An Algoclay-Based Decontaminant Decreases Exposure to Aflatoxin B1, Ochratoxin A, and Deoxynivalenol in a Toxicokinetic Model, as well as Supports Intestinal Morphology, and Decreases Liver Oxidative Stress in Broiler Chickens Fed a Diet Naturally Contaminated with Deoxynivalenol
Abstract
1. Introduction
2. Results
2.1. Experiment 1: Toxicokinetic Assay
2.2. Experiment 2: Naturally Contaminated Diets
2.2.1. Growth Performance
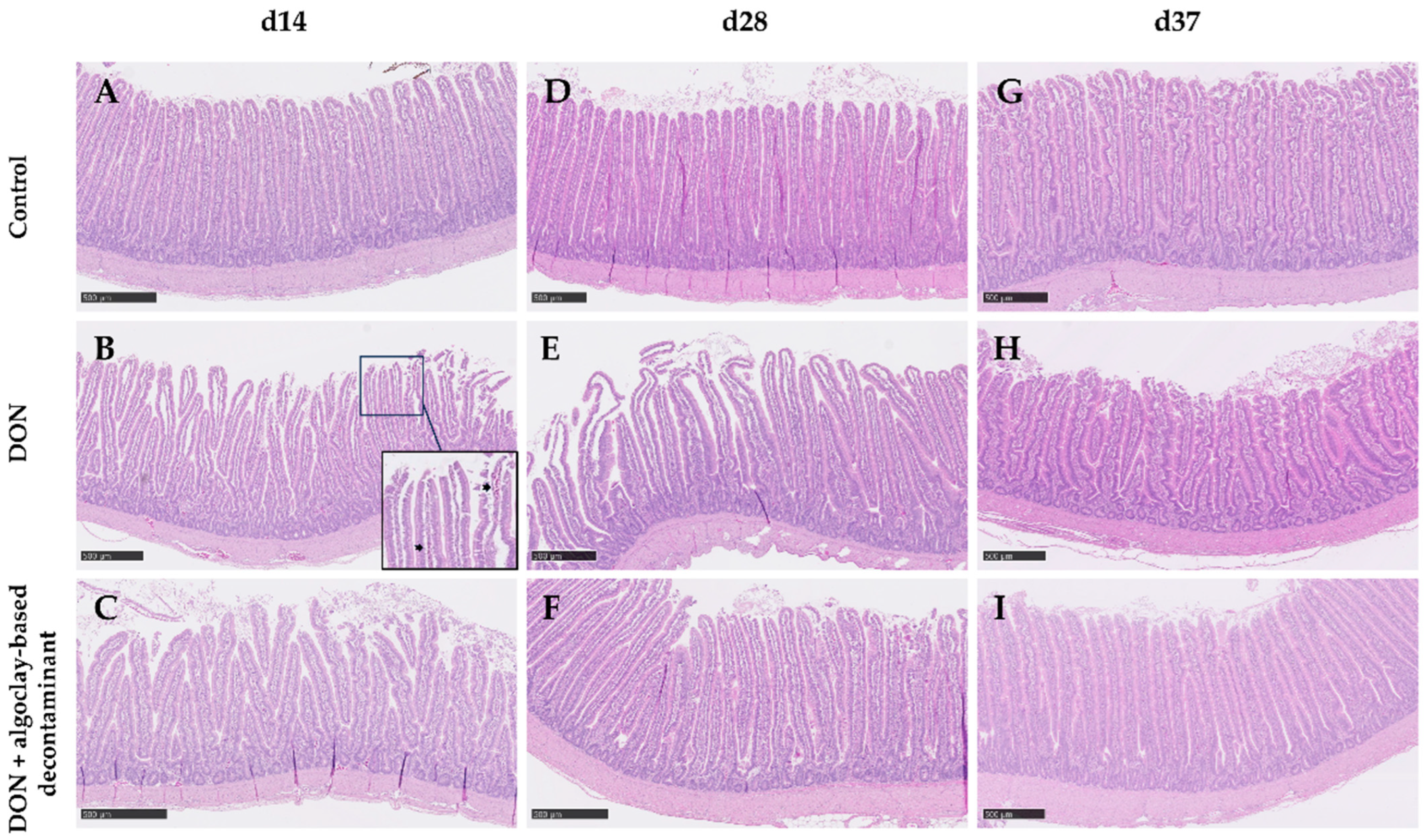
2.2.2. Intestinal Analysis
2.2.3. mRNA Expression of Markers for Oxidative Stress and Metabolism in the Liver
2.2.4. DON and DON-3 Sulphate in the Serum
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Experiment 1: Toxicokinetic Assay
5.1.1. Chemicals, Products, and Reagents
5.1.2. Broiler Chickens
5.1.3. Mycotoxin Administration and Blood Sampling
5.1.4. Analysis Method
5.2. Experiment 2: Naturally Contaminated Diet
5.2.1. Broiler Chickens
5.2.2. Diets and Experimental Design
5.2.3. Growth Performance
5.2.4. Jejunum Morphometry and Scoring
5.2.5. mRNA Expression of Markers for Liver Function
5.2.6. Serum and Plasma Analysis of DON and DON-3S
5.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Declaration of AI-Assisted Technology in the Writing Process
Abbreviations
| ACTB | β-actin |
| AFB1 | Aflatoxin B1 |
| AMEn: | Nitrogen-corrected apparent metabolizable energy |
| ANOVA | Analysis of variance |
| AOAC | Association of Official Analytical Chemists |
| AUC | Area under the curve |
| BW | Body weight |
| BWG | Body weight gain |
| Ca | Calcium |
| CD | Crypt depth |
| cDNA | Complementary deoxyribonucleic acid |
| Cl | Chlorine |
| Cmax | Maximal plasma concentration |
| CPT1 | Carnitine palmitoyltransferase-1 |
| dEB | Dietary electrolyte balance |
| DM | Dry matter |
| DON | Deoxynivalenol |
| DON-3G | Deoxynivalenol-3-Glucoside |
| DON-3S | Deoxynivalenol-3-sulphate |
| EFSA | European Food Safety Authority |
| EU | European Union |
| FCR | Feed conversion ratio |
| FI | Feed intake |
| GOI | Gene of interest |
| GSS | glutathione synthetase |
| HKG | Housekeeping genes |
| HMGCR | 3-hydroxy-3-methylglutaryl-coenzyme A reductase |
| HPRT | Hypoxanthine phosphoribosyltransferase |
| iNOS | inducible nitric oxide synthase |
| K | Potassium |
| Mg | Magnesium |
| Na | Sodium |
| OTA | Ochratoxin A |
| P | Phosphorus |
| p.a. | Post-administration |
| PAS | Periodic Acid–Schiff |
| qPCR | Quantitative polymerase chain reaction |
| Relative F | relative oral bioavailability |
| RNA | Ribonucleic acid |
| SEM | Standard error of the mean |
| TK | Toxicokinetics |
| Tmax | Time at maximal plasma concentration. |
| VH | Villus height |
| VH:CD ratio | Villus height:Crypt depth ratio |
References
- Osselaere, A.; Santos, R.; Hautekiet, V.; De Backer, P.; Chiers, K.; Ducatelle, R.; Croubels, S. Deoxynivalenol impairs hepatic and intestinal gene expression of selected oxidative stress, tight junction and inflammation proteins in broiler chickens, but addition of an adsorbing agent shifts the effects to the distal parts of the small intestine. PLoS ONE 2013, 8, e69014. [Google Scholar] [CrossRef] [PubMed]
- EC-European Commission. Commission Recommendation 2016/1319/EC of 29 July 2016 Amending Commission Recommendation 2006/576/EC on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union. 2016, L208, 58–60. [Google Scholar]
- Santos, R.R.; Oosterveer-van der Doelen, M.A.M.; Tersteeg-Zijderveld, M.H.G.; Molist, F.; Mezes, M.; Gehring, R. Susceptibility of broiler chickens. Animals 2021, 11, 989. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.R.; van Eerden, E. Impaired performance of broiler chickens fed diets naturally contaminated with moderate levels of deoxynivalenol. Toxins 2021, 13, 170. [Google Scholar] [CrossRef] [PubMed]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Leblanc, J.-C.; Nielsen, E.; et al. Scientific Opinion on the assessment of information as regards the toxicity of deoxynivalenol for horses and poultry. EFSA J. 2023, 21, e07806. [Google Scholar]
- Santos, R.R.; Awati, A.; Roubos-van den Hil, P.J.; van Kempen, T.A.T.G.; Tersteg-Zijderveld, M.H.G.; Koolmees, P.A.; Smits, C.; Fink-Gremmels, J. Effects of a feed additive blend on broilers challenged with heat stress. Avian Pathol. 2019, 48, 582–601. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Oswald, I.P. Effect of deoxynivalenol and other Type B trichothecenes on the intestine: A review. Toxins 2014, 6, 1615–1643. [Google Scholar] [CrossRef] [PubMed]
- Kubena, L.F.; Edrington, T.S.; Harvey, R.B.; Buckley, S.A.; Phillips, T.D.; Rottinghaus, G.E.; Casper, H.H. Individual and combined effects of fumonisin b1 present in Fusarium moniliforme culture material and T-2 Toxin or deoxynivalenol in broiler chicks. Poult. Sci. 1997, 76, 1239–1247. [Google Scholar] [CrossRef]
- Kulcsar, S.; Kovesi, B.; Balogh, K.; Zandoki, E.; Ancsin, Z.; Marta, B.E.; Mezes, M. Effects of Fusarium mycotoxin exposure on lipid peroxidation and glutathione redox system in the liver of laying hens. Antioxidants 2021, 10, 1313. [Google Scholar] [CrossRef]
- Del Vesco, A.P.; Khatlab, A.S.; Goes, E.S.R.; Utsunomiya, K.S.; Vieira, J.S.; Oliveira Neto, A.R. Age-related oxidative stress and antioxidant capacity in heat-stressed broilers. Animal 2017, 11, 1783–1790. [Google Scholar] [CrossRef]
- Adesso, S.; Autore, G.; Quaroni, A.; Popolo, A.; Severino, L.; Marzocco, S. The food contaminants nivalenol and deoxynivalenol induce inflammation in intestinal epithelial cells by regulating reactive oxygen species release. Nutrients 2017, 9, 1343. [Google Scholar] [CrossRef]
- Yarru, L.P.; Settivari, R.S.; Gowda, N.K.S.; Antoniou, E.; Ledoux, D.R.; Rottinghaus, G.E. Effects of turmeric (Curcuma longa) on the expression of hepatic genes associated with biotransformation, antioxidant, and immune systems in broiler chicks fed aflatoxin. Poult. Sci. 2009, 88, 2620–2627. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.T.; Yudell, B.E.; Loor, J.J. Regulation of energy metabolism by long-chain fatty acids. Prog. Lipid Res. 2014, 53, 124–144. [Google Scholar] [CrossRef]
- Sanchez, A.L.B.; Wang, Q.; Thiam, M.; Wang, Z.; Zhang, J.; Zhang, Q.; Zhang, N.; Li, Q.; Wen, J.; Zhao, G. Liver transcriptome response to heat stress in Beijing You and Guang Ming broilers. Genes 2022, 13, 416. [Google Scholar] [CrossRef] [PubMed]
- Maresca, M.; Mahfoud, R.; Garmy, N.; Fantini, J. The mycotoxin deoxynivalenol affects nutrient absorption in human intestinal epithelial cells. J. Nutr. 2002, 132, 2723–2731. [Google Scholar] [CrossRef] [PubMed]
- Barbouche, R.; Gaigé, S.; Airalt, C.; Poirot, K.; Dallaporta, M.; Troadec, J.D. The food contaminant deoxynivalenol provokes metabolic impairments resulting in non-alcoholic fatty liver (NAFL) in mice. Sci. Rep. 2020, 10, 12072. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Duan, Y.; Hu, Y.; Sun, L.; Wang, S.; Fu, W.; Ni, Y.; Zhao, R. Exogenous administration of chronic corticosterone affects hepatic cholesterol metabolism in broiler chickens showing long or short tonic immobility. Comp. Bioch Physiol. A 2016, 191, 53–58. [Google Scholar] [CrossRef] [PubMed]
- European Union Register of Feed Additives Pursuant to Regulation (EC) no 1831/2003 Appendixes 3e & 4(I). Annex I: List of Additives. Available online: http://ec.europa.eu/food/food/animalnutrition/feedadditives/comm_register_feed_additives_1831-03.pdf (accessed on 4 January 2015).
- Burt, R. Soil Survey Investigations Report No 45; USDANRCS: Lincoln, NE, USA, 2011.
- Jaynes, W.F.; Zartman, R.E.; Hudnall, W.H. Aflatoxin B1 adsorption by clays from water and corn meal. Appl. Clay Sci. 2007, 36, 197–205. [Google Scholar] [CrossRef]
- Laza, A.L. Développement D’une Nouvelle Alternative Naturelle en Alimentation et Hygiène Animale à Base D’argile et D’algues. Ph.D. Thesis, de Haute Alsace, Mulhouse, France, 2006. [Google Scholar]
- Balusson, H.; Laza-Knoerr, A.L.; Balusson, S.; Blouin, S.; Vintan, D. Method for Preparing an Intercalated and/or Exfoliated Organophillic Clay from Clay and Macroalgae, Corresponding Fertiliser Product, Food Supplement for Animals and Fish Feed. Patent number EP2674397, 20 December 2013. [Google Scholar]
- Boudergue, C.; Burel, C.; Dragacci, S.; Favrot, M.C.; Fremy, J.M.; Massimi, C.; Prigent, P.; Debongnie, P.; Pussemier, L.; Boudra, H.; et al. Review of Mycotoxin-Detoxifying Agents Used as Feed Additives: Mode of Action, Efficacy and Feed/Food Safety; EFSA Supporting Publications: Parma, Italy, 2009; Volume 6, p. 22. 192p. [Google Scholar]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Statement on the establishment of guidelines for the assessment of additives from the functional group ‘substances for reduction of the contamination of feed by mycotoxins’. EFSA J. 2010, 8, 1693. [Google Scholar] [CrossRef]
- Lauwers, M.; De Baere, S.; Letor, B.; Rychlik, M.; Croubels, S.; Devreese, M. Multi LC-MS/MS and LC-HRMS methods for determination of 24 mycotoxins including major phase I and II biomarker metabolites in biological matrices from pigs and broiler chickens. Toxins 2019, 11, 171. [Google Scholar] [CrossRef]
- Devreese, M.; Antonissen, G.; Broekaert, N.; De Mil, T.; De Baere, S.; Vanhaecke, L.; De Backer, P.; Croubels, S. Toxicokinetic study and oral bioavailability of deoxynivalenol in turkey poults, and comparative biotransformation between broilers and turkeys. World Mycotoxin J. 2015, 8, 533–539. [Google Scholar] [CrossRef]
- Devreese, M.; Croubels, S.; De Baere, S.; Gehring, R.; Antonissen, G. Comparative toxicokinetics and plasma protein binding of ochratoxin A in four avian species. Agric. Food Chem. 2018, 66, 2129–2135. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.; Chakor, A.; Creepy, E.E.; Kane, A.; Roschenthaler, R.; Dirheimer, G. Evidence for an enterohepatic circulation of ochratoxin A in mice. Toxicology 1988, 48, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; Bastos, M.L.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; Gropp, J.; et al. Safety and efficacy of bentonite as a feed additive for all animal species. EFSA J. 2017, 15, 5096. [Google Scholar]
- Jaynes, W.F.; Zartman, R.E. Aflatoxin Toxicity Reduction in Feed by Enhanced Binding to Surface-Modified Clay Additives. Toxins 2011, 3, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Rejeb, R.; Baere, S.; Devreese, M.; Ducatelle, R.; Croubels, S.; Ayed, M.H.; Ghorbal, A.; Antonissen, G. Calcination improves the in vivo efficacy of a montmorillonite clay to bind aflatoxin G1 in broiler chickens: A toxicokinetic approach. Toxins 2020, 18, 660. [Google Scholar] [CrossRef]
- Joannis-Cassan, C.; Tozlovanu, M.; Hadjeba-Medjdoub, K.; Ballet, N.; Pfohl-Leszkowicz, A. Binding of zearalenone, aflatoxin B1, and ochratoxin A by yeast-based products: A method for quantification of adsorption performance. J. Food Prot. 2011, 74, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, M.; Masek, A. Saccharomyces cerevisiae cell wall components as tools for ochratoxin a decontamination. Toxins 2015, 7, 1151–1162. [Google Scholar] [CrossRef]
- Sabater-Vilar, M.; Malekinejad, H.; Selman, M.H.J.; van der Doelen, M.A.M.; Fink-Gremmels, J. In vitro assessment of adsorbents aiming to prevent deoxynivalenol and zearalenone mycotoxicosis. Mycopathologia 2007, 163, 81–90. [Google Scholar] [CrossRef]
- Awad, W.A.; Ghareeb, K.; Bohm, J.; Zentek, J. Decontamination and detoxification strategies for the Fusarium mycotoxin deoxynivalenol in animal feed and the effectiveness of microbial biodegradation. Food Addit. Contam. 2010, 27, 510–520. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Liu, S.; Wu, Y.; Zhou, Q.; Zhang, Y.; Zheng, X.; Han, Y.; Xie, C.; Liu, N. Adsorption of deoxynivalenol by pillared montmorillonite. Food Chem. 2021, 343, 128391. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef]
- Aviagen, N.L. Ross 308/Ross 308 FF Broiler: Performance Objectives; Aviagen, Inc.: Huntsville, AL, USA, 2022. [Google Scholar]
- OECD-FAO. Agricultural Outlook 2022–2031; OECD-FAO: Rome, Italy; Paris, France, 2022. [Google Scholar]
- Osei, E.; Jafri, S.H.; Saleh, A.; Gassman, P.W.; Gallego, O. Simulated climate changes impacts on corn and soybean yields in Buchanan County, Iowa. Agriculture 2023, 13, 268. [Google Scholar] [CrossRef]
- Bryla, M.; Pierzgalski, A.; Zapasnik, A.; Uwineza, P.A.; Ksieniewicz-Wozniak, E.; Modrzewska, M.; Waskiewicz, A. Recent research on Fusarium mycotoxins in maize—A review. Foods 2022, 11, 3465. [Google Scholar] [CrossRef]
- Colovic, R.; Puvaca, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Duragic, O.; Kos, J.; Pinotti, L. Decontamination of mycotoxin-contaminated feedstuffs and compound feed. Toxins 2019, 11, 617. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, X.; Chai, X.; Jiao, Y.; Sun, J.; Wang, S.; Yu, H.; Feng, X. Curcumin mitigates oxidative damage in broiler liver and ileum caused by AFB1-contaminated feed through Nrf2 signaling pathway. Animals 2024, 14, 409. [Google Scholar] [CrossRef]
- Weaver, A.C.; Weaver, D.M.; Adams, N.; Yiannikouris, A. Use of yeast cell wall extract for growing pigs consuming feed contaminated with mycotoxins below or above regulatory guidelines: A meta-analysis with meta-regression. Toxins 2023, 15, 596. [Google Scholar] [CrossRef]
- Wan, S.; Sun, N.; Li, H.; Khan, A.; Zheng, X.; Sun, Y.; Fan, R. Deoxynivalenol damages the intestinal barrier and biota of the broiler chickens. BMC Vet. Res. 2022, 18, 311. [Google Scholar] [CrossRef]
- Wang, A.; Hogan, N.S. Performance effects of feed-borne Fusarium mycotoxins on broiler chickens: Influences of timing and duration of exposure. Anim. Nutr. 2019, 5, 32–40. [Google Scholar] [CrossRef]
- Moore, R.; Carlson, S.; Madara, J.L. Villus contraction aids repair of intestinal epithelium after injury. Am. J. Physiol. 1989, 257, 274–283. [Google Scholar] [CrossRef]
- Antonissen, G.; Van Immerseel, F.; Pasmans, F.; Ducatelle, R.; Haesebrouck, F.; Timbermont, L.; Verlinden, M.; Janssens, G.P.J.; Eeckhaut, V.; Eeckhout, M.; et al. The mycotoxin deoxynivalenol predisposes for the development of Clostridium perfringens-induced necrotic enteritis in broiler chickens. PLoS ONE 2014, 9, e108775. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.R.; Ravindran, V.; Svihus, B. Pelleting of broiler diets: An overview with emphasis on pellet quality and nutritional value. Anim. Feed Sci. Technol. 2013, 179, 1–23. [Google Scholar] [CrossRef]
- Nakade, M.; Pelyhe, C.; Kovesi, B.; Balogh, K.; Kovacs, B.; Szabo-Fodor, J.; Zandoki, E.; Mezes, M.; Erdelyi, M. Short-term effects of T-2 toxin or deoxynivalenol on glutathione status and expression of its regulatory genes in chicken. Acta Vet. Hung. 2018, 66, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Devreese, M.; Osselaere, A.; Goossens, J.; Vandenbroucke, V.; De Baere, S.; Eeckhout, M.; De Backer, P.; Croubels, S. New bolus models for in vivo efficacy testing of mycotoxin-detoxifying agents in relation to EFSA guidelines, assessed using deoxynivalenol in broiler chickens. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Lauwers, M.; Croubels, S.; Letor, B.; Gougolias, C.; Devreese, M. Biomarkers for exposure as a tool for efficacy testing of a mycotoxin detoxifier in broiler chickens and pigs. Toxins 2019, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.R.; Awati, A.; Roubos-van den Hil, P.J.; Tersteeg-Zijderveld, M.H.; Koolmees, P.A.; Fink-Gremmels, J. Quantitative histo-morphometric analysis of heat-stress-related damage in the small intestine of broiler chickens. Avian Pathol. 2015, 44, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Varasteh, S.; Braber, S.; Akbari, P.; Garssen, J.; Fink-Gremmels, J. Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto-oligosaccharides. PLoS ONE 2015, 10, e0138975. [Google Scholar] [CrossRef] [PubMed]
- Milanova, A.; Santos, R.R.; Lashev, L.; Koinarski, V.; Fink-Gremmels, J. Influence of experimentally induced Eimeria tenella infection on gene expression of some host response factors in chickens. Bulg. J. Vet. Med. 2016, 19, 47–56. [Google Scholar] [CrossRef]
- Dridi, S.; Decuypere, E.; Buyse, J. Cerulenin upregulates heat shock protein-70 gene expression in chicken muscle. Poult. Sci. 2013, 92, 2745–2753. [Google Scholar] [CrossRef]
- Hu, Y.; Feng, Y.; Ding, Z.; Lv, L.; Sui, Y.; Sun, Q.; Abobaker, H.; Cai, D.; Zhao, R. Maternal betaine supplementation decreases hepatic cholesterol deposition in chicken offspring with epigenetic modulation of SREBP2 and CYP7A1 genes. Poult. Sci. 2020, 99, 3111–3120. [Google Scholar] [CrossRef] [PubMed]

| Toxicokinetic Parameter | Mycotoxins | Mycotoxins + Algoclay-Based Decontaminant | p-Value | SEM |
|---|---|---|---|---|
| AUC0–8h (h.ng/mL) | 9.44 b | 3.39 a | <0.001 | 0.98 |
| Cmax (ng/mL) | 8.65 b | 2.07 a | <0.01 | 1.72 |
| Tmax (h) | 0.26 b | 3.44 a | 0.03 | 1.21 |
| Relative F (%) | 100 | 35.9 |
| Toxicokinetic Parameter | Mycotoxins | Mycotoxins + Algoclay-Based Decontaminant | p-Value | SEM |
|---|---|---|---|---|
| AUC0–24h (h.ng/mL) | 205.99 b | 114.74 a | <0.001 | 18.48 |
| Cmax (ng/mL) | 31.45 | 26.48 | 0.37 | 5.38 |
| Tmax (h) | 3.28 | 1.75 | 0.31 | 1.45 |
| Relative F (%) | 100 | 55.7 |
| Toxicokinetic Parameter | Mycotoxins | Mycotoxins + Algoclay-Based Decontaminant | p-Value | SEM |
|---|---|---|---|---|
| AUC0–12h (response.h) | 760.9 b | 457.6 a | 0.01 | 92.9 |
| Cmax (response) | 346.1 | 270.1 | 0.48 | 105.0 |
| Tmax (h) | 1.31 | 0.91 | 0.25 | 0.33 |
| Relative F (%) | 100 | 60.1 |
| Diets | p-Value | SEM | |||
|---|---|---|---|---|---|
| Control | DON Diet | DON + Algoclay-Based Decontaminant | |||
| d0–14 | |||||
| BW d14 | 534 | 550 | 557 | 0.06 | 6.4 |
| BWG (g) | 494 | 510 | 517 | 0.06 | 6.38 |
| FI (g) | 553 | 549 | 554 | 0.89 | 7.33 |
| FCR (g/g) | 1.119 b | 1.076 a | 1.071 a | <0.0001 | 0.0064 |
| Mortality (%) | 1.0 | 2.0 | 0.5 | 0.49 | 0.88 |
| d14–28 | |||||
| BW d28 | 1774 a | 1859 b | 1870 b | 0.01 | 19.9 |
| BWG (g) | 1240 a | 1309 b | 1312 b | 0.01 | 15.6 |
| FI (g) | 1692 | 1753 | 1749 | 0.08 | 19.3 |
| FCR (g/g) | 1.366 b | 1.340 a | 1.332 a | 0.01 | 0.0070 |
| Mortality (%) | 0.5 | 1.0 | 1.5 | 0.58 | 0.66 |
| d28–37 | |||||
| BW d37 | 2694 | 2762 | 2773 | 0.12 | 27.6 |
| BWG (g) | 920 | 903 | 903 | 0.78 | 19.6 |
| FI (g) | 1566 | 1599 | 1575 | 0.56 | 22.0 |
| FCR (g/g) | 1.705 | 1.775 | 1.749 | 0.14 | 0.0234 |
| Mortality (%) | 0.5 | 1.5 | 1.5 | 0.67 | 0.88 |
| d0–37 | |||||
| BWG (g) | 2654 | 2722 | 2733 | 0.12 | 27.6 |
| FI (g) | 3811 | 3901 | 3877 | 0.23 | 36.9 |
| FCR (g/g) | 1.436 | 1.433 | 1.419 | 0.16 | 0.0062 |
| Mortality (%) | 2.0 | 4.5 | 3.5 | 0.49 | 1.45 |
| Diets | p-Value | SEM | |||
|---|---|---|---|---|---|
| Control | DON Diet | DON + Algoclay-Based Decontaminant | |||
| d14 | |||||
| Villus height (µm) | 730 | 613 | 703 | 0.19 | 45.2 |
| Crypt depth (µm) | 182 | 178 | 192 | 0.62 | 10.2 |
| VH:CD | 4.17 | 3.58 | 3.77 | 0.16 | 0.21 |
| Villus area (mm2) | 96 | 77 | 78 | 0.30 | 9.21 |
| Score | 0.54 | 0.83 | 0.84 | 0.13 | 0.11 |
| d28 | |||||
| Villus height (µm) | 990 | 765 | 873 | 0.15 | 76.4 |
| Crypt depth (µm) | 200 | 189 | 224 | 0.19 | 13.2 |
| VH:CD | 5.06 | 4.27 | 4.18 | 0.13 | 0.32 |
| Villus area (mm2) | 135 | 116 | 152 | 0.26 | 15.1 |
| Score | 0.51 a | 1.14 b | 0.68 a,b | 0.04 | 0.16 |
| d37 | |||||
| Villus height (µm) | 1187 b | 884 a | 1030 a,b | 0.01 | 59.3 |
| Crypt depth (µm) | 224 | 225 | 201 | 0.50 | 15.9 |
| VH:CD | 5.58 b | 4.13 a | 5.41 b | 0.02 | 0.33 |
| Villus area (mm2) | 96 | 77 | 78 | 0.67 | 24.4 |
| Score | 0.69 | 0.93 | 0.53 | 0.24 | 0.16 |
| Diets | p-Value | SEM | |||
|---|---|---|---|---|---|
| Control | DON Diet | DON + Algoclay-Based Decontaminant | |||
| d14 | |||||
| GSS | 1.00 | 0.84 | 0.93 | 0.61 | 0.12 |
| iNOS | 1.00 | 1.06 | 0.95 | 0.66 | 0.09 |
| CPT1 | 1.00 | 0.99 | 1.10 | 0.74 | 0.11 |
| d28 | |||||
| GSS | 1.00 | 0.69 | 0.57 | 0.68 | 0.35 |
| iNOS | 1.00 | 1.03 | 1.16 | 0.81 | 0.17 |
| CPT1 | 1.00 | 0.68 | 1.29 | 0.11 | 0.19 |
| d37 | |||||
| GSS | 1.00 a | 2.01 b | 1.05 a | 0.01 | 0.20 |
| iNOS | 1.00 | 1.09 | 0.89 | 0.27 | 0.09 |
| CPT1 | 1.00 | 1.32 | 1.20 | 0.79 | 0.32 |
| Mycotoxins (mg/kg) | Control | DON | DON + Algoclay-Based Decontaminant |
|---|---|---|---|
| Starter (d0–14) | |||
| DON | 0.19 | 2.82 | 2.91 |
| 3 + 15 Ac-DON | <LOQ | 0.06 | 0.10 |
| DON-3G | 0.06 | 0.40 | 0.51 |
| Zearalenone | <LOQ | 0.16 | 0.20 |
| Fumonisins B1 + B2 | 0.05 | 0.29 | 0.29 |
| Beauvericin | 0.01 | 0.02 | 0.02 |
| Enniatin B | 0.02 | 0.02 | 0.02 |
| Enniatin B1 | 0.01 | 0.01 | 0.01 |
| Grower (d14–28) | |||
| DON | 0.19 | 2.69 | 2.66 |
| 3 + 15 Ac-DON | <LOQ | 0.08 | 0.11 |
| DON-3G | 0.04 | 0.37 | 0.44 |
| Zearalenone | <LOQ | 0.26 | 0.23 |
| Fumonisins B1 + B2 | <LOQ | 0.19 | 0.09 |
| Beauvericin | 0.01 | 0.02 | 0.02 |
| Enniatin B | 0.02 | 0.02 | 0.02 |
| Enniatin B1 | 0.01 | 0.01 | 0.01 |
| Finisher (d28–37) | |||
| DON | 0.25 | 2.71 | 2.81 |
| 3 + 15 Ac-DON | <LOQ | - | 0.11 |
| DON-3G | 0.06 | 0.49 | 0.49 |
| Zearalenone | <LOQ | 0.23 | 0.24 |
| Fumonisins B1 + B2 | 0.04 | 0.08 | 0.13 |
| Beauvericin | 0.01 | 0.02 | 0.02 |
| Enniatin B | 0.03 | 0.03 | 0.03 |
| Enniatin B1 | 0.01 | 0.01 | 0.01 |
| Ingredients (%) | Starter (d0–14) | Grower (d14–28) | Finisher (d28–37) |
|---|---|---|---|
| Corn | 45.00 | 45.00 | 45.00 |
| Soybean meal | 33.55 | 29.55 | 24.81 |
| Wheat | 9.71 | 12.50 | 16.55 |
| Barley | 5.00 | 5.00 | 5.00 |
| Soybean oil | 0.00 | 0.08 | 0.34 |
| Animal fat | 3.23 | 4.36 | 4.79 |
| Salt | 0.36 | 0.23 | 0.11 |
| Limestone | 0.80 | 0.80 | 0.82 |
| Monocalcium Phosphate | 1.13 | 1.15 | 1.19 |
| Sodium Bicarbonate | 0.00 | 0.19 | 0.37 |
| Lysine HCl | 0.26 | 0.24 | 0.21 |
| DL-Methionine | 0.31 | 0.26 | 0.20 |
| Threonine | 0.08 | 0.06 | 0.06 |
| Tryptophane | 0.002 | 0.00 | 0.00 |
| Valine | 0.03 | 0.01 | 0.01 |
| Choline chloride | 0.06 | 0.06 | 0.06 |
| Vitamin and mineral premix | 0.50 | 0.50 | 0.50 |
| Nutrients | |||
| AMEn, kcal/kg | 2.900 | 3.000 | 3.075 |
| DM, g/kg | 880 | 881 | 882 |
| Ash, g/kg | 52.51 | 49.94 | 47.37 |
| Crude protein, g/kg | 218 | 201 | 182 |
| Crude fat, g/kg | 57.90 | 69.99 | 77.01 |
| Crude fiber, g/kg | 22.81 | 22.11 | 21.44 |
| Starch, g/kg | 368 | 385 | 408 |
| Sugar, g/kg | 31.18 | 29.33 | 27.34 |
| Ca, g/kg | 6.00 | 5.95 | 5.95 |
| P, g/kg | 6.12 | 6.01 | 5.93 |
| Mg, g/kg | 1.64 | 1.56 | 1.47 |
| K, g/kg | 9.51 | 8.74 | 7.87 |
| Na, g/kg | 1.50 | 1.50 | 1.50 |
| Cl, g/kg | 3.26 | 2.46 | 1.66 |
| dEB, meq | 217 | 220 | 220 |
| Genes | Primer Sequence | Annealing Tº | Reference |
|---|---|---|---|
| HKG | |||
| ACTB | F: ATGTGGATCAGCAAGCAGGAGTA R: TTTATGCGCATTTATGGGTTTTGT | 61 | [55] |
| HPRT | F: CGTTGCTGTCTCTACTTAAGCAG R: GATATCCCACACTTCGAGGAG T | 61 | [6] |
| GOI | |||
| Oxidative stress | |||
| GSS | F: GTGCCAGTTCCAGTTTTCTTATG R: TCCCACAGTAAAGCCAAGAG | 61.0 | [10] |
| iNOS | F: GGACAAGGGCCATTGCACCA R: TCCATCAGCGCTGCGCACAA | 61.0 | [56] |
| Metabolism | |||
| CPT1 | F: AAGGGTACAGCAAAGAAGATCCA R: CCACAGGTGTCCAACAATAGGAG | 61.0 | [57] |
| HMGCR | F: TTGGATAGAGGGAAGAGGGAAG R: CTCGTAGTTGTATTCGGTAA | 55.7 | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallissot, M.; Rodriguez, M.A.; Devreese, M.; Van herteryck, I.; Molist, F.; Santos, R.R. An Algoclay-Based Decontaminant Decreases Exposure to Aflatoxin B1, Ochratoxin A, and Deoxynivalenol in a Toxicokinetic Model, as well as Supports Intestinal Morphology, and Decreases Liver Oxidative Stress in Broiler Chickens Fed a Diet Naturally Contaminated with Deoxynivalenol. Toxins 2024, 16, 207. https://doi.org/10.3390/toxins16050207
Gallissot M, Rodriguez MA, Devreese M, Van herteryck I, Molist F, Santos RR. An Algoclay-Based Decontaminant Decreases Exposure to Aflatoxin B1, Ochratoxin A, and Deoxynivalenol in a Toxicokinetic Model, as well as Supports Intestinal Morphology, and Decreases Liver Oxidative Stress in Broiler Chickens Fed a Diet Naturally Contaminated with Deoxynivalenol. Toxins. 2024; 16(5):207. https://doi.org/10.3390/toxins16050207
Chicago/Turabian StyleGallissot, Marie, Maria A. Rodriguez, Mathias Devreese, Isis Van herteryck, Francesc Molist, and Regiane R. Santos. 2024. "An Algoclay-Based Decontaminant Decreases Exposure to Aflatoxin B1, Ochratoxin A, and Deoxynivalenol in a Toxicokinetic Model, as well as Supports Intestinal Morphology, and Decreases Liver Oxidative Stress in Broiler Chickens Fed a Diet Naturally Contaminated with Deoxynivalenol" Toxins 16, no. 5: 207. https://doi.org/10.3390/toxins16050207
APA StyleGallissot, M., Rodriguez, M. A., Devreese, M., Van herteryck, I., Molist, F., & Santos, R. R. (2024). An Algoclay-Based Decontaminant Decreases Exposure to Aflatoxin B1, Ochratoxin A, and Deoxynivalenol in a Toxicokinetic Model, as well as Supports Intestinal Morphology, and Decreases Liver Oxidative Stress in Broiler Chickens Fed a Diet Naturally Contaminated with Deoxynivalenol. Toxins, 16(5), 207. https://doi.org/10.3390/toxins16050207







