Environmental Factors Impacting the Development of Toxic Cyanobacterial Proliferations in a Central Texas Reservoir
Abstract
1. Introduction
2. Results
2.1. Species Information
2.2. CyanoHAP Toxicity
2.3. Flow Conditions
2.4. Water Quality Conditions
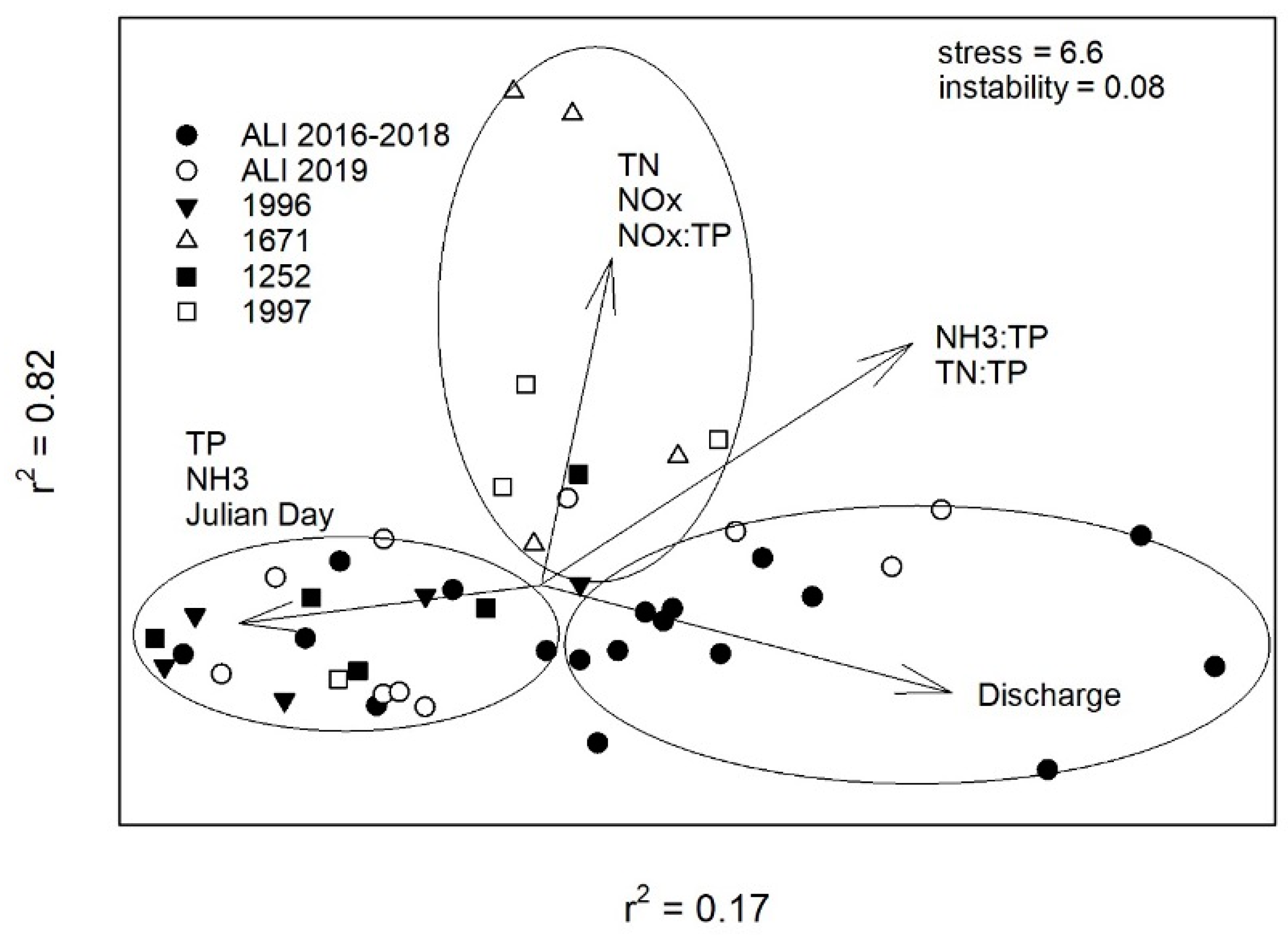
2.5. Water Quality Ordination
3. Discussion
4. Materials and Methods
4.1. Site Description
4.2. Sample Collection
4.3. Long-Term Monitoring
4.4. HPLC-MS Analysis
4.5. 16S and 23S rRNA Barcode Analysis
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wood, S.A.; Kelly, L.T.; Bouma-Gregson, K.; Humber, J.-F.; Laughinghouse, H.D.; Lazorchak, J.; McAllister, T.G.; McQueen, A.; Pokrzywinski, K.; Puddick, J.; et al. Toxic benthic freshwater cyanobacterial proliferations: Challenges and solutions for enhancing knowledge and improving monitoring and mitigation. Freshw. Biol. 2020, 65, 1824–1842. [Google Scholar] [CrossRef] [PubMed]
- Codd, G.A.; Morrison, L.F.; Metcalf, J.S. Cyanobacterial toxins: Risk management for health protection. Toxicol. Appl. Pharmacol. 2005, 203, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Perri, K.A.; Sullivan, J.M.; Boyer, G.L. Harmful algal blooms in Sodus Bay, Lake Ontario: A comparison of nutrients, marina presence, and cyanobacterial toxins. J. Great Lakes Res. 2015, 41, 326–337. [Google Scholar] [CrossRef]
- Brooks, B.W.; Lazorchak, J.M.; Howard, M.D.A.; Johnson, M.-V.; Morton, S.L.; Perkins, D.A.K.; Reavie, E.D.; Scott, G.I.; Smith, S.A.; Steevens, J.A. Are harmful algal blooms becoming the greatest inland water quality threat to public heath and aquatic ecosystems? Environ. Toxicol. Chem. 2016, 35, 6–13. [Google Scholar] [CrossRef]
- Puschner, B.; Hoff, B.; Tor, E.R. Diagnosis of anatoxin-a poisoning in dogs from North America. J. Vet. Diag. Investig. 2008, 20, 89–92. [Google Scholar] [CrossRef]
- Thomson-Laing, G.; Puddick, J.; Laroche, O.; Fulton, S.; Steiner, K.; Heath, M.W.; Wood, S.A. Broad and fine scale variability in bacterial diversity and cyanotoxin quotas in benthic cyanobacterial mats. Front. Microbiol. 2020, 11, 129. [Google Scholar] [CrossRef]
- Méjean, A.; Paci, G.; Gautier, V.; Ploux, O. Biosynthesis of anatoxin-a and analogues (anatoxins) in cyanobacteria. Toxicon 2014, 91, 15–22. [Google Scholar] [CrossRef]
- Faassen, E.J.; Harkema, L.; Begeman, L.; Lurling, M. First report of (homo)anatoxin-a and dog neurotoxicosis after ingestion of bentic cyanobacteria in The Netherlands. Toxicon 2012, 60, 378–384. [Google Scholar] [CrossRef]
- Wood, S.A.; Puddick, J.; Fleming, R.; Huessner, A.H. Detection of anatoxin-producing Phormidium in a New Zealand farm pond and an associated dog death. N. Z. J. Bot. 2017, 55, 36–46. [Google Scholar] [CrossRef]
- Bauer, F.; Fastner, J.; Bartha-Dima, B.; Breuer, W.; Falkenau, A.; Mayer, C.; Raeder, U. Mass occurrence of anatoxin-a- and dihydroanatoxin-a-producing Tychonema sp. in mesotrophic reservoir Mandichosee (River Lech, Germany) as a cause of neurotoxicosis in dogs. Toxins 2020, 12, 726. [Google Scholar] [CrossRef] [PubMed]
- Puddick, J.; van Ginkel, R.; Page, C.D.; Murray, J.S.; Greenhough, H.E.; Bowater, J.; Selwood, A.I.; Wood, S.A.; Prinsep, M.R.; Truman, P.; et al. Acute toxicity of dihydroanatoxin-a from Microcoleus autumnalis in comparison to anatoxin-a. Chemosphere 2021, 263, 127937. [Google Scholar] [CrossRef]
- Feist, S.M.; Lance, R.F. Genetic detection of freshwater harmful algal blooms: A review focused on the use of environmental DNA (eDNA) in Microcystis aeruginosa and Prymnesium parvum. Harmful Algae 2021, 110, 102124. [Google Scholar] [CrossRef]
- Yuan, J.; Yoon, K.-J. Overview of PCR methods applied for the identification of freshwater toxigenic cyanobacteria. In Cyanobacteria: Recent Advances in Taxonomy and Applications, 1st ed.; Hoxxein, W.N., Ed.; IntechOpen: London, UK, 2021; pp. 1–24. [Google Scholar]
- Knoll, L.B.; Sarnelle, O.; Hamilton, S.K.; Kissman, C.E.H.; Wilson, A.E.; Rose, J.B.; Morgan, M.R. Invasive zebra mussels (Dreissena polymorpha) increase cyanobacterial toxin concentrations in low-nutrient lakes. Can. J. Fish. Aquat. Sci. 2008, 65, 448–455. [Google Scholar] [CrossRef]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacterial blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Paerl, H.W.; Barnard, M.A. Mitigating the global expansion of harmful cyanobacterial blooms: Moving targets in a human- and climatically- altered world. Harmful Algae 2020, 96, 101845. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microbio. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef]
- Celikkol, S.; Fortin, N.; Tromas, N.; Andriananjamanantosa, H.; Greer, C.W. Bioavailable nutrients (N and P) and precipitation patterns drive cyanobacterial blooms in Missisquoi Bay, Lake Champlain. Microoganisms 2021, 9, 2097. [Google Scholar] [CrossRef] [PubMed]
- Cadel-Six, S.; Peyraud-Thomas, C.; Brient, L.; de Marsac, N.T.; Rippka, R.; Méjean, A. Different genotypes of anatoxin-producing cyanobacteria coexist in the Tarn River, France. Appl. Environ. Microbiol. 2007, 73, 7605–7614. [Google Scholar] [CrossRef] [PubMed]
- Hamill, K.D. Toxicity in benthic freshwater cyanobacteria (blue-green algae): First observations in New Zealand. N. Z. J. Mar. Fresh. 2001, 35, 1057–1059. [Google Scholar] [CrossRef]
- Wood, S.A.; Rasmussen, J.P.; Holland, P.T.; Campbell, R.C.; Crowe, A.L.M. First report of the cyanotoxin anatoxin-a from Aphanizomenon issatschenkoi (Cyanobacteria). J. Phycol. 2007, 43, 356–365. [Google Scholar] [CrossRef]
- Fastner, J.; Beulker, C.; Geiser, B.; Hoffmann, A.; Kröger, R.; Teske, K.; Hoppe, J.; Mundhenk, L.; Neurath, H.; Sagebiel, D.; et al. Fatal neurotoicosis in dogs associated with tychoplantic, anatoxin-a producing Tychonema sp. in a mesotrpohic Lake Tegel, Berlin. Toxins 2018, 10, 60. [Google Scholar] [CrossRef]
- Frederickson, A.; Richter, A.; Perri, K.A.; Manning, S.R. First confirmed case of canine mortality due to dihydroanatoxin-a in Central Texas, USA. Toxins 2023, 15, 485. [Google Scholar] [CrossRef] [PubMed]
- Manning, S.R.; Perri, K.A.; Bellinger, B.J. Bloom announcement: First reports of dog mortalities associated with neurotoxic filamentous cyanobacterial mats at recreational sites in Lady Bird Lake, Austin, Texas. Data Brief 2020, 33, 106344. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, B.J. Contact Recreation Considerations for Lady Bird Lake. 2020. Available online: https://services.austintexas.gov/watershed_protection/publications/document.cfm?id=352244 (accessed on 22 September 2023).
- Perri, K.A.; Manning, S.R.; Ashworth, M.A.; Bellinger, B.J. Expanded Molecular, Biochemical, and Statistical Analyses of a 2019 Toxic Cyanobacterial Proliferation Event in Lady Bird Lake, Austin, Texas; Mendeley Data: 2024. Available online: https://data.mendeley.com/datasets/f94329byhk/1 (accessed on 22 September 2023).
- Bellinger, B.J.; Richter, A.; Porras, A.; Davis, S.L. Drought and management effects on biophysicochemistry in a rapidly-flushed reservoir. Lake Reserv. Manag. 2018, 34, 182–198. [Google Scholar] [CrossRef]
- Austin Parks and Recreation Annual Concession Report 2018. Available online: https://services.austintexas.gov/edims/document.cfm?id=310317 (accessed on 22 September 2023).
- Austin Parks and Recreation Annual Concession Report 2019. Available online: https://services.austintexas.gov/edims/document.cfm?id=332209 (accessed on 22 September 2023).
- Wood, S.A.; Heath, M.W.; Kuhajek, J.; Ryan, K.G. Fine-scale spatial variability in anatoxin-a and homoanatoxin-a concentrations in benthic cyanobacterial mats: Implication for monitoring and management. J. Appl. Microbiol. 2010, 109, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; Cohen, M.; Chapuis-Hugon, F.; Pichon, V.; Mazmouz, R.; Méjean, A.; Ploux, O. Synthesis, configuration assignment, and simultaneous quantification by liquid chromatography coupled to tandem mass spectrometry, of dihydroanatoxin-a and dihydrohomoanatoxin-a together with the parent toxins, in axenic cyanobacterial strains and in environmental samples. Toxicon 2012, 60, 1404–1414. [Google Scholar] [PubMed]
- Méjean, A.; Dalle, K.; Paci, G.; Bouchonnet, S.; Mann, S.; Pichon, V.; Ploux, O. Dihydroanatoxin-a is biosynthesized from proline in Cylindrospermum stagnale PCC 7417: Isotopic incorporation experiments and mass spectrometry analysis. J. Nat. Prod. 2016, 779, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Heath, M.W.; Wood, S.A.; Barbieri, R.F.; Young, R.G.; Ryan, K.G. Effects of nitrogen and phosphorus on anatoxin-a, homoanatoxin-a, dihydroanatoxin-a and dihydrohomoanatoxin-a production by Phormidium autumnale. Toxicon 2014, 92, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.A.; Smith, F.M.J.; Heath, M.W.; Palfroy, T.; Gaw, S.; Young, R.G.; Ryan, K.G. Within-mat variability in anatoxin-a and homoanatoxin-a production amon benthic Phormidium (Cyanobacteria) strains. Toxins 2012, 4, 900–912. [Google Scholar] [CrossRef]
- Gamez, T.E.; Groeger, A.W.; Manning, S.R. Dynamic phytoplankton community structure in a subtropical reservoir during an extended drought, Central Texas, USA. Aquat. Biol. 2022, 85, 7. [Google Scholar] [CrossRef]
- Strategies for Preventing and Managing Benthic Harmful Cyanobacterial Blooms. Available online: https://hcb-2.itrcweb.org (accessed on 22 September 2023).
- Sabater, S.; Vilalta, E.; Gaudes, A.; Guasch, H.; Muñoz, I.; Romaní, A. Ecological implications of mass growth of benthic cyanobacteria in rivers. Aquat. Microb. Ecol. 2003, 32, 175–184. [Google Scholar] [CrossRef]
- Tee, H.S.; Waite, D.; Payne, L.; Middleditch, M.; Wood, S.; Handley, K.M. Tools for successful proliferation: Diverse strategies of nutrient acquisition by a benthic cyanobacterium. ISME J. 2020, 14, 2164–2178. [Google Scholar] [CrossRef] [PubMed]
- Glibert, P.M. Harmful algae at the complex nexus of eutrophication and climate change. Harmful Algae 2020, 91, 101583. [Google Scholar] [CrossRef] [PubMed]
- James, W.F.; Barko, J.W.; Eakin, H.L. Nutrient regeneration by the zebra mussel (Dreissena polymorpha). J. Freshw. Ecol. 1997, 12, 209–216. [Google Scholar] [CrossRef]
- James, W.F.; Barko, J.W.; Avis, M.; Eakin, H.L.; Rogala, J.T.; Miller, A.C. Filtration and excretion by zebra mussels: Implications for water quality impacts in Lake Pepin, Upper Mississippi River. J. Freshw. Ecol. 2000, 15, 429–437. [Google Scholar] [CrossRef]
- Conroy, J.D.; Edwards, W.J.; Pontius, R.A.; Kane, D.D.; Zhang, H.; Shea, J.F.; Richey, J.N.; Culver, D.A. Soluble nitrogen and phosphorus excretion of exotic freshwater mussles (Dreissena spp.): Potential impacts for nutrient remineralization in western Lake Erie. Freshw. Biol. 2005, 50, 1146–1162. [Google Scholar] [CrossRef]
- Boussiba, S.; Gibson, J. Ammonia translocation in cyanobacteria. FEMS Microbiol. Rev. 1991, 88, 1–14. [Google Scholar]
- De Freene, P.; Cougnon, M.; Janssens, G.P.J.; Vangansbeke, P. Nutrient fertilization by dogs in peri-urban ecosystems. Ecol. Solut. Evid. 2021, 3, e12128. [Google Scholar] [CrossRef]
- Richter, A. Creation of a Multi-Metric Index for Describing the Environmental Integrity of Austin-Area Lakes. Available online: https://services.austintexas.gov/watershed_protection/publications/document.cfm?id=196479 (accessed on 22 September 2023).
- Janse, I.; Meima, M.; Kardinaal, W.E.A.; Zward, G. High-resolution differentiation of cyanobacterial by using rRNA-internal transcribed spacer denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 2003, 69, 6634–6643. [Google Scholar] [CrossRef]
- Del Campo, E.M.; Del Hoyo, A.; Royo, C.; Casano, L.M.; Álvarez, R.; Barreno, E. A single primer pair gives a specific ortholog amplicon in a wide range of Cyanobacteria and plastid-bearing organisms: Applicability in inventory of reference material from collections and phylogenetic analysis. Mol. Phylogenetics Evol. 2010, 57, 1323–1328. [Google Scholar] [CrossRef]
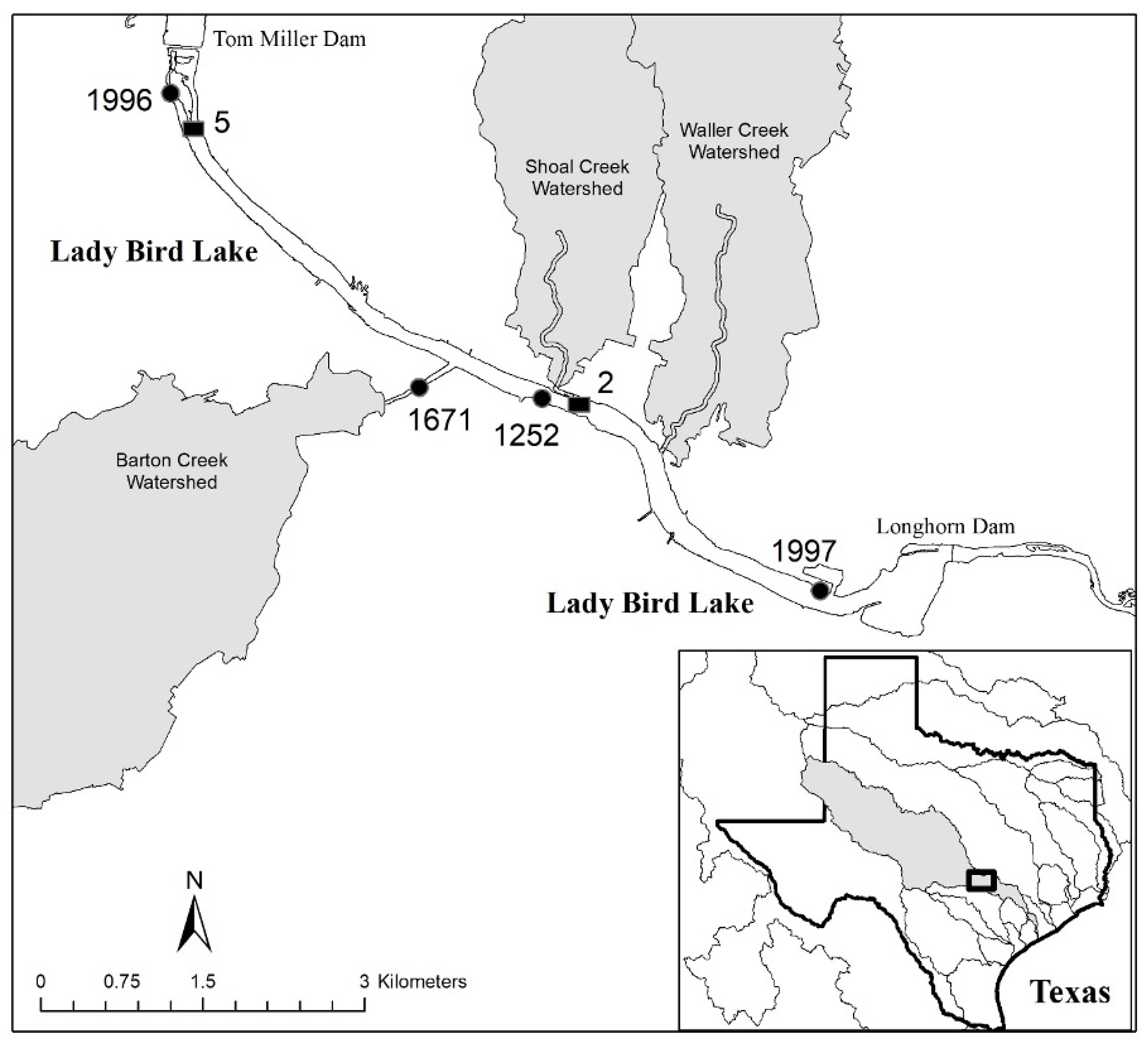

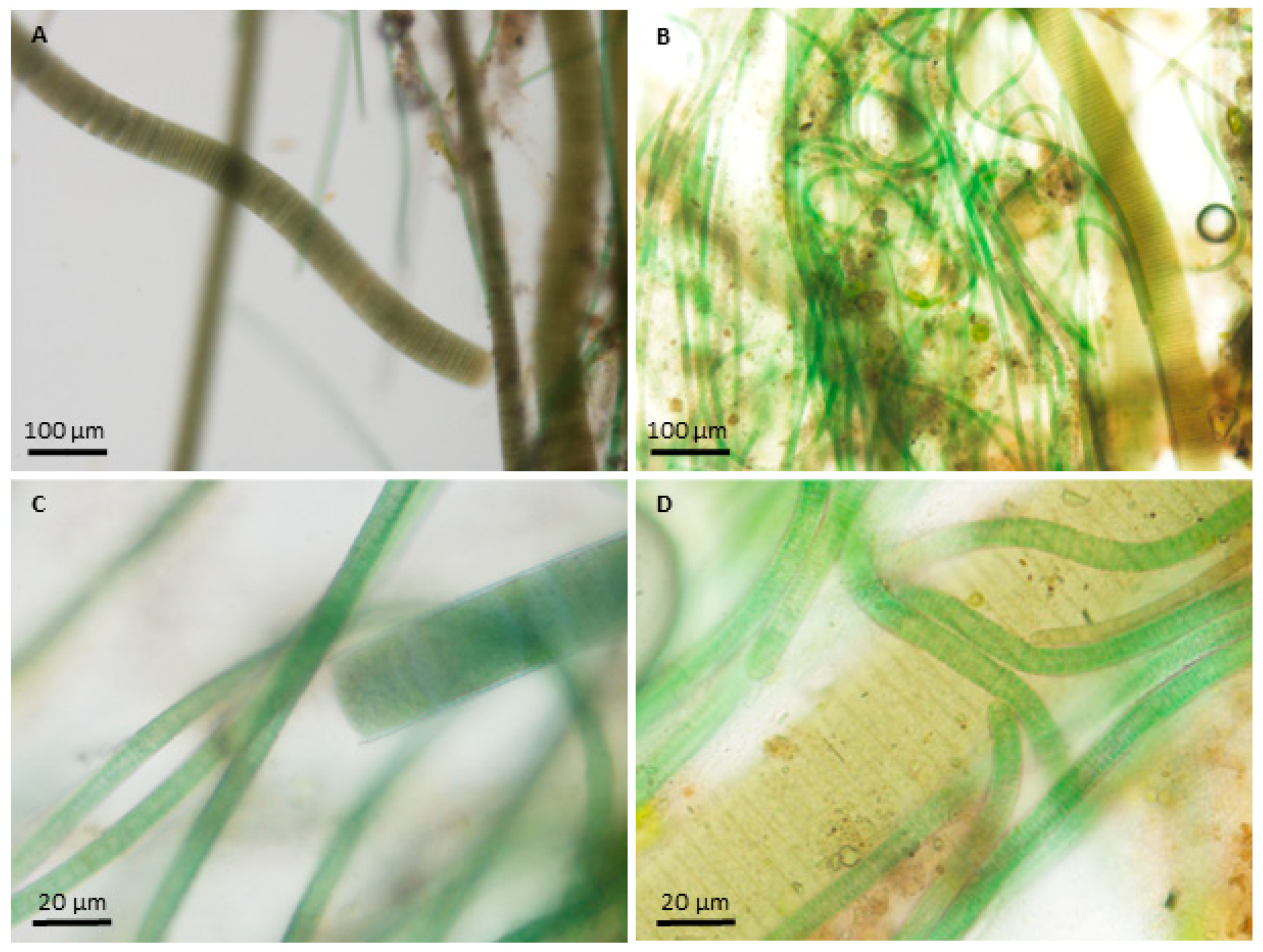
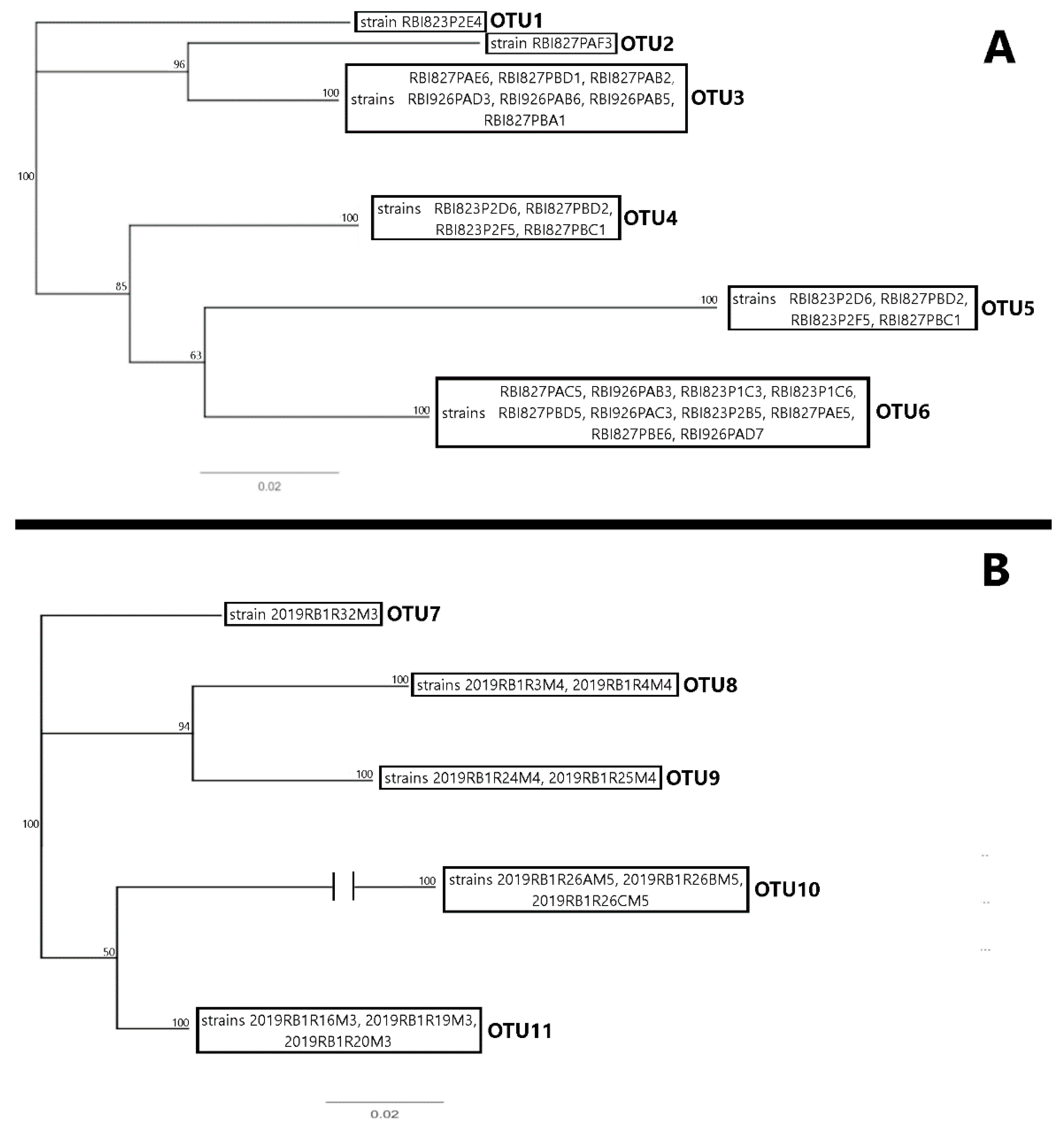
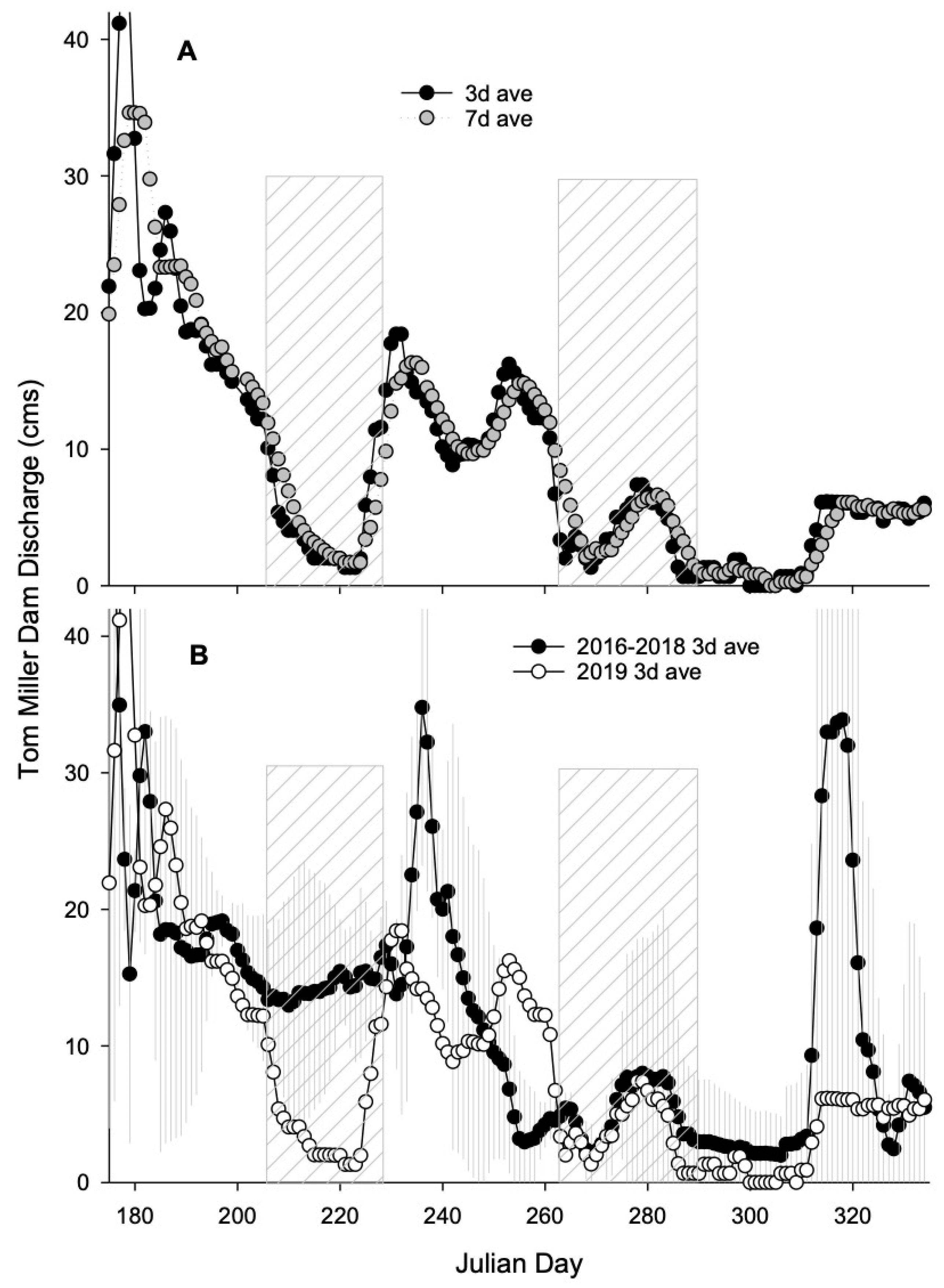
| Site(s) | NH3-N (µg/L) | NOx-N (µg/L) | TKN (µg/L) | TN (µg/L) | TP (µg/L) | N:P (Molar) |
|---|---|---|---|---|---|---|
| 2 and 5 * | 8.0 ± 0.01 | 199.0 ± 198.0 | 411.0 ± 82.9 | 610.0 ± 212.0 | 14.1 ± 9.3 | 123.4 ± 53.4 |
| 2 and 5 ** | 19.1 ± 18.2 + | 476.0 ± 488.0 ++ | 377.0 ± 49.9 | 853.0 ± 494.0 | 26.4 ± 15.5 + | 98.0 ± 70.6 + |
| 1996 # | 41.0 ± 7.7 | 112.5 ± 41.2 | 401.0 ± 28.7 | 513.5 ± 40.8 | 26.0 ± 20.8 | 87.1 ± 72.7 |
| 1671 # | 8.0 ± 0.0 ŧ | 1520.0 ± 176.9 | 184.7 ± 61.4 | 1704.7 ± 230.5 | 18.6 ± 9.3 | 259.4 ± 168.1 |
| 1252 # | 24.0 ± 20.3 | 393.8 ± 149.4 | 404.5 ± 56.5 | 798.3 ± 200.8 | 17.7 ± 6.5 | 119.1 ± 74.3 |
| 1997 # | 26.9 ± 32.7 | 390.7 ± 115.7 | 399.3 ± 16.6 | 790.0 ± 113.8 | 17.5 ± 16.4 | 165.6 ± 108.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perri, K.A.; Bellinger, B.J.; Ashworth, M.P.; Manning, S.R. Environmental Factors Impacting the Development of Toxic Cyanobacterial Proliferations in a Central Texas Reservoir. Toxins 2024, 16, 91. https://doi.org/10.3390/toxins16020091
Perri KA, Bellinger BJ, Ashworth MP, Manning SR. Environmental Factors Impacting the Development of Toxic Cyanobacterial Proliferations in a Central Texas Reservoir. Toxins. 2024; 16(2):91. https://doi.org/10.3390/toxins16020091
Chicago/Turabian StylePerri, Katherine A., Brent J. Bellinger, Matt P. Ashworth, and Schonna R. Manning. 2024. "Environmental Factors Impacting the Development of Toxic Cyanobacterial Proliferations in a Central Texas Reservoir" Toxins 16, no. 2: 91. https://doi.org/10.3390/toxins16020091
APA StylePerri, K. A., Bellinger, B. J., Ashworth, M. P., & Manning, S. R. (2024). Environmental Factors Impacting the Development of Toxic Cyanobacterial Proliferations in a Central Texas Reservoir. Toxins, 16(2), 91. https://doi.org/10.3390/toxins16020091






