Aflatoxin M1 Contamination in Dairy Milk in Kathmandu, Nepal
Abstract
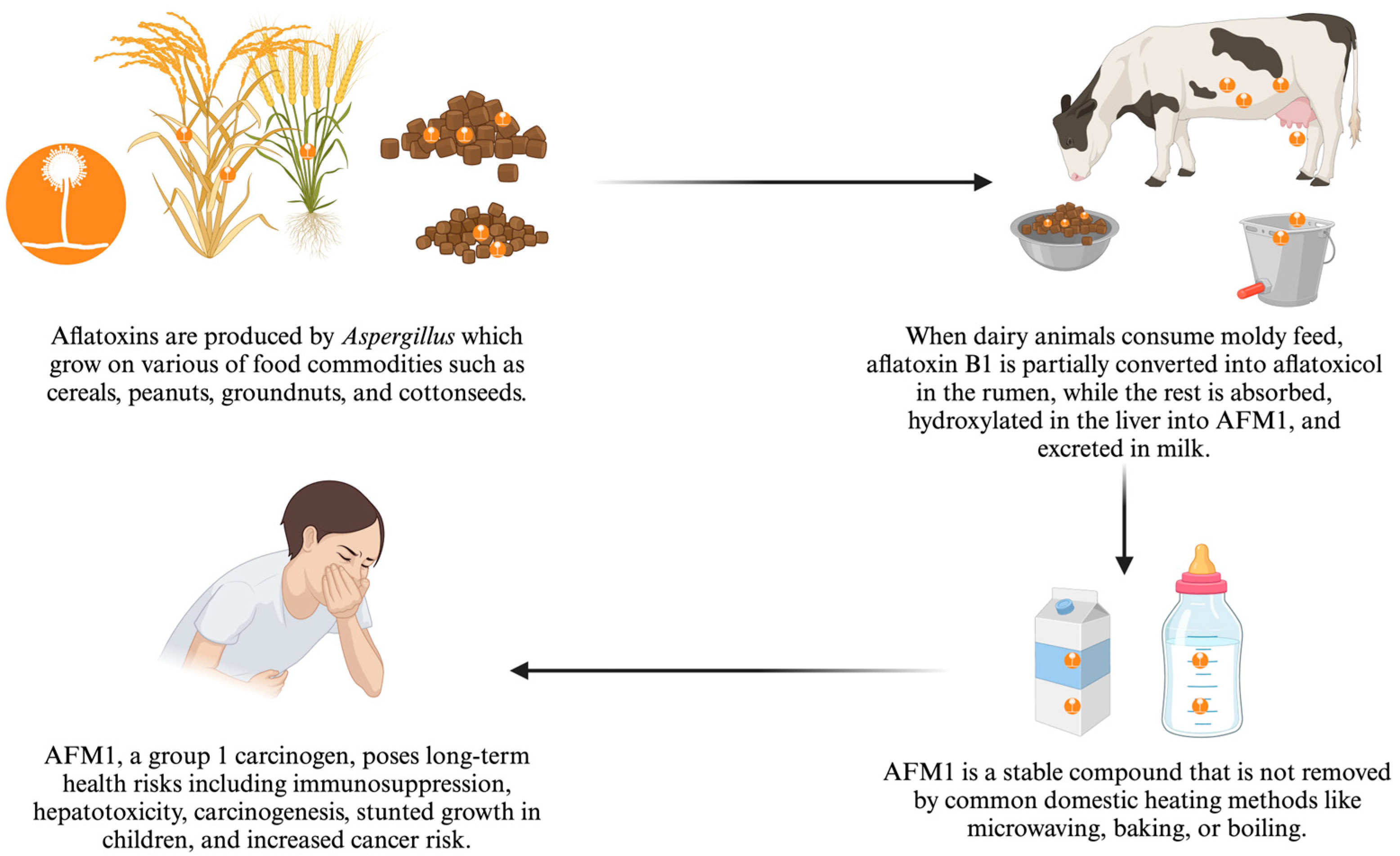
1. Introduction
2. Results
2.1. Aflatoxin Contamination
2.2. Farm Characteristics
2.3. Feeding Practice
2.4. Storage Practice
3. Discussion
4. Materials and Methods
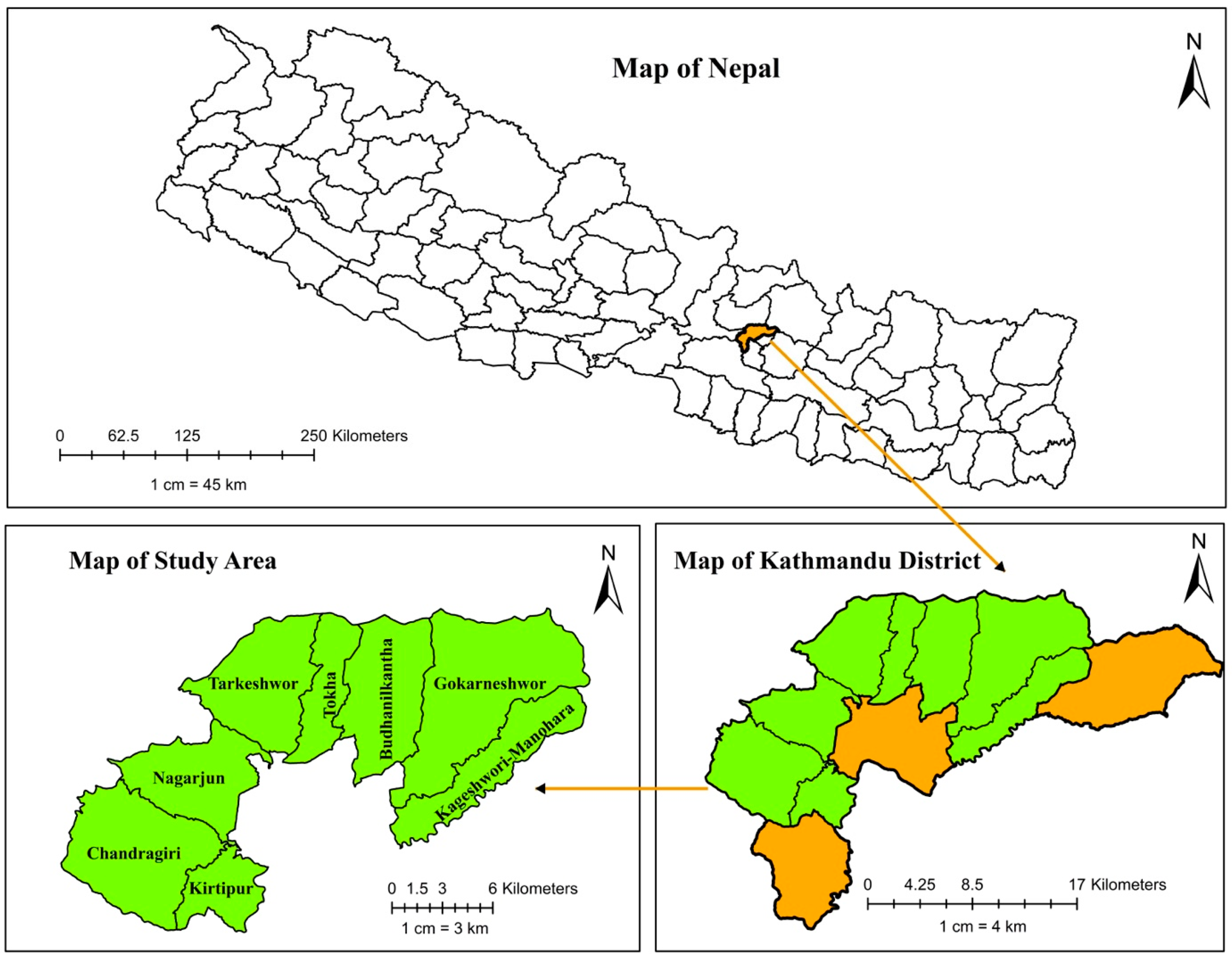
4.1. Study Design and Study Area
4.2. Sample Collection
4.3. Laboratory Analysis
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Zain, M.E. Impact of Mycotoxins on Humans and Animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef]
- Kumar, A.; Pathak, H.; Bhadauria, S.; Sudan, J. Aflatoxin Contamination in Food Crops: Causes, Detection, and Management: A Review. Food Prod Process Nutr. 2021, 3, 17. [Google Scholar] [CrossRef]
- Giovati, L.; Magliani, W.; Ciociola, T.; Santinoli, C.; Conti, S.; Polonelli, L. AFM1 in Milk: Physical, Biological, and Prophylactic Methods to Mitigate Contamination. Toxins 2015, 7, 4330–4349. [Google Scholar] [CrossRef]
- Esam, R.M.; Hafez, R.S.; Khafaga, N.I.M.; Fahim, K.M.; Ibrahim Ahmed, L. Assessment of Aflatoxin M1 and B1 in Some Dairy Products with Referring to the Analytical Performances of Enzyme-Linked Immunosorbent Assay in Comparison to High-Performance Liquid Chromatography. Vet. World 2022, 15, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Al-Delaimy, K.; Mahmoud, I. Aflatoxin M1 in Milk and Milk Products in Jordan and Methods for Its Reduction: A Preliminary Study. BJAST 2015, 6, 597–605. [Google Scholar] [CrossRef]
- Iha, M.H.; Barbosa, C.B.; Okada, I.A.; Trucksess, M.W. Occurrence of Aflatoxin M1 in Dairy Products in Brazil. Food Control 2011, 22, 1971–1974. [Google Scholar] [CrossRef]
- Omeiza, G.K.; Mwanza, M.; Enem, S.I.; Godwin, E.; Adeiza, M.A.; Okoli, C. Reducing Efficiencies of the Commonly Used Heat Treatment Methods and Fermentation Processes on Aflatoxin M1 in Naturally Contaminated Fresh Cow Milk. OJVM 2018, 8, 134–145. [Google Scholar] [CrossRef]
- Deveci, O.; Sezgin, E. Changes in Concentration of Aflatoxin M1 during Manufacture and Storage of Skim Milk Powder. J. Food Prot. 2006, 69, 682–685. [Google Scholar] [CrossRef]
- Kang’ethe, E.K.; Lang’a, K.A. Aflatoxin B1 and M1 Contamination of Animal Feeds and Milk from Urban Centers in Kenya. Afr. Health Sci. 2009, 9, 218–226. [Google Scholar] [PubMed]
- Sharma, H.; Jadhav, V.J.; Garg, S.R. Aflatoxin M1 in Milk in Hisar City, Haryana, India and Risk Assessment. Food Addit. Contam. Part B 2020, 13, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Akhtar, S.; Levin, R.E.; Ismail, T.; Riaz, M.; Amir, M. Aflatoxin M1: Prevalence and Decontamination Strategies in Milk and Milk Products. Crit. Rev. Microbiol. 2016, 42, 418–427. [Google Scholar] [CrossRef]
- Ferrari, L.; Rizzi, N.; Grandi, E.; Clerici, E.; Tirloni, E.; Stella, S.; Bernardi, C.E.M.; Pinotti, L. Compliance between Food and Feed Safety: Eight-Year Survey (2013–2021) of Aflatoxin M1 in Raw Milk and Aflatoxin B1 in Feed in Northern Italy. Toxins 2023, 15, 168. [Google Scholar] [CrossRef] [PubMed]
- Paniel, N.; Radoi, A.; Marty, J.-L. Development of an Electrochemical Biosensor for the Detection of Aflatoxin M1 in Milk. Sensors 2010, 10, 9439–9448. [Google Scholar] [CrossRef]
- Kiarie, G.M.; Dominguez-Salas, P.; Kang’ethe, S.K.; Grace, D.; Lindahl, J. Aflatoxin Exposure among Young Children in Urban Low-Income Areas of Nairobi and Association with Child Growth. AJFAND 2016, 16, 10967–10990. [Google Scholar] [CrossRef]
- Abdulrazzaq, Y.M.; Osman, N.; Yousif, Z.M.; Al-Falahi, S. Aflatoxin M1 in Breast-Milk of UAE Women. Ann. Trop. Paediatr. 2003, 23, 173–179. [Google Scholar] [CrossRef]
- Mahdavi, R.; Nikniaz, L.; Arefhosseini, S.R.; Vahed Jabbari, M. Determination of Aflatoxin M1 in Breast Milk Samples in Tabriz–Iran. Matern. Child Health J. 2010, 14, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Ahlberg, S.; Grace, D.; Kiarie, G.; Kirino, Y.; Lindahl, J. A Risk Assessment of Aflatoxin M1 Exposure in Low and Mid-Income Dairy Consumers in Kenya. Toxins 2018, 10, 348. [Google Scholar] [CrossRef]
- Shahat, A.; MA, S.; Mohamed, A.F.; Abdel-Wahhab, P.M. Correlation Study Between Aflatoxin M 1 and Hepatitis C Virus in Egyptian Patients with Chronic Liver Disease. World J. Med. Sci. 2012, 7, 224–231. [Google Scholar] [CrossRef]
- Jallow, A.; Xie, H.; Tang, X.; Qi, Z.; Li, P. Worldwide Aflatoxin Contamination of Agricultural Products and Foods: From Occurrence to Control. Comp. Rev. Food Sci. Food Safe 2021, 20, 2332–2381. [Google Scholar] [CrossRef] [PubMed]
- Torović, L. Aflatoxin M 1 in Processed Milk and Infant Formulae and Corresponding Exposure of Adult Population in Serbia in 2013–2014. Food Addit. Contam. Part B 2015, 8, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Mollayusefian, I.; Ranaei, V.; Pilevar, Z.; Cabral-Pinto, M.M.S.; Rostami, A.; Nematolahi, A.; Khedher, K.M.; Thai, V.N.; Fakhri, Y.; Mousavi Khaneghah, A. The Concentration of Aflatoxin M1 in Raw and Pasteurized Milk: A Worldwide Systematic Review and Meta-Analysis. Trends Food Sci. Technol. 2021, 115, 22–30. [Google Scholar] [CrossRef]
- Chamlagain, S.; Dahal, T. Status of Production and Distribution of Fresh Milk by Villagers in Bariyarpatti, Sohpur. J. Mgt. 2020, 3, 110–121. [Google Scholar] [CrossRef]
- Pokharel, A.; Webb, P.; Andrews-Trevino, J.; Lamichhane, A.; Shrestha, R.; Acharya, S.; Davis, D.; Baral, K.; Wang, J.-S.; Xue, K.; et al. Prevalence and Associated Factors of Breastmilk Aflatoxin M1 Levels in Mothers from Banke, Nepal. Food Control 2021, 126, 108069. [Google Scholar] [CrossRef]
- Asghar, M.A.; Ahmed, A.; Asghar, M.A. Aflatoxin M 1 in Fresh Milk Collected from Local Markets of Karachi, Pakistan. Food Addit. Contam. Part B 2018, 11, 167–174. [Google Scholar] [CrossRef]
- Dhavan, A.S.; Choudary, M.R. Incidence of Aflatoxins in Animal Feedstuffs: A Decade’s Scenario in India. J. Aoac Int. 1995, 78, 693–697. [Google Scholar] [CrossRef]
- Hattimare, D.; Shakya, S.; Patyal, A.; Chandrakar, C.; Kumar, A. Occurrence and Exposure Assessment of Aflatoxin M1 in Milk and Milk Products in India. J. Food Sci. Technol. 2022, 59, 2460–2468. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Asi, M.R.; Jinap, S. Variation of Aflatoxin M1 Contamination in Milk and Milk Products Collected during Winter and Summer Seasons. Food Control 2013, 34, 714–718. [Google Scholar] [CrossRef]
- Rahmani, J.; Alipour, S.; Miri, A.; Fakhri, Y.; Riahi, S.-M.; Keramati, H.; Moradi, M.; Amanidaz, N.; Pouya, R.H.; Bahmani, Z.; et al. The Prevalence of Aflatoxin M1 in Milk of Middle East Region: A Systematic Review, Meta-Analysis and Probabilistic Health Risk Assessment. Food Chem. Toxicol. 2018, 118, 653–666. [Google Scholar] [CrossRef]
- Tadesse, B.T.; Ashley, E.A.; Ongarello, S.; Havumaki, J.; Wijegoonewardena, M.; González, I.J.; Dittrich, S. Antimicrobial Resistance in Africa: A Systematic Review. BMC Infect. Dis. 2017, 17, 616. [Google Scholar] [CrossRef]
- Gizachew, D.; Szonyi, B.; Tegegne, A.; Hanson, J.; Grace, D. Aflatoxin Contamination of Milk and Dairy Feeds in the Greater Addis Ababa Milk Shed, Ethiopia. Food Control 2016, 59, 773–779. [Google Scholar] [CrossRef]
- Kuboka, M.M.; Imungi, J.K.; Njue, L.; Mutua, F.; Grace, D.; Lindahl, J.F. Occurrence of Aflatoxin M1 in Raw Milk Traded in Peri-Urban Nairobi, and the Effect of Boiling and Fermentation. Infect. Ecol. Epidemiol. 2019, 9, 1625703. [Google Scholar] [CrossRef] [PubMed]
- Mulunda, M.; Mike, D. Occurrence of Aflatoxin M1 from Rural Subsistence and Commercial Farms from Selected Areas of South Africa. Food Control 2014, 39, 92–96. [Google Scholar] [CrossRef]
- Sifuentes dos Santos, J.; França, V.; Katto, S.; Santana, E.H. Aflatoxin M1 in Pasteurized, UHT Milk and Milk Powder Commercialized in Londrina, Brazil and Estimation of Exposure. Arch. Latinoam. Nutr. 2015, 65, 181–185. [Google Scholar]
- Ruangwises, S.; Saipan, P.; Ruangwises, N. Occurrence of Aflatoxin M1 in Raw and Pasteurized Goat Milk in Thailand. In Aflatoxins—Recent Advances and Future Prospects; Razzaghi-Abyaneh, M., Ed.; InTech: Penang, Malaysia, 2013; ISBN 978-953-51-0904-4. [Google Scholar]
- Lin, L.-C.; Liu, F.-M.; Fu, Y.-M.; Shih, D.Y.-C. Survey of Aflatoxin M1 Contamination of Dairy Products in Taiwan. J. Food Drug Anal. 2020, 12, 8. [Google Scholar] [CrossRef]
- Sumantri, I.; Purwanti, F.; Nuryono, N.; Agus, A. Estimation of Aflatoxin M1 Exposure through Consumption of Various Dairy Milk Products in Yogyakarta, Indonesia (Estimasi Paparan Aflatoksin M1 Melalui Konsumsi Berbagai Produk Susu Di Yogyakarta, Indonesia). J. Vet. 2019, 20, 58. [Google Scholar] [CrossRef]
- Assaf, J.C.; Nahle, S.; Chokr, A.; Louka, N.; Atoui, A.; El Khoury, A. Assorted Methods for Decontamination of Aflatoxin M1 in Milk Using Microbial Adsorbents. Toxins 2019, 11, 304. [Google Scholar] [CrossRef]
- Ashiq, S. Natural Occurrence of Mycotoxins in Food and Feed: Pakistan Perspective. Comp. Rev. Food Sci. Food Safe 2015, 14, 159–175. [Google Scholar] [CrossRef]
- Mamo, F.T.; Abate, B.A.; Tesfaye, K.; Nie, C.; Wang, G.; Liu, Y. Mycotoxins in Ethiopia: A Review on Prevalence, Economic and Health Impacts. Toxins 2020, 12, 648. [Google Scholar] [CrossRef]
- Yunus, A.W.; Ullah, A.; Lindahl, J.F.; Anwar, Z.; Ullah, A.; Saif, S.; Ali, M.; Zahur, A.B.; Irshad, H.; Javaid, S.; et al. Aflatoxin Contamination of Milk Produced in Peri-Urban Farms of Pakistan: Prevalence and Contributory Factors. Front. Microbiol. 2020, 11, 159. [Google Scholar] [CrossRef]
- Patyal, A.; Gill, J.P.S.; Bedi, J.S.; Aulakh, R.S. Assessment of Aflatoxin Contamination in Dairy Animal Concentrate Feed from Punjab, India. Environ. Sci. Pollut. Res. 2021, 28, 37705–37715. [Google Scholar] [CrossRef] [PubMed]
- Tadele, F.; Demissie, B.; Amsalu, A.; Demelash, H.; Mengist, Z.; Ambelu, A.; Yenew, C. Aflatoxin Contamination of Animal Feeds and Its Predictors among Dairy Farms in Northwest Ethiopia: One Health Approach Implications. Front. Vet. Sci. 2023, 10, 1123573. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.R.; Karki, D. Prevalence of Aflatoxin B1 and B2 in Poultary Feed. Nepal Agric. Res. J. 2009, 9, 109–112. [Google Scholar] [CrossRef]
- Joshi, P.; Chauysrinule, C.; Mahakarnchanakul, W.; Maneeboon, T. Multi-Mycotoxin Contamination, Mold Incidence and Risk Assessment of Aflatoxin in Maize Kernels Originating from Nepal. Microbiol. Res. 2022, 13, 258–277. [Google Scholar] [CrossRef]
- Gautam, D.N.; Bhatta, R.; Bhandary, M.R. Assessment of Aflatoxin B1 Level in Chilli, Maize and Groundnut Samples from Kathmandu Valley. J. Food Sci. Technol. Nepal 2008, 4, 57–60. [Google Scholar]
- Pokhrel, P. Postharvest Handling and Prevalence of Aflatoxin Contamination in Nepalese Maize Produce. J. Food Sci. Technol. Nepal 2016, 9, 11–19. [Google Scholar] [CrossRef]
- Akbar, N.; Nasir, M.; Naeem, N.; Ahmad, M.; Saeed, F.; Anjum, F.M.; Iqbal, S.; Imran, M.; Tufail, T.; Shah, F.; et al. Assessment of Aflatoxin in Milk and Feed Samples and Impact of Seasonal Variations in the Punjab, Pakistan. Food Sci. Nutr. 2020, 8, 2699–2709. [Google Scholar] [CrossRef]
- Phillips, S.I.; Wareing, P.W.; Dutta, A.; Panigrahi, S.; Medlock, V. The Mycoflora and Incidence of Aflatoxin, Zearalenone and Sterigmatocystin in Dairy Feed and Forage Samples from Eastern India and Bangladesh. Mycopathologia 1996, 133, 15–21. [Google Scholar] [CrossRef]
- Neme, K.; Mohammed, A. Mycotoxin Occurrence in Grains and the Role of Postharvest Management as a Mitigation Strategies. A Review. Food Control 2017, 78, 412–425. [Google Scholar] [CrossRef]
- Adenitan, A.A.; Awoyale, W.; Akinwande, B.A.; Busie, M.-D.; Michael, S. Mycotoxin Profiles of Solar Tent-Dried and Open Sun-Dried Plantain Chips. Food Control 2021, 119, 107467. [Google Scholar] [CrossRef]
- PHLIL, N. Feed the Future Innovation Lab for the Reduction of Post-Harvest Loss. PHLIL Nepal Buy-In: Final Report; USAID: Washington, DC, USA, 2020; p. 78. Available online: https://www.k-state.edu/phl/project-countries/projectcountries_docs/PHLIL%20Nepal%20Report%20-%20final%202020.pdf (accessed on 16 October 2024).
- KoboToolbox. Available online: https://www.kobotoolbox.org/ (accessed on 22 August 2024).
- Ishler, V.A.; Becker, C. Concentrates for Dairy Cattle. Available online: https://extension.psu.edu/concentrates-for-dairy-cattle (accessed on 16 October 2024).

| Sample | Sample Size | Samples Exceeding EU Limit-AFM1 (%) |
|---|---|---|
| Municipalities | ||
| Budanilkantha | 12 | 100 |
| Kirtipur | 15 | 100 |
| Tokha | 13 | 77.8 |
| Tarkeswor | 6 | 100 |
| Gokernswor | 7 | 100 |
| Kageswori-manohara | 6 | 100 |
| Chandragiri | 7 | 100 |
| Nagarjun | 7 | 100 |
| Sample categories | ||
| Farms’ raw milk | 48 | 95.8 |
| Dairy retailers’ milk | 25 | 100 |
| Packet milk | 11 | 100 |
| Farms Characteristics | Number (%) | Samples Exceeding EU Limit-AFM1 (%) |
|---|---|---|
| A. Location | ||
| Urban | 33 (68.8%) | 100% |
| Pre-Urban | 15 (31.3%) | 86.7% |
| B. Rearing Practice | ||
| Intensive | 42 (87.5%) | 97.6% |
| Semi-Intensive | 6 (12.5%) | 83.3% |
| C. Herd Size | ||
| 0–10 | 29 (60.4%) | 93.1% |
| 11–20 | 10 (20.8%) | 100% |
| Above 20 | 9 (18.8%) | 100% |
| Inclusion in Animal Feed Diet | Response | Number (%) | Samples Exceeding EU Limit-AFM1 (%) |
|---|---|---|---|
| Cut and carry forage | Yes | 46 (95.8%) | 95.7 |
| No | 2 (4.2%) | 100 | |
| Silage | Yes | 3 (6.3%) | 100 |
| No | 45 (93.8%) | 95.6 | |
| Concentrates | Yes | 48 (100%) | 95.8% |
| No | 0 (0%) | ||
| Inclusion of unusual feedstuffs in animal diet | Yes | 23 (47.9%) | 100% |
| No | 25 (52.1%) | 92.0% | |
| Types of concentrate in animal diet | Only commercial pellet | 11 (22.9%) | 100% |
| Only homemade concentrate | 2 (4.2%) | 50% | |
| Mixtures of both | 35 (72.9%) | 97.1% |
| Storage Practice of Animal Feed | Frequency (%) | Samples Exceeding EU Limit-AFM1 (%) | |
|---|---|---|---|
| Storage of dry fodder (straw) | Sack (outdoor) | 18 (40%) | 100% |
| Shed (open) | 16 (35.6%) | 93.8% | |
| Shed (separate compartment) | 3 (6.7%) | 100% | |
| Room | 6 (13.3%) | 100% | |
| Others | 2 (4.4%) | 100% | |
| Storage of concentrates | Room (closed) | 24 (50%) | 91.7% |
| Room (Open) | 7 (14.6%) | 100% | |
| Shed (open and/or in compartment) | 15 (31.3%) | 100% | |
| others | 2 (4.2%) | 100% | |
| Floor of storage facilities | Raised | 24 (50%) | 100% |
| Floored (Unraised) | 24 (50%) | 91.7% | |
| Regular monitoring of temperature, humidity and moisture in storage facilities | Yes | 0 (0%) | |
| No | 48 (100%) | 95.8% | |
| Have you seen mold infestation in storage facilities? | Yes | 13 (27.1%) | 100% |
| No | 35 (72.9%) | 94.3% | |
| Have you seen rodent and insect infestation in storage facilities? | Yes | 33 (68.8%) | 100% |
| No | 15 (31.3%) | 86.7% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kafle, S.; Paudel, M.; Shrestha, C.; Kathayat, K.B.; Sapkota, R.C.; Tiwari, A.; Subedi, D. Aflatoxin M1 Contamination in Dairy Milk in Kathmandu, Nepal. Toxins 2024, 16, 468. https://doi.org/10.3390/toxins16110468
Kafle S, Paudel M, Shrestha C, Kathayat KB, Sapkota RC, Tiwari A, Subedi D. Aflatoxin M1 Contamination in Dairy Milk in Kathmandu, Nepal. Toxins. 2024; 16(11):468. https://doi.org/10.3390/toxins16110468
Chicago/Turabian StyleKafle, Sujan, Madhav Paudel, Chanda Shrestha, Khadak Bahadur Kathayat, Ram Chandra Sapkota, Ananda Tiwari, and Deepak Subedi. 2024. "Aflatoxin M1 Contamination in Dairy Milk in Kathmandu, Nepal" Toxins 16, no. 11: 468. https://doi.org/10.3390/toxins16110468
APA StyleKafle, S., Paudel, M., Shrestha, C., Kathayat, K. B., Sapkota, R. C., Tiwari, A., & Subedi, D. (2024). Aflatoxin M1 Contamination in Dairy Milk in Kathmandu, Nepal. Toxins, 16(11), 468. https://doi.org/10.3390/toxins16110468










