/s1: Figures S1–S6: HRESIMS, 1D, and 2D NMR spectra of compound 1; Figures S7–S12: HRESIMS, 1D, and 2D NMR spectra of compound 2; Figures S13–S18: HRESIMS, 1D, and 2D NMR spectra of compound 3; Figures S19–S24: HRESIMS, 1D, and 2D NMR spectra of compound 4; Figure S25: Apoptosis of three cell lines; Table S1: spectral data for DTX-1; Table S2: Previously isolated diol esters.
Author Contributions
Formal analysis and experiments, Y.K.J., Y.K. and S.L.; conceptualization, J.-R.R. and E.J.J.; methodology, S.M.; resources, Y.K.J.; data curation, S.M.; writing—original draft preparation, J.-R.R.; writing—review and editing, E.J.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation (NRF) grant by the Korea government (MSIP) (NRF-2020R1A2C2102603) and UN Decade Implementation Research Group project (RS-2023-00256732) funded by the Ministry of Oceans and Fisheries, Republic of Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in this article and Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lee, J.S.; Igarashi, T.; Fraga, S.; Dahl, E.; Hovgaard, P.; Yasumoto, T. Determination of diarrhetic shellfish toxins in various dinoflagellate species. J. Appl. Phycol. 1989, 1, 147–152. [Google Scholar] [CrossRef]
- Vale, P.; Veloso, V.; Amorim, A. Toxin composition of a Prorocentrum lima strain isolated from the Portuguese coast. Toxicon 2009, 54, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, T.; Murata, M.; Oshima, Y.; Sano, M.; Matsumoto, G.K.; Calardy, J. Diarrhetic shellfish toxins. Tetrahedron 1985, 41, 1019–1025. [Google Scholar] [CrossRef]
- Cohen, P.; Holmes, C.F.B.; Tsukitani, Y. Okadaic acid: A new probe for the study of cellular regulation. Trend Biochem. Sci. 1990, 15, 98–102. [Google Scholar] [CrossRef]
- Dominguez, H.J.; Paz, B.; Daranas, A.H.; Norte, M.; Franco, J.M.; Fernández, J.J. Dinoflagellate polyether within the yessotoxin, pectenotoxin and okadaic acid toxin groups: Characterization, analysis and human health implication. Toxicon 2010, 56, 191–217. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Marr, J.; Defreitas, A.S.W.; Quilliam, M.A.; Walter, J.A.; Wright, J.L.C.; Pleasance, S. New diol esters isolated from cultures of the dinoflagellates Prorocentrum lima and Prorocentrum concavum. J. Nat. Prod. 1992, 55, 1631–1637. [Google Scholar] [CrossRef]
- Suárez-Gómez, B.; Souto, M.L.; Norte, S.M.; Fernández, J.J. Isolation and structural determination of DTX-6, a new okadaic acid derivative. J. Nat. Prod. 2001, 64, 1363–1364. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Gómez, B.; Souto, M.L.; Cruz, P.G.; Fernández, J.J.; Norte, M. New targets in diarrhetic shellfish poisoning control. J. Nat. Prod. 2005, 68, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Norte, M.; Padilla, A.; Fernández, J.; Souto, M.L. Structure determination and biosynthetic origin of two ester derivatives of okadaic acid isolated from Prorocentrum lima. Tetrahedron 1994, 50, 9175–9180. [Google Scholar] [CrossRef]
- Lee, S.; Hwang, B.S.; Kim, H.S.; Yih, W.; Jeong, E.J.; Rho, J.-R. A new diol ester derivative of dinophysistoxin-1 from cultures of Prorocentrum lima collected in South Korea. Bull. Korean Chem. Soc. 2015, 36, 395–398. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Pan, J.; Liang, J.; Zhou, Y.; Wu, J. Identification of the okadaic acid-based toxin profile of a marine dinoflagellate strain Prorocentrum lima by LC-MS/MS and NMR spectroscopic data. J. Sep. Sci. 2012, 35, 782–789. [Google Scholar] [CrossRef]
- Hu, T.; Curtis, J.M.; Walter, J.A.; McLachlan, J.L.; Wright, J.L.C. Two new water-soluble DSP toxin derivatives from the dinoflagellate Prorocentrum maculosum: Possible storage and excretion products. Tetrahedron Lett. 1995, 36, 9273–9276. [Google Scholar] [CrossRef]
- Hu, T.; Curtis, J.M.; Walter, J.A.; Wright, J.L.C. Identification of DTX-4 a new water-soluble phosphatase inhibitor from the toxic dinoflagellate Prorocentrum lima. J. Chem. Soc. Chem. Commun. 1995, 42, 597–599. [Google Scholar] [CrossRef]
- Cruz, P.C.; Daranas, A.H.; Fernández, J.J.; Souto, M.L.; Norte, M. DTX5c, a new OA sulphate ester derivative from cultures of Prorocentrum belizeanum. Toxicon 2006, 47, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; LeBlanc, P.; Burton, I.W.; Walter, J.A.; McCarron, P.; Melanson, J.E.; Strangman, W.K.; Wright, J.L.C. Sulfated diesters of okadaic acid and DTX-1: Self-protective precursors of diarrhetic shellfish poisoning (DSP) toxins. Harmful Algae 2017, 63, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Choi, D.H.; Lee, Y.; Rho, J.-R. Three new okadaic acid derivatives isolated from a benthic Dinoflagellate prorocentrum lima. J. Korean Magn. Reson. 2024, 28, 25–31. [Google Scholar]
- Hu, T.; Burton, I.; Curtis, J.M.; Quilliam, M.A.; Walter, J.A.; Windust, A.J.; Wright, J.L.C. Oxidative transformation of a naturally occurring okadaic acid diol ester by the diatom Thalassiosira weissflogii. Tetrahedron Lett. 1999, 40, 3981–3984. [Google Scholar] [CrossRef]
- Fernández, J. J.; Suarez-Gomez, B.; Souto, M. L.; Norte, M. Identification of New Okadaic Acid Derivatives from Laboratory Cultures of Prorocentrum lima. J. Nat. Prod 2003, 66, 1294–1296. [Google Scholar]
- Yasumoto, T.; Murata, M.; Lee, J. S.; Torigoe, K. Mycotoxins and Phycotoxins ’88; Elsevier Science Ltd: Amsterdam, The Netherland, 1989; pp. 373–382. [Google Scholar]
- Miles, C. O.; Wilkins, A.L.; Hawkes, A.D.; Jensen, D.J.; Cooney, J.M.; Larsen, K.; Petersen, D.; Rise, F.; Beuzenberg, V.; MacKenzie, A.L. Isolation and identification of a cis-C8-diol-ester of okadaic acid from Dinophysis acuta in New Zealand. Toxicon 2006, 48, 195–203. [Google Scholar]
- Wu, H.; Chen, J.; Peng, J.; Zhong, Y.; Zheng, G.; Guo, M.; Tan, Z.; Zhai, Y.; Lu, S. Nontarget screening and toxicity evaluation of diol esters of okadaic acid and dinophysistoxins reveal intraspecies difference of Prorocentrum lima. Environ. Sci. Technol. 2020, 54, 12366–12375. [Google Scholar] [CrossRef]
- Lincoln, A.M.; Andrew, I.S.; Paul, M.; Lesley, R. Benthic dinoflagellate toxins in two warm-temperature estuaries: Rangaunu and Parengarenga Harbours, Northland, New Zealand. Harmful Algae 2011, 10, 559–566. [Google Scholar]
- Rodríguez, F.; Riobó, P.; Crespín, G.D.; Daranas, A.H.; de Vera, C.R.; Norte, M.; Fernández, J.J.; Fraga, S. The toxic benthic dinoflagellate Prorocentrum maculosum Faust is a synonym of Prorocentrum hoffmannianum Faust. Harmful Algae 2018, 78, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, H.; Zheng, G.; Zhong, Y.; Tan, Z. Variation profile of diarrhetic shellfish toxins and diol esters derivatives of Prorocentrum lima during growth by high-resolution mass spectrometry. Toxicon 2023, 232, 107224. [Google Scholar] [CrossRef] [PubMed]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).