Glucose and Oxygen Levels Modulate the Pore-Forming Effects of Cholesterol-Dependent Cytolysin Pneumolysin from Streptococcus pneumoniae
Abstract
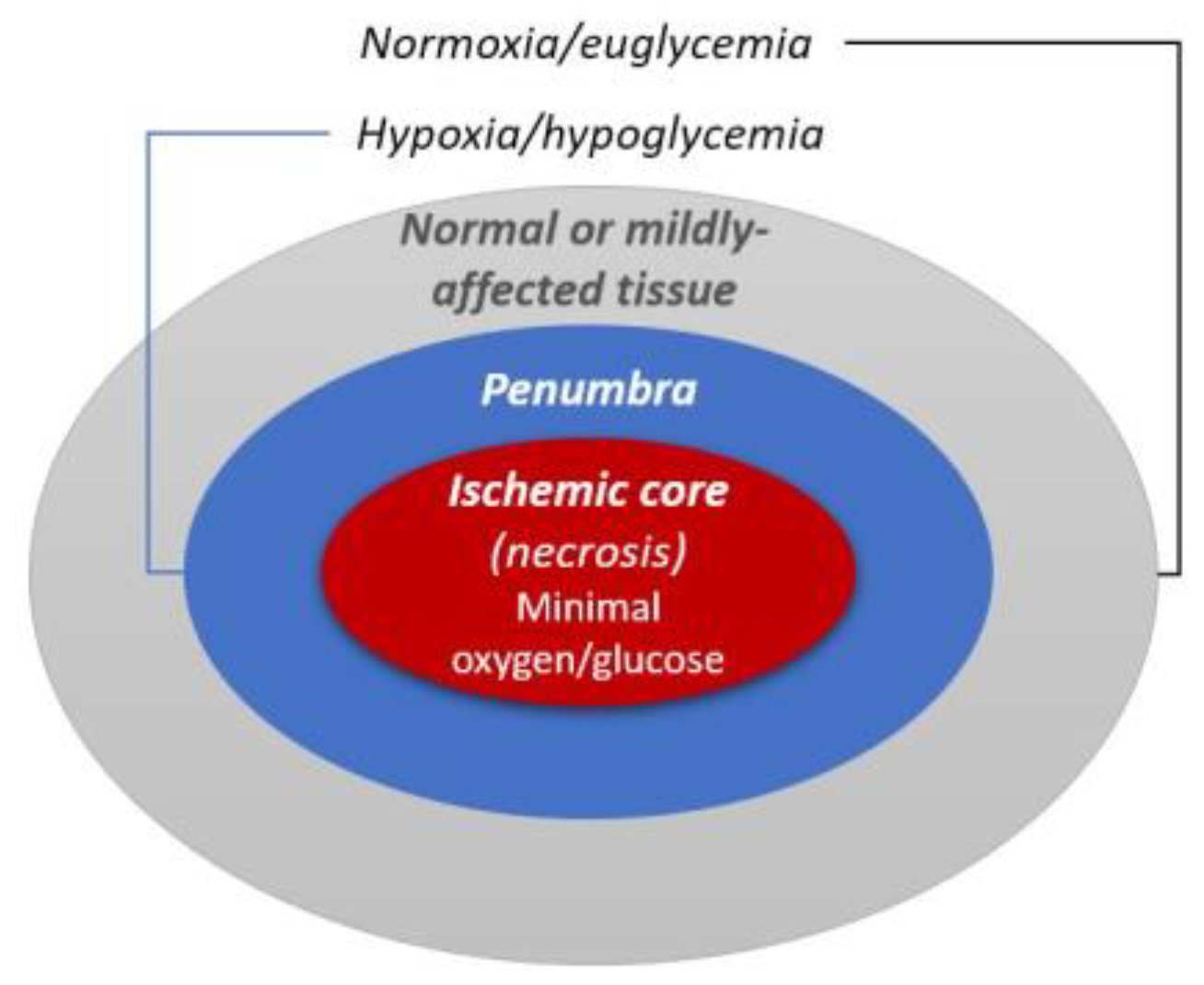
1. Introduction
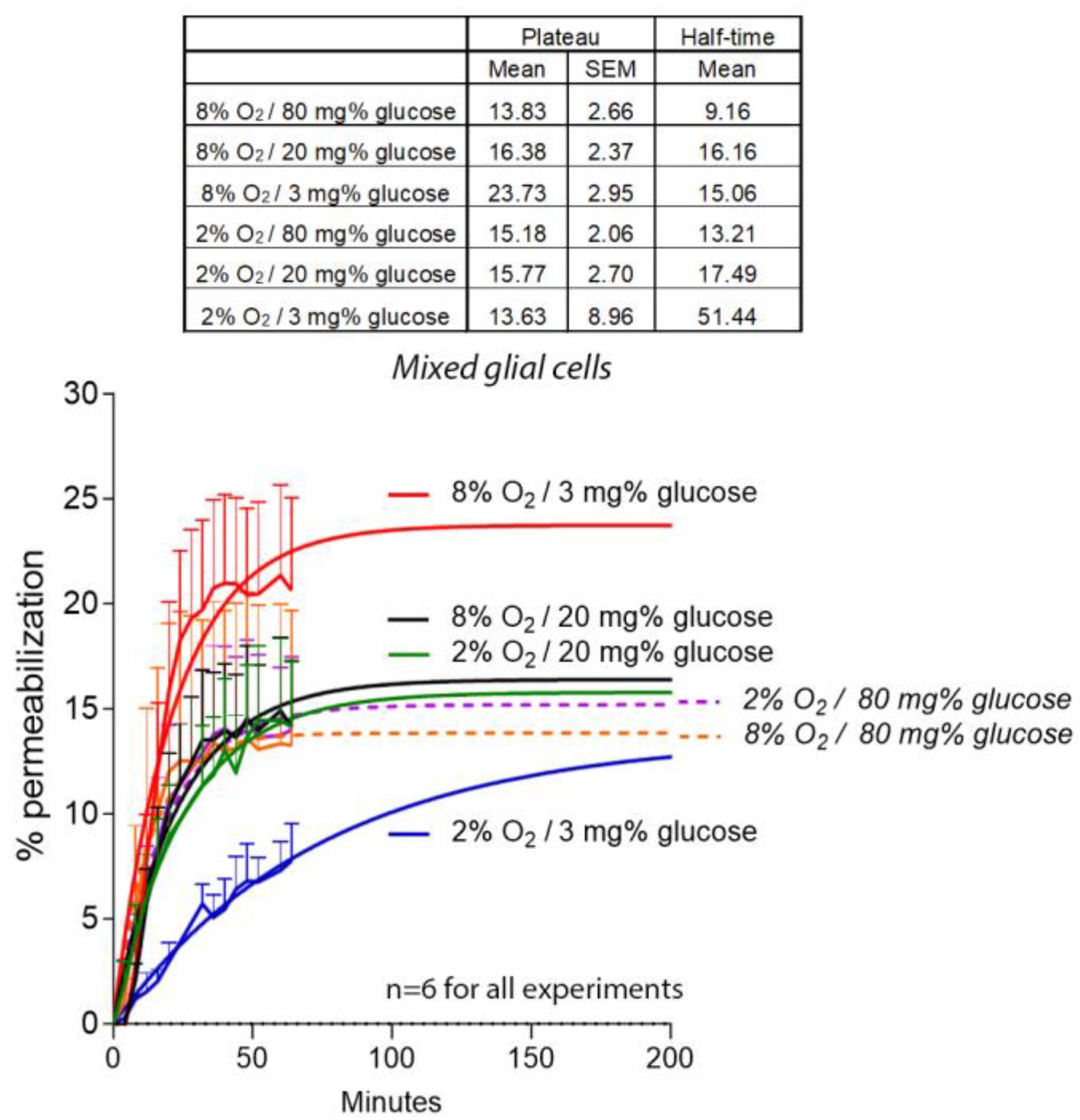
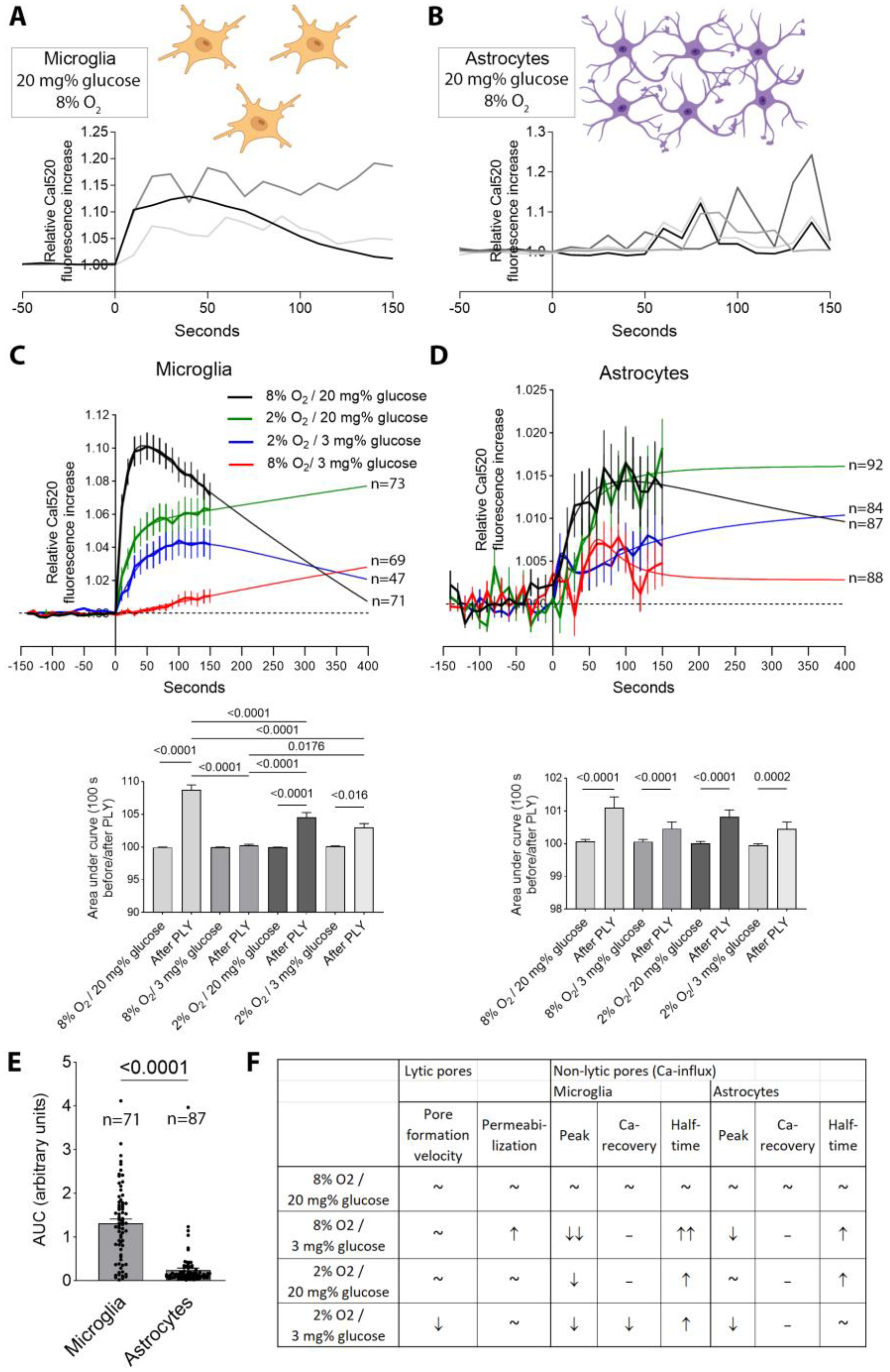
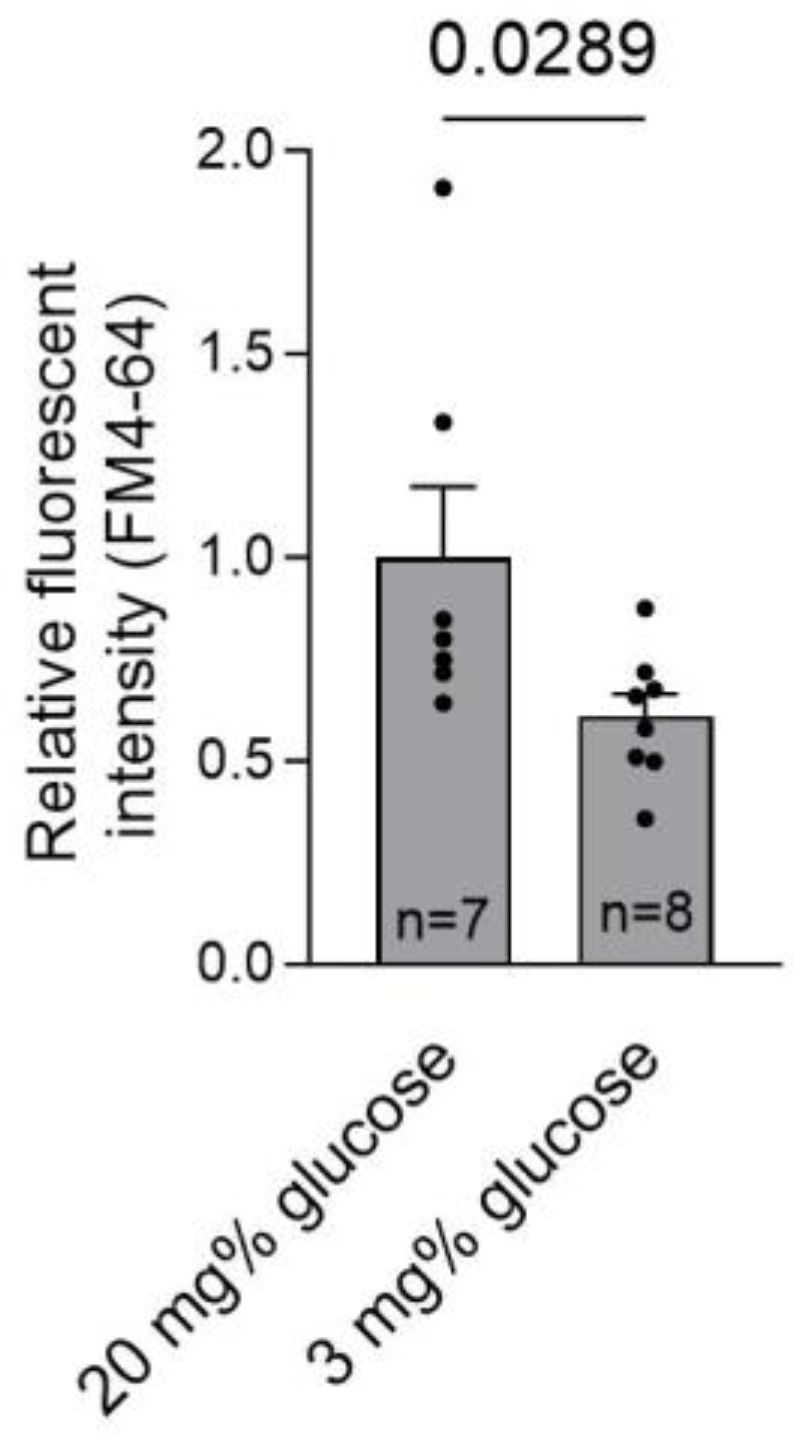
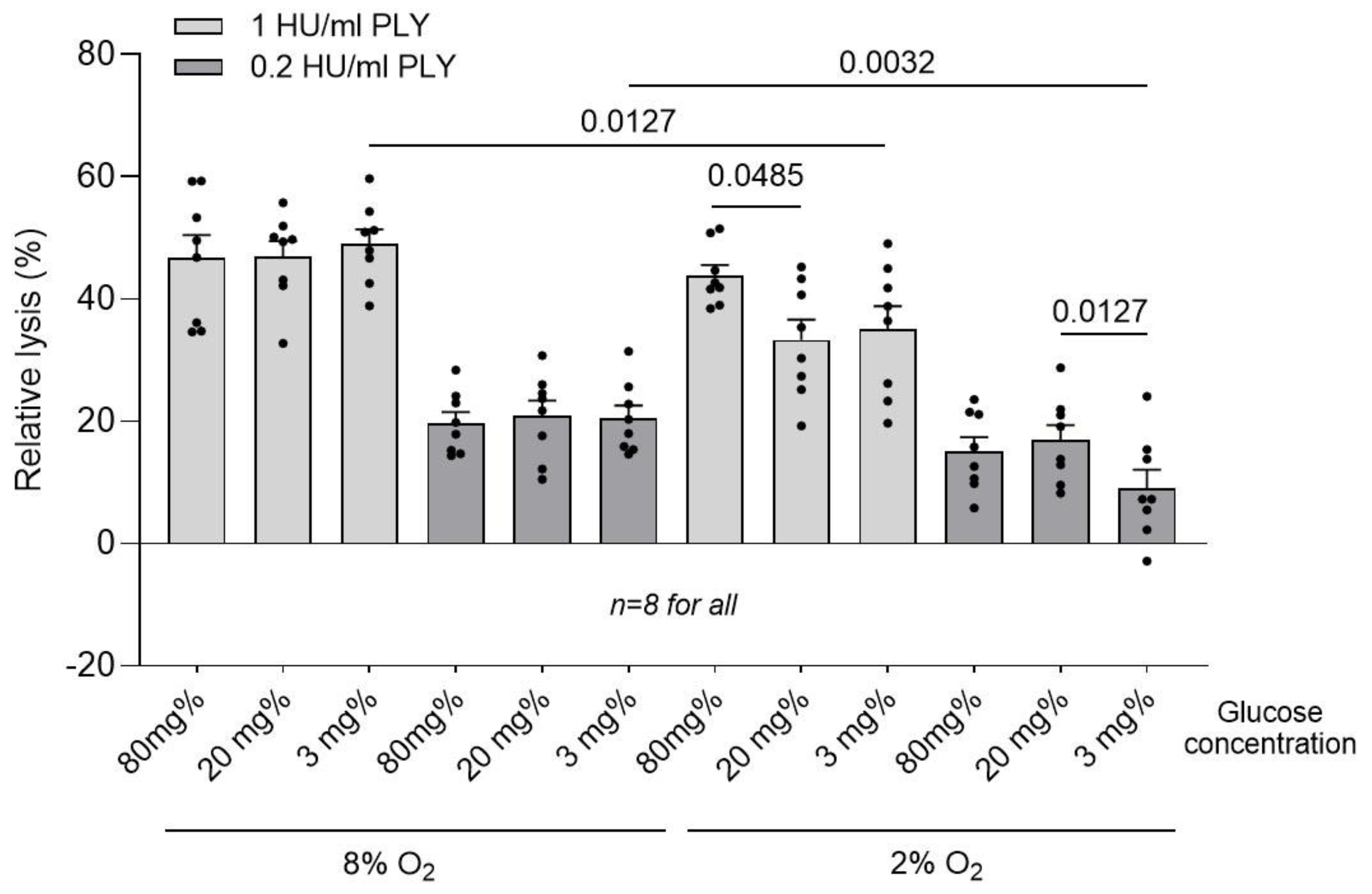
2. Results
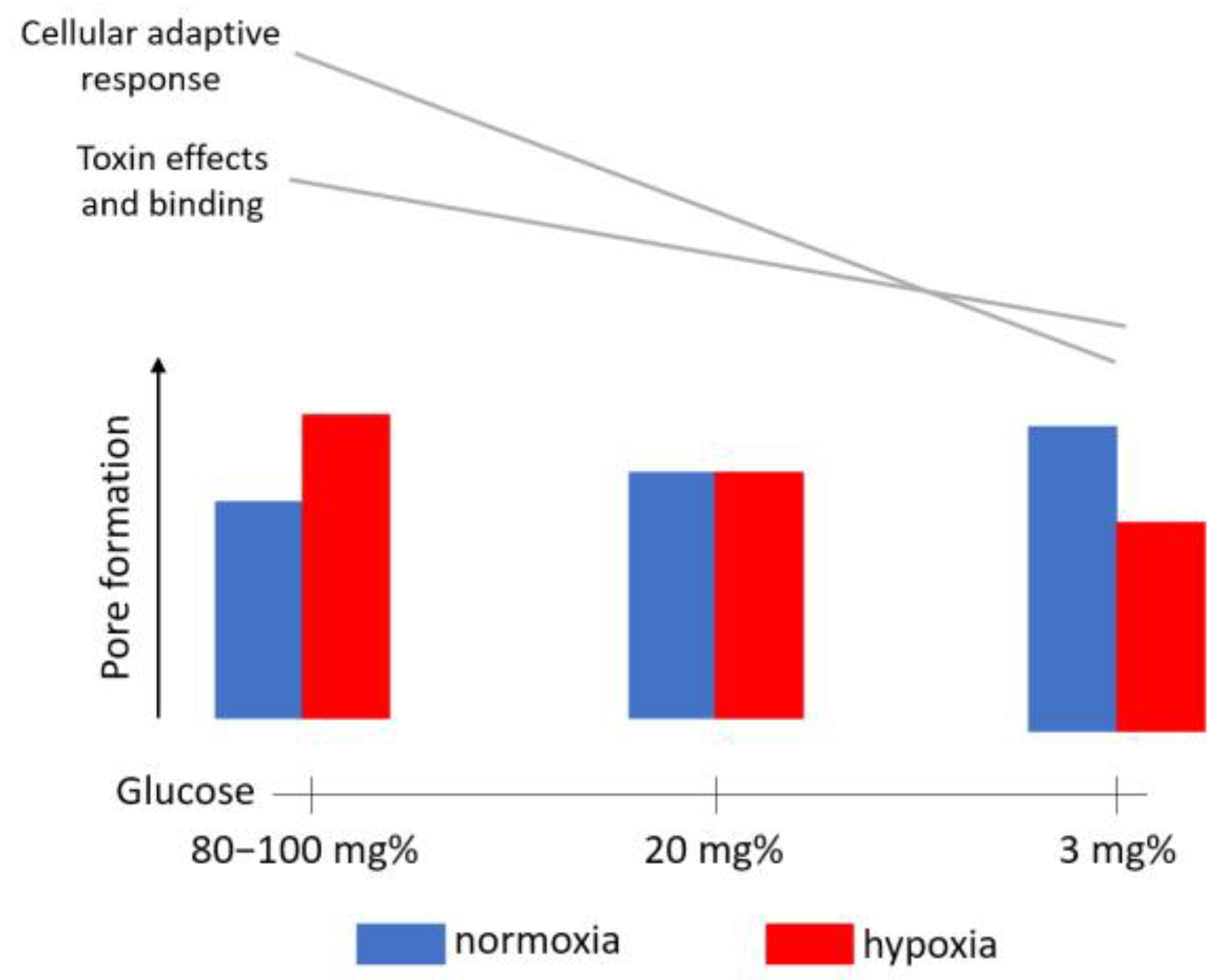
3. Discussion
4. Materials and Methods
4.1. Recombinant PLY Preparation
4.2. Cell Culture and Tissue Culture
4.3. LDH Assay
4.4. Cell Culture Ischemia Model System
4.5. Microscopy Live Imaging Assays (Permeabilization, Calcium Measurement, Endocytosis)
4.6. Filipin Staining
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gessner, B.D.; Mueller, J.E.; Yaro, S. African Meningitis Belt Pneumococcal Disease Epidemiology Indicates a Need for an Effective Serotype 1 Containing Vaccine, Including for Older Children and Adults. BMC Infect. Dis. 2010, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Saez-Llorens, X.; McCracken, G.H.J.; McCracken, G.H.J. Bacterial Meningitis in Children. Lancet 2003, 361, 2139–2148. [Google Scholar] [CrossRef] [PubMed]
- Hirst, R.A.A.; Gosai, B.; Rutman, A.; Guerin, C.J.J.; Nicotera, P.; Andrew, P.W.W.; O’Callaghan, C.; O’Callaghan, C. Streptococcus Pneumoniae Deficient in Pneumolysin or Autolysin Has Reduced Virulence in Meningitis. J. Infect. Dis. 2008, 197, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Benton, K.A.; Everson, M.P.; Briles, D.E. A Pneumolysin-Negative Mutant of Streptococcus Pneumoniae Causes Chronic Bacteremia Rather than Acute Sepsis in Mice. Infect. Immun. 1995, 63, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Canvin, J.R.; Marvin, A.P.; Sivakumaran, M.; Paton, J.C.; Boulnois, G.J.; Andrew, P.W.; Mitchell, T.J. The Role of Pneumolysin and Autolysin in the Pathology of Pneumonia and Septicemia in Mice Infected with a Type 2 Pneumococcus. J. Infect. Dis. 1995, 172, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Spreer, A.; Kerstan, H.; Böttcher, T.; Gerber, J.; Siemer, A.; Zysk, G.; Mitchell, T.J.; Eiffert, H.; Nau, R.; Bottcher, T.; et al. Reduced Release of Pneumolysin by Streptococcus Pneumoniae in Vitro and in Vivo after Treatment with Nonbacteriolytic Antibiotics in Comparison to Ceftriaxone. Antimicrob. Agents Chemother. 2003, 47, 2649–2654. [Google Scholar] [CrossRef] [PubMed]
- Tilley, S.J.; Orlova, E.V.; Gilbert, R.J.; Andrew, P.W.; Saibil, H.R. Structural Basis of Pore Formation by the Bacterial Toxin Pneumolysin. Cell 2005, 121, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Förtsch, C.; Hupp, S.; Ma, J.; Mitchell, T.J.; Maier, E.; Benz, R.; Iliev, A.I.; Förtsch, C.; Hupp, S.; Ma, J.; et al. Changes in Astrocyte Shape Induced by Sublytic Concentrations of the Cholesterol-Dependent Cytolysin Pneumolysin Still Require Pore-Forming Capacity. Toxins 2011, 3, 43–62. [Google Scholar] [CrossRef] [PubMed]
- Iliev, A.I.; Djannatian, J.R.; Opazo, F.; Gerber, J.; Nau, R.; Mitchell, T.J.; Wouters, F.S. Rapid Microtubule Bundling and Stabilization by the Streptococcus Pneumoniae Neurotoxin Pneumolysin in a Cholesterol-Dependent, Non-Lytic and Src-Kinase Dependent Manner Inhibits Intracellular Trafficking. Mol. Microbiol. 2009, 71, 461–477. [Google Scholar] [CrossRef]
- Hupp, S.; Förtsch, C.; Graber, F.; Mitchell, T.J.; Iliev, A.I. Pneumolysin Boosts the Neuroinflammatory Response to Streptococcus Pneumoniae through Enhanced Endocytosis. Nat. Commun. 2022, 13, 5032. [Google Scholar] [CrossRef]
- Stringaris, A.K.; Geisenhainer, J.; Bergmann, F.; Balshusemann, C.; Lee, U.; Zysk, G.; Mitchell, T.J.; Keller, B.U.; Kuhnt, U.; Gerber, J.; et al. Neurotoxicity of Pneumolysin, a Major Pneumococcal Virulence Factor, Involves Calcium Influx and Depends on Activation of P38 Mitogen-Activated Protein Kinase. Neurobiol. Dis. 2002, 11, 355–368. [Google Scholar] [CrossRef]
- Wippel, C.; Maurer, J.; Förtsch, C.; Hupp, S.; Bohl, A.; Ma, J.; Mitchell, T.J.; Bunkowski, S.; Brück, W.; Nau, R.; et al. Bacterial Cytolysin during Meningitis Disrupts the Regulation of Glutamate in the Brain, Leading to Synaptic Damage. PLoS Pathog. 2013, 9, e1003380. [Google Scholar] [CrossRef]
- Nau, R.; Gerber, J.; Bunkowski, S.; Bruck, W. Axonal Injury, a Neglected Cause of CNS Damage in Bacterial Meningitis. Neurology 2004, 62, 509–511. [Google Scholar] [CrossRef]
- Pfister, H.W.; Borasio, G.D.; Dirnagl, U.; Bauer, M.; Einhäupl, K.M. Cerebrovascular Complications of Bacterial Meningitis in Adults. Neurology 1992, 42, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Devraj, G.; Guérit, S.; Seele, J.; Spitzer, D.; Macas, J.; Khel, M.I.; Heidemann, R.; Braczynski, A.K.; Ballhorn, W.; Günther, S.; et al. HIF-1α Is Involved in Blood–Brain Barrier Dysfunction and Paracellular Migration of Bacteria in Pneumococcal Meningitis. Acta Neuropathol. 2020, 140, 183. [Google Scholar] [CrossRef] [PubMed]
- Vergouwen, M.D.I.; Schut, E.S.; Troost, D.; Van De Beek, D. Diffuse Cerebral Intravascular Coagulation and Cerebral Infarction in Pneumococcal Meningitis. Neurocrit. Care 2010, 13, 217–227. [Google Scholar] [CrossRef]
- Marrie, T.J.; Myers, K.A. Thrombotic Microangiopathy Associated with Streptococcus Pneumoniae Bacteremia: Case Report and Review. Clin. Infect. Dis. 1993, 17, 1037–1040. [Google Scholar] [CrossRef]
- Ramos-Cabrer, P.; Campos, F.; Sobrino, T.; Castillo, J. Targeting the Ischemic Penumbra. Stroke 2011, 42, S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Bewersdorf, J.P.; Grandgirard, D.; Koedel, U.; Leib, S.L. Novel and Preclinical Treatment Strategies in Pneumococcal Meningitis. Curr. Opin. Infect. Dis. 2018, 31, 85–92. [Google Scholar] [CrossRef]
- Gruetter, R.; Novotny, E.J.; Boulware, S.D.; Rothman, D.L.; Mason, G.F.; Shulman, G.I.; Shulman, R.G.; Tamborlane, W.V. Direct Measurement of Brain Glucose Concentrations in Humans by 13C NMR Spectroscopy. Proc. Natl. Acad. Sci. USA 1992, 89, 1109. [Google Scholar] [CrossRef]
- Rinderknecht, H.; Ehnert, S.; Braun, B.; Histing, T.; Nussler, A.K.; Linnemann, C. The Art of Inducing Hypoxia. Oxygen 2021, 1, 46–61. [Google Scholar] [CrossRef]
- Hupp, S.; Tomov, N.S.; Bischoff, C.; Baronti, D.; Iliev, A.I. Easy to Build Cost-Effective Acute Brain Slice Incubation System for Parallel Analysis of Multiple Treatment Conditions. J. Neurosci. Methods 2022, 366, 109405. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, D.G.; Avshalumov, M.V.; Rice, M.E. Brain Edema Induced by in Vitro Ischemia: Causal Factors and Neuroprotection. J. Neurochem. 2003, 85, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Schurr, A.; Payne, R.S.; Rigor, B.M. Protection by Mk-801 against Hypoxia-, Excitotoxin-, and Depolarization-Induced Neuronal Damage in Vitro. Neurochem. Int. 1995, 26, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Silver, I.; Erecinska, M. Extracellular Glucose Concentration in Mammalian Brain: Continuous Monitoring of Changes during Increased Neuronal Activity and upon Limitation in Oxygen Supply in Normo-, Hypo-, and Hyperglycemic Animals. J. Neurosci. 1994, 14, 5068–5076. [Google Scholar] [CrossRef]
- Hupp, S.; Grandgirard, D.; Mitchell, T.J.; Leib, S.L.; Hathaway, L.J.; Iliev, A.I. Pneumolysin and the Bacterial Capsule of Streptococcus Pneumoniae Cooperatively Inhibit Taxis and Motility of Microglia. J. Neuroinflamm. 2019, 16, 105. [Google Scholar] [CrossRef] [PubMed]
- Los, F.C.O.; Kao, C.Y.; Smitham, J.; McDonald, K.L.; Ha, C.; Peixoto, C.A.; Aroian, R.V. RAB-5- and RAB-11-Dependent Vesicle-Trafficking Pathways Are Required for Plasma Membrane Repair after Attack by Bacterial Pore-Forming Toxin. Cell Host Microbe 2011, 9, 147–157. [Google Scholar] [CrossRef]
- Wertham, F. THE CEREBRAL LESIONS IN PURULENT MENINGITIS. Arch. Neurol. Psychiatry 1931, 26, 549–582. [Google Scholar] [CrossRef]
- Klobassa, D.S.; Zoehrer, B.; Paulke-Korinek, M.; Gruber-Sedlmayr, U.; Pfurtscheller, K.; Strenger, V.; Sonnleitner, A.; Kerbl, R.; Ausserer, B.; Arocker, W.; et al. The Burden of Pneumococcal Meningitis in Austrian Children between 2001 and 2008. Eur. J. Pediatr. 2014, 173, 871–878. [Google Scholar] [CrossRef]
- Snyder, R.D.; Stovring, J.; Cushing, A.H.; Davis, L.E.; Hardy, T.L. Cerebral Infarction in Childhood Bacterial Meningitis. J. Neurol. Neurosurg. Psychiatry 1981, 44, 581–585. [Google Scholar] [CrossRef]
- Leib, S.L.; Leppert, D.; Clements, J.; Täuber, M.G. Matrix Metalloproteinases Contribute to Brain Damage in Experimental Pneumococcal Meningitis. Infect. Immun. 2000, 68, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Goitein, K.J.; Tamir, I. Cerebral Perfusion Pressure in Central Nervous System Infections of Infancy and Childhood. J. Pediatr. 1983, 103, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, P.; Ahlm, C.; Ericsson, M.; Gothefors, L.; Naredi, S.; Koskinen, L.O.D. Reducing Intracranial Pressure May Increase Survival among Patients with Bacterial Meningitis. Clin. Infect. Dis. 2004, 38, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Orihuela, C.J.; Gao, G.; Francis, K.P.; Yu, J.; Tuomanen, E.I. Tissue-Specific Contributions of Pneumococcal Virulence Factors to Pathogenesis. J. Infect. Dis. 2004, 190, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Beurg, M.; Hafidi, A.; Skinner, L.; Cowan, G.; Hondarrague, Y.; Mitchell, T.J.; Dulon, D. The Mechanism of Pneumolysin-Induced Cochlear Hair Cell Death in the Rat. J. Physiol. 2005, 568, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Hupp, S.; Heimeroth, V.; Wippel, C.; Fortsch, C.; Ma, J.; Mitchell, T.J.; Iliev, A.I.; Förtsch, C.; Ma, J.; Mitchell, T.J.; et al. Astrocytic Tissue Remodeling by the Meningitis Neurotoxin Pneumolysin Facilitates Pathogen Tissue Penetration and Produces Interstitial Brain Edema. Glia 2012, 60, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Wolfmeier, H.; Radecke, J.; Schoenauer, R.; Koeffel, R.; Babiychuk, V.S.; Drücker, P.; Hathaway, L.J.; Mitchell, T.J.; Zuber, B.; Draeger, A.; et al. Active Release of Pneumolysin Prepores and Pores by Mammalian Cells Undergoing a Streptococcus Pneumoniae Attack. Biochim. Biophys. Acta 2016, 1860, 2498–2509. [Google Scholar] [CrossRef] [PubMed]
- Iliev, A.I.; Djannatian, J.R.; Nau, R.; Mitchell, T.J.; Wouters, F.S. Cholesterol-Dependent Actin Remodeling via RhoA and Rac1 Activation by the Streptococcus Pneumoniae Toxin Pneumolysin. Proc. Natl. Acad. Sci. USA 2007, 104, 2897–2902. [Google Scholar] [CrossRef] [PubMed]
- Idone, V.; Tam, C.; Goss, J.W.; Toomre, D.; Pypaert, M.; Andrews, N.W. Repair of Injured Plasma Membrane by Rapid Ca2+-Dependent Endocytosis. J. Cell Biol. 2008, 180, 905–914. [Google Scholar] [CrossRef]
- Farber, J.L. Biology of Disease: Membrane Injury and Calcium Homeostasis in the Pathogenesis of Coagulative Necrosis. Lab. Investig. 1982, 47, 114–123. [Google Scholar]
- Schanne, F.A.X.; Kane, A.B.; Young, E.E.; Farber, J.L. Calcium Dependence of Toxic Cell Death: A Final Common Pathway. Science 1979, 206, 700–702. [Google Scholar] [CrossRef] [PubMed]
- Baines, C.P.; Kaiser, R.A.; Purcell, N.H.; Blair, N.S.; Osinska, H.; Hambleton, M.A.; Brunskill, E.W.; Sayen, M.R.; Gottlieb, R.A.; Dorn, G.W.; et al. Loss of Cyclophilin D Reveals a Critical Role for Mitochondrial Permeability Transition in Cell Death. Nature 2005, 434, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.; Cabanes, D.; Sarmento Mesquita, F.; Sousa, S. Mechanisms Protecting Host Cells against Bacterial Pore-Forming Toxins. Cell. Mol. Life Sci. 2019, 76, 1319. [Google Scholar] [CrossRef] [PubMed]
- Nollmann, M.; Gilbert, R.; Mitchell, T.; Sferrazza, M.; Byron, O. The Role of Cholesterol in the Activity of Pneumolysin, a Bacterial Protein Toxin. Biophys. J. 2004, 86, 3141–3151. [Google Scholar] [CrossRef] [PubMed]
- Botto, L.; Beretta, E.; Bulbarelli, A.; Rivolta, I.; Lettiero, B.; Leone, B.E.; Miserocchi, G.; Palestini, P. Hypoxia-Induced Modifications in Plasma Membranes and Lipid Microdomains in A549 Cells and Primary Human Alveolar Cells. J. Cell. Biochem. 2008, 105, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Zuniga-Hertz, J.P.; Patel, H.H. The Evolution of Cholesterol-Rich Membrane in Oxygen Adaption: The Respiratory System as a Model. Front. Physiol. 2019, 10, 1340. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Bensinger, S.J. Reprogramming Cholesterol Metabolism in Macrophages and Its Role in Host Defense against Cholesterol-Dependent Cytolysins. Cell. Mol. Immunol. 2022, 19, 327–336. [Google Scholar] [CrossRef]
- Yoshimura, S.I.; Banno, Y.; Nakashima, S.; Takenaka, K.; Sakai, H.; Nishimura, Y.; Sakai, N.; Shimizu, S.; Eguchi, Y.; Tsujimoto, Y.; et al. Ceramide Formation Leads to Caspase-3 Activation during Hypoxic PC12 Cell Death. Inhibitory Effects of Bcl-2 on Ceramide Formation and Caspase-3 Activation. J. Biol. Chem. 1998, 273, 6921–6927. [Google Scholar] [CrossRef]
- Kristian, T. Metabolic Stages, Mitochondria and Calcium in Hypoxic/Ischemic Brain Damage. Cell Calcium 2004, 36, 221–233. [Google Scholar] [CrossRef]
- Keyel, P.A.; Heid, M.E.; Salter, R.D. Macrophage Responses to Bacterial Toxins: A Balance between Activation and Suppression. Immunol. Res. 2011, 50, 118–123. [Google Scholar] [CrossRef]
- Prinz, M.; Priller, J.; Sisodia, S.S.; Ransohoff, R.M. Heterogeneity of CNS Myeloid Cells and Their Roles in Neurodegeneration. Nat. Neurosci. 2011, 14, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, T.J.; Walker, J.A.; Saunders, F.K.; Andrew, P.W.; Boulnois, G.J. Expression of the Pneumolysin Gene in Escherichia Coli: Rapid Purification and Biological Properties. Biochim. Biophys. Acta 1989, 1007, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Norcross, E.W.; Quincy C Moore, I.; Onwubiko, C.; King, L.B.; Fratkin, J.; Marquart, M.E.; Moore, Q.C.; Onwubiko, C.; King, L.B.; et al. A Comparison of Pneumolysin Activity and Concentration in Vitro and in Vivo in a Rabbit Endophthalmitis Model. Clin. Ophthalmol. 2008, 2, 793–800. [Google Scholar] [CrossRef] [PubMed][Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffet, M.S.; Tomov, N.S.; Hupp, S.; Mitchell, T.J.; Iliev, A.I. Glucose and Oxygen Levels Modulate the Pore-Forming Effects of Cholesterol-Dependent Cytolysin Pneumolysin from Streptococcus pneumoniae. Toxins 2024, 16, 232. https://doi.org/10.3390/toxins16060232
Hoffet MS, Tomov NS, Hupp S, Mitchell TJ, Iliev AI. Glucose and Oxygen Levels Modulate the Pore-Forming Effects of Cholesterol-Dependent Cytolysin Pneumolysin from Streptococcus pneumoniae. Toxins. 2024; 16(6):232. https://doi.org/10.3390/toxins16060232
Chicago/Turabian StyleHoffet, Michelle Salomé, Nikola S. Tomov, Sabrina Hupp, Timothy J. Mitchell, and Asparouh I. Iliev. 2024. "Glucose and Oxygen Levels Modulate the Pore-Forming Effects of Cholesterol-Dependent Cytolysin Pneumolysin from Streptococcus pneumoniae" Toxins 16, no. 6: 232. https://doi.org/10.3390/toxins16060232
APA StyleHoffet, M. S., Tomov, N. S., Hupp, S., Mitchell, T. J., & Iliev, A. I. (2024). Glucose and Oxygen Levels Modulate the Pore-Forming Effects of Cholesterol-Dependent Cytolysin Pneumolysin from Streptococcus pneumoniae. Toxins, 16(6), 232. https://doi.org/10.3390/toxins16060232




