Inhibition of Aflatoxin Production in Aspergillus flavus by a Klebsiella sp. and Its Metabolite Cyclo(l-Ala-Gly)
Abstract
1. Introduction
2. Results
2.1. Isolation and Identification of the Strain KTTM
2.2. Isolation and Identification of the AF Production Inhibitor Produced by the Strain KTTM
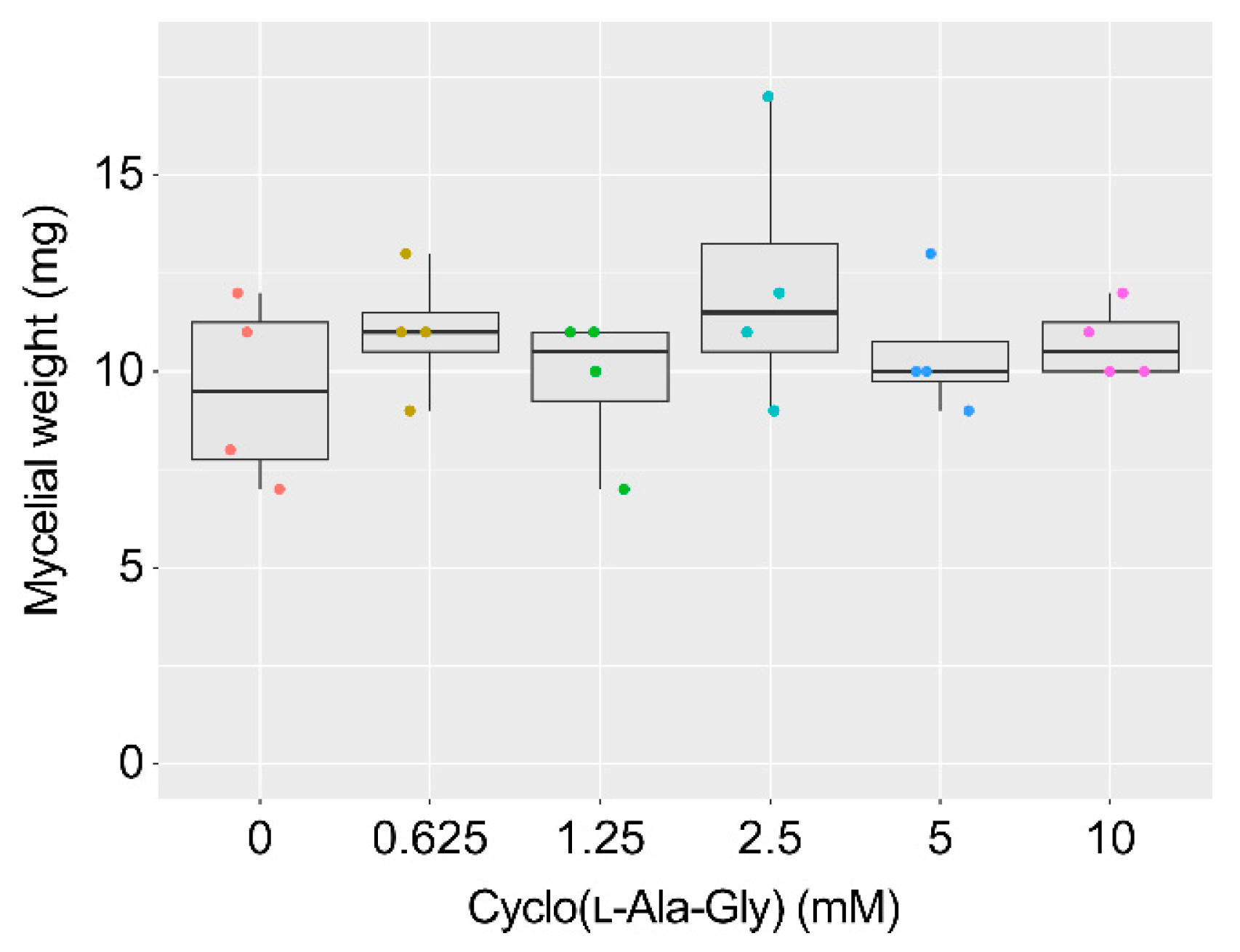
2.3. AF Production Inhibition by Cyclo(l-Ala-Gly) and Related Compounds
2.4. Inhibition of AF Production by the Strain KTTM
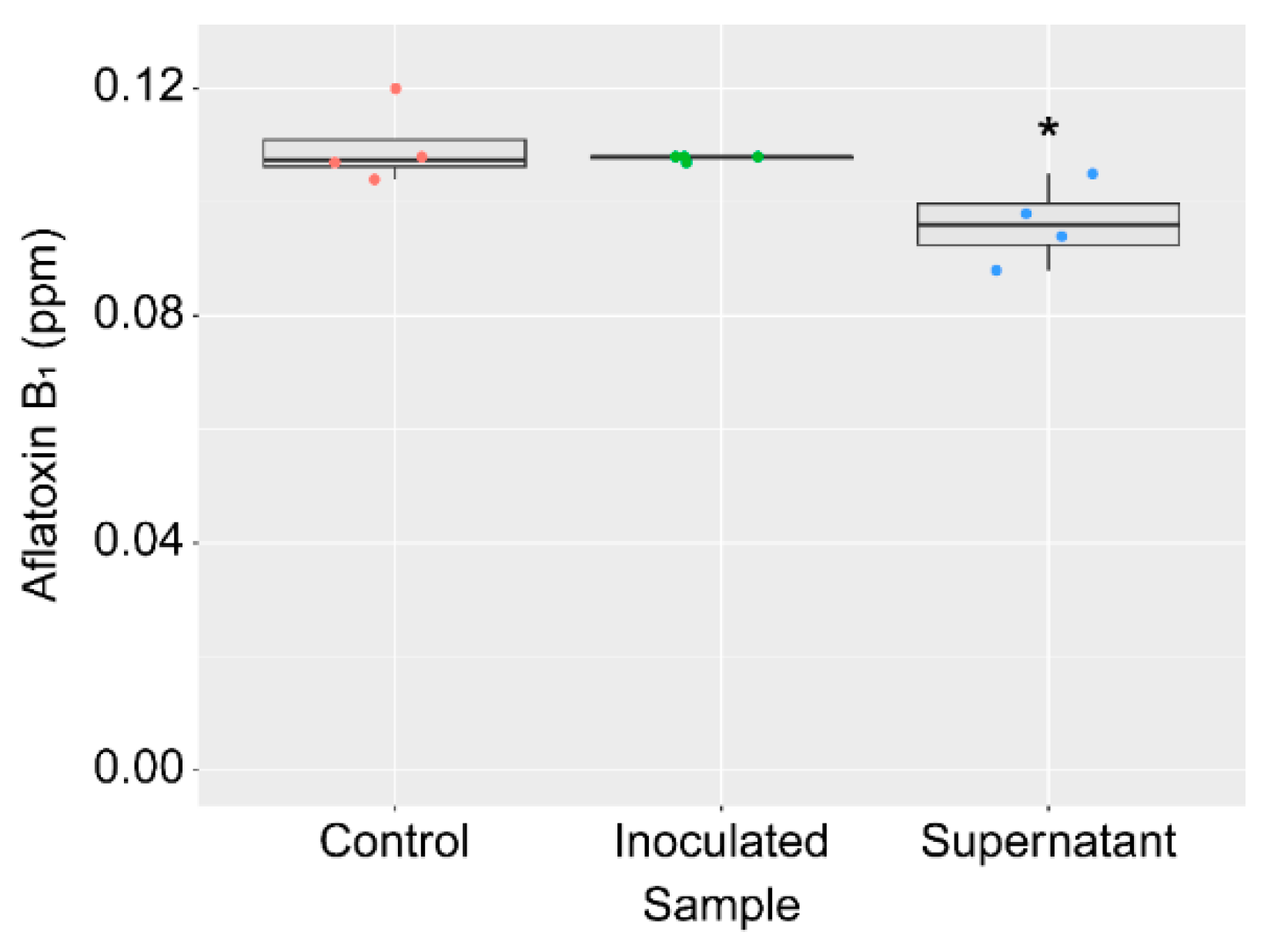
2.5. AF Degradation Activity of the Strain KTTM
2.6. Effects of Cyclo(l-Ala-Gly) and Related Compounds on A. flavus Glutathione-S-Transferase Activity
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Strains and Culture
5.2. Assay Method in Liquid Culture
5.3. Assay Method Using Peanuts
5.4. Isolation of the Active Component from the Strain KTTM
5.5. Preparation of the Cyclo(d-Ala-Gly), Cyclo(l-Abu(2)-Gly), Cyclo(Gly-Gly), Cyclo(l-Val-Gly), Cyclo(l-Nva-Gly), and Cyclo(l-Leu-Gly)
5.6. AF Degradation Activity of the Strain KTTM
5.7. Preparation of the Recombinant AfGST
5.8. Measurement of the GST Activity of AfGST-FLAG
5.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klich, M.A. Aspergillus flavus: The major producer of aflatoxin. Mol. Plant Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef]
- Pickova, D.; Ostry, V.; Toman, J.; Malir, F. Aflatoxins: History, significant milestones, recent data on their toxicity and ways to mitigation. Toxins 2021, 13, 399. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, N.J.; Bowers, E.; Hurburgh, C.; Wu, F. Potential economic losses to the US corn industry from aflatoxin contamination. Food Addit. Contam. Part A 2016, 33, 540–550. [Google Scholar] [CrossRef]
- Hamid, A.S.; Tesfamariam, I.G.; Zhang, Y.; Zhang, Z.G. Aflatoxin B1-induced hepatocellular carcinoma in developing countries: Geographical distribution, mechanism of action and prevention (review). Oncol. Lett. 2013, 5, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar] [CrossRef]
- Logrieco, A.F.; Miller, J.D.; Eskola, M.; Krska, R.; Ayalew, A.; Bandyopadhyay, R.; Battilani, P.; Bhatnagar, D.; Chulze, S.; Saeger, S.D.; et al. The Mycotox charter: Increasing awareness of, and concerted action for, minimizing mycotoxin exposure worldwide. Toxins 2018, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.K.; Wilkinson, J.R.; Zablotowicz, R.M.; Accinelli, C.; Abel, C.A.; Bruns, H.A.; Weaver, M.A. Ecology of Aspergillus flavus, regulation of aflatoxin production, and management strategies to reduce aflatoxin contamination of corn. Toxin Rev. 2009, 28, 142–153. [Google Scholar] [CrossRef]
- Sakuda, S.; Yoshinari, T.; Furukawa, T.; Jermnak, U.; Takagi, K.; Iimura, K.; Yamamoto, T.; Suzuki, M.; Nagasawa, H. Search for aflatoxin and trichothecene production inhibitors and analysis of their modes of action. Biosci. Biotechnol. Biochem. 2016, 80, 43–52. [Google Scholar] [CrossRef]
- Furukawa, T.; Katayama, H.; Oikawa, A.; Negishi, L.; Ichikawa, T.; Suzuki, M.; Murase, K.; Takayama, S.; Sakuda, S. Dioctatin activates ClpP to degrade mitochondrial components and inhibits aflatoxin production. Cell Chem. Biol. 2020, 27, 1396–1409. [Google Scholar] [CrossRef]
- Brown, R.L.; Cotty, P.J.; Cleveland, T.E. Reduction in aflatoxin content of maize by atoxigenic strains of Aspergillus flavus. J. Food Prot. 1991, 54, 623–626. [Google Scholar] [CrossRef][Green Version]
- Abbas, H.K.; Weaver, M.A.; Horn, B.W.; Carbone, I.; Monacell, J.T.; Shier, W.T. Selection of Aspergillus flavus isolates for biological control of aflatoxins in corn. Toxin Rev. 2011, 30, 59–70. [Google Scholar] [CrossRef]
- Moore, G.G. Practical considerations will ensure the continued success of pre-harvest biocontrol using non-aflatoxigenic Aspergillus flavus strains. Crit. Rev. Food Sci. Nutr. 2022, 62, 4208–4255. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Peer review of the pesticide risk assessment of the active substance Aspergillus flavus strain MUCL54911. EFSA J. 2022, 20, e07202. [Google Scholar]
- Peles, F.; Sipos, P.; Kovacs, S.; Gyori, Z.; Pocsi, I.; Pusztahelyi, T. Biological control and mitigation of aflatoxin contamination in commodities. Toxins 2021, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhang, Q.; Zhang, W.; Mao, J.; Li, P. Control of aflatoxigenic molds by antagonistic microorganisms: Inhibitory behaviors, bioactive compounds, related mechanisms, and influencing factors. Toxins 2020, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Jermnak, U.; Chinaphuti, A.; Poapolathep, A.; Kawai, R.; Nagasawa, H.; Sakuda, S. Prevention of aflatoxin contamination by a soil bacterium of Stenotrophomonas sp. that produces aflatoxin production inhibitors. Microbiology 2013, 159, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.S.; Song, Y.; Sakuno, E.; Nakajima, H.; Nakagawa, H.; Yabe, K. Cyclo(L-Leucyl-L-Prolyl) produced by Achromobacter xylosoxidans inhibits aflatoxin production by Aspergillus parasiticus. Appl. Environ. Microbiol. 2004, 70, 7466–7473. [Google Scholar] [CrossRef] [PubMed]
- Ning, M.; Zhang, S.; Xie, Y.; Wang, W.; Gao, Y. Aflatoxin B1 removal by three bacterial strains and optimization of fermentation process parameters. Biotechnol. Appl. Biochem. 2019, 66, 930–938. [Google Scholar] [CrossRef]
- Iimura, K.; Furukawa, T.; Yamamoto, T.; Negishi, L.; Suzuki, M.; Sakuda, S. The mode of action of cyclo(L-Ala-L-Pro) in inhibiting aflatoxin production of Aspergillus flavus. Toxins 2017, 9, 219. [Google Scholar] [CrossRef]
- Borgman, P.; Lopez, R.D.; Lane, A.L. The expanding spectrum of diketopiperazine natural product biosynthetic pathways containing cyclodipeptide synthases. Org. Biomol. Chem. 2019, 17, 2305–2314. [Google Scholar] [CrossRef]
- Liu, S.; Liu, M.; Wu, H.; Wang, Q.; Li, W.; Huang, S.; Feng, J. A new isomer and other metabolites isolated from Alternaria alternata. Chem. Nat. Compd. 2021, 57, 844–847. [Google Scholar] [CrossRef]
- Chen, L.; Guo, Q.F.; Ma, J.W.; Kang, W.J. Chemical constituents of Bacillus coagulans LL1103. Chem. Nat. Compd. 2018, 54, 419–420. [Google Scholar] [CrossRef]
- Li, X.J.; Tang, H.Y.; Duan, J.L.; Gao, J.M.; Xue, Q.H. Bioactive alkaloids produced by Pseudomonas brassicacearum subsp. neoaurantiaca, an endophytic bacterium from Salvia miltiorrhiza. Nat. Prod. Res. 2013, 27, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Thajudeen, H.; Park, K.; Moon, S.S.; Hong, I.S. An efficient green synthesis of proline-based cyclic dipeptides under water-mediated catalyst-free conditions. Tetrahedron Lett. 2010, 51, 1303–1305. [Google Scholar] [CrossRef]



| Compound | IC50 (mM) |
|---|---|
| cyclo(l-Ala-Gly) | 0.75 |
| cyclo(d-Ala-Gly) | >10 |
| cyclo(l-Ala-L-Pro) | 1.9 |
| cyclo(l-Abu(2)-Gly) | 0.01 |
| cyclo(l-Nva-Gly) | 0.09 |
| cyclo(l-Val-Gly) | 0.04 |
| cyclo(l-Leu-Gly) | 4.2 |
| cyclo(Gly-Gly) | >5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakuda, S.; Sunaoka, M.; Terada, M.; Sakoda, A.; Ishijima, N.; Hakoshima, N.; Uchida, K.; Enomoto, H.; Furukawa, T. Inhibition of Aflatoxin Production in Aspergillus flavus by a Klebsiella sp. and Its Metabolite Cyclo(l-Ala-Gly). Toxins 2024, 16, 141. https://doi.org/10.3390/toxins16030141
Sakuda S, Sunaoka M, Terada M, Sakoda A, Ishijima N, Hakoshima N, Uchida K, Enomoto H, Furukawa T. Inhibition of Aflatoxin Production in Aspergillus flavus by a Klebsiella sp. and Its Metabolite Cyclo(l-Ala-Gly). Toxins. 2024; 16(3):141. https://doi.org/10.3390/toxins16030141
Chicago/Turabian StyleSakuda, Shohei, Masaki Sunaoka, Maho Terada, Ayaka Sakoda, Natsumi Ishijima, Noriko Hakoshima, Kenichi Uchida, Hirofumi Enomoto, and Tomohiro Furukawa. 2024. "Inhibition of Aflatoxin Production in Aspergillus flavus by a Klebsiella sp. and Its Metabolite Cyclo(l-Ala-Gly)" Toxins 16, no. 3: 141. https://doi.org/10.3390/toxins16030141
APA StyleSakuda, S., Sunaoka, M., Terada, M., Sakoda, A., Ishijima, N., Hakoshima, N., Uchida, K., Enomoto, H., & Furukawa, T. (2024). Inhibition of Aflatoxin Production in Aspergillus flavus by a Klebsiella sp. and Its Metabolite Cyclo(l-Ala-Gly). Toxins, 16(3), 141. https://doi.org/10.3390/toxins16030141






