Scoliidines: Neuroprotective Peptides in Solitary Scoliid Wasp Venoms
Abstract
1. Introduction
2. Results
2.1. Studies of Crude Venom Extract by Liquid Chromatography-Electrospray Ionization-Mass Spectrometry (LC-ESI-MS)
2.1.1. On-Line Mass Fingerprinting
2.1.2. Identification of Small Molecules
2.1.3. Peptide Sequencing
2.2. Biological Characterization of γ-, δ- and ε-Scoliidines
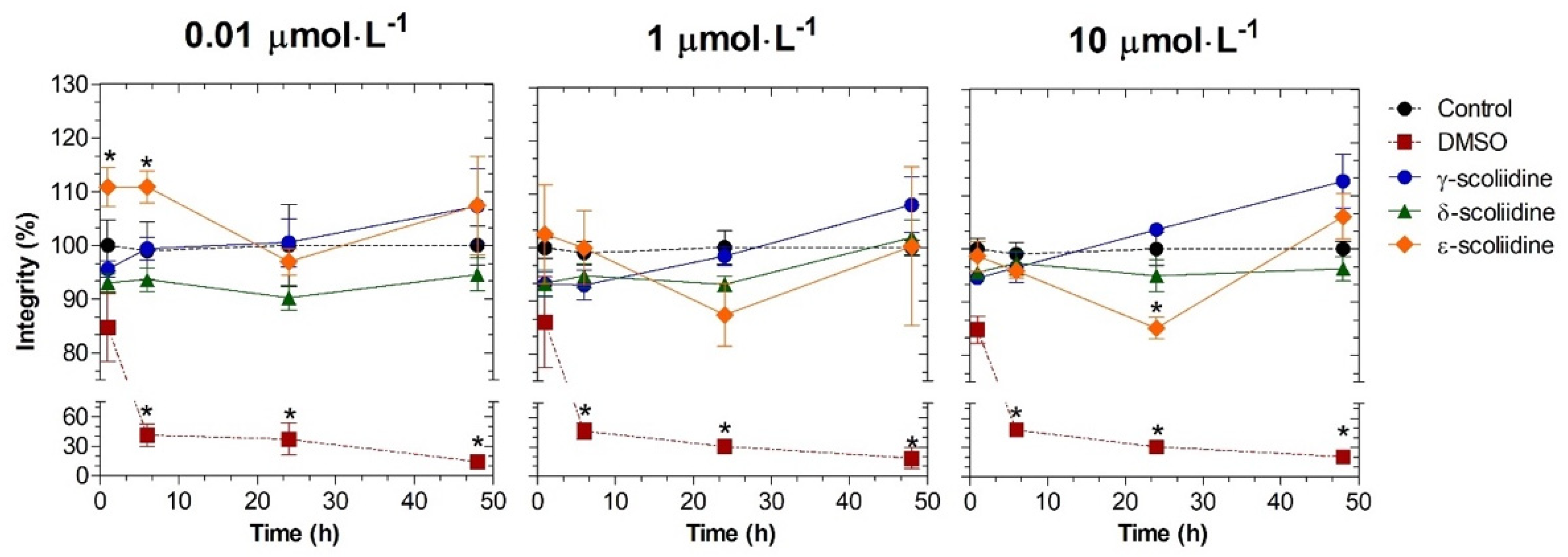
2.2.1. Toxicological Profile in Neuronal PC12 Cells
2.2.2. Scoliidines-Mediated Neuroprotection
2.2.3. Effects of γ-, δ- and ε-Scoliidines on AChE Activity
2.2.4. Effects of γ-, δ- and ε-Scoliidines on ACE and NEP Activities
2.3. In Silico Analyses
2.3.1. Hemolytic Activity
2.3.2. Toxic Activity
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Wasp Collection
5.2. Reagents and Cell Line
5.3. LC-ESI-MS
5.4. MALDI-TOF MS
5.5. Peptide Synthesis
5.6. ACE and NEP Activities
5.7. Stability Tests of Peptides
5.8. Measurement of AChE Activity
5.9. Toxicity Studies on the Integrity Cell
5.10. Effects of Scoliidines on Changes Induced by Oxidative Stress
5.11. Prediction of Hemolytic Peptides
5.12. Prediction of Toxic Peptides
5.13. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piek, T. (Ed.) Venoms of the Hymenoptera: Biochemical, Pharmacological and Behavioural Aspects; Academic Press: London, UK, 1986. [Google Scholar]
- Nakajima, T. Pharmacological Biochemistry of Vespid Venoms. In Venoms of the Hymenoptera: Biochemical, Pharmacological and Behavioural Aspects; Piek, T., Ed.; Academic Press: London, UK, 1986; pp. 309–327. [Google Scholar]
- Silva, J.; Monge-Fuentes, V.; Gomes, F.; Lopes, K.; dos Anjos, L.; Campos, G.; Arena, C.; Biolochi, A.; Gonçalves, J.; Galante, P.; et al. Pharmacological alternatives for the treatment of neurodegenerative disorders: Wasp and bee venoms and their components as new neuroactive tools. Toxins 2015, 7, 3179–3209. [Google Scholar] [CrossRef]
- Abd El-Wahed, A.; Yosri, N.; Sakr, H.H.; Du, M.; Algethami, A.F.M.; Zhao, C.; Abdelazeem, A.H.; Tahir, H.E.; Masry, S.H.D.; Abdel-Daim, M.M.; et al. Wasp venom biochemical components and their potential in biological applications and nanotechnological interventions. Toxins 2021, 13, 206. [Google Scholar] [CrossRef]
- Luo, L.; Kamau, P.M.; Lai, R. Bioactive peptides and proteins from wasp venoms. Biomolecules 2022, 12, 527. [Google Scholar] [CrossRef]
- O’Neill, K.M. Solitary Wasps: Behavior and Natural History; Cornell University Press: Ithaca, NY, USA, 2001; ISBN 0-8014-3721-0. [Google Scholar]
- Lee, S.H.; Baek, J.H.; Yoon, K.A. Differential properties of venom peptides and proteins in solitary vs. social hunting wasps. Toxins 2016, 8, 32. [Google Scholar] [CrossRef]
- White, S.R.; Kadavakollu, S. Bradykinin in Hemipepsis ustulata: A novel method for safely milking wasps. Toxicon 2016, 117, 49–52. [Google Scholar] [CrossRef]
- Nolasco, M.; Biondi, I.; Pimenta, D.C.; Branco, A. Extraction and preliminary chemical characterization of the venom of the spider wasp Pepsis decorata (Hymenoptera: Pompilidae). Toxicon 2018, 150, 74–76. [Google Scholar] [CrossRef]
- Moore, E.L.; Arvidson, R.; Banks, C.; Urenda, J.L.; Duong, E.; Mohammed, H.; Adams, M.E. Ampulexins: A new family of peptides in venom of the emerald jewel wasp, Ampulex Compressa. Biochemistry 2018, 57, 1907–1916. [Google Scholar] [CrossRef]
- Kotea, S.; Faktorb, J.; Dapica, I.; Mayordomoa, M.Y.; Kocikowskia, M.; Kagansky, A.; Goodletta, D.; Vojtesek, B.; Huppa, T.; Wilcockson, D.; et al. Analysis of venom sac constituents from the solitary, aculeate wasp Cerceris rybyensis. Toxicon 2019, 169, 1–4. [Google Scholar] [CrossRef]
- Huicab-Uribe, M.A.; Verdel-Aranda, K.; Martínez-Hernández, A.; Zamudio, F.Z.; Jiménez-Vargas, J.M.; Lara-Reyna, J. Molecular composition of the paralyzing venom of three solitary wasps (Hymenoptera: Pompilidae) collected in southeast Mexico. Toxicon 2019, 168, 98–102. [Google Scholar] [CrossRef]
- Jensen, T.; Walker, A.A.; Nguyen, S.H.; Jin, A.-H.; Deuis, J.R.; Vetter, I.; King, G.F.; Schmidt, J.O.; Robinson, S.D. Venom chemistry underlying the painful stings of velvet ants (Hymenoptera: Mutillidae). Cell. Mol. Life Sci. 2021, 78, 5163–5177. [Google Scholar] [CrossRef]
- Dashevsky, D.; Rodriguez, J. A short review of the venoms and toxins of spider wasps (Hymenoptera: Pompilidae). Toxins 2021, 13, 774. [Google Scholar] [CrossRef]
- Nolasco, M.; Mariano, D.O.C.; Pimenta, D.C.; Biondi, I.; Branco, A. Proteomic analysis of venom from a Spider Hawk, Pepsis decorata. J. Venom. Anim. Toxins Incl. Trop. Dis. 2023, 29, e20220090. [Google Scholar] [CrossRef]
- Eldefrawi, A.T.; Eldefrawi, M.E.; Konno, K.; Mansour, N.A.; Nakanishi, K.; Oltz, E.; Usherwood, P.N.R. Structure and synthesis of a potent glutamate receptor antagonist in wasp venom. Proc. Natl. Acad. Sci. USA 1988, 85, 4910–4913. [Google Scholar] [CrossRef]
- Piek, T.; Hue, B. Philanthotoxins, a new class of neuroactive polyamines, block nicotinic transmission in the insect CNS. Comp. Biochem. Physiol. 1989, 93, 403–406. [Google Scholar] [CrossRef]
- Yasuhara, T.; Mantel, P.; Nakajima, T.; Piek, T. Two kinins isolated from an extract of the venom reservoirs of the solitary wasp Megascolia flavifrons. Toxicon 1987, 25, 527–535. [Google Scholar] [CrossRef]
- Piek, T.; Hue, B.; Mantel, P.; Nakajima, T.; Pelhate, M.; Yasuhara, T. Threonine6-bradykinin in the venom of the wasp Colpa interrupta (F.) presynaptically blocks nicotinic synaptic transmission in the insect CNS. Comp. Biochem. Physiol. 1990, 96, 157–162. [Google Scholar] [CrossRef]
- Piek, T.; Hue, B.; Mony, L.; Nakajima, T.; Pelhate, M.; Yasuhara, T. Block of synaptic transmission in insect CNS by toxins from the venom of the WASP Megascolia flavifrons (FAB.). Comp. Biochem. Physiol. 1987, 87, 287–295. [Google Scholar] [CrossRef]
- Konno, K.; Kazuma, K.; Nihei, K. Peptide toxins in solitary wasp venoms. Toxins 2016, 8, 114. [Google Scholar] [CrossRef]
- Cabrera, M.P.D.S.; Rangel, M.; Ruggiero Neto, J.; Konno, K. Chemical and biological characteristics of antimicrobial-helical peptides found in solitary wasp venoms and their interaction with model membranes. Toxins 2019, 11, 559. [Google Scholar] [CrossRef]
- Hernández, C.; Konno, K.; Salceda, E.; Vega, R.; Zaharenko, A.J.; Soto, E. Sa12b peptide from solitary wasp inhibits ASIC currents in rat dorsal root ganglion neurons. Toxins 2019, 10, 585. [Google Scholar] [CrossRef]
- Nihei, K.; Peigneur, S.; Tytgat, J.; Lange, A.B.; Konno, K. Isolation and characterization of FMRFamide-like peptides in the venoms of solitary sphecid wasps. Peptides 2021, 142, 170575. [Google Scholar] [CrossRef]
- Alberto-Silva, C.; Portaro, F.; Kodama, R.; Pantaleão, H.; Rangel, M.; Nihei, K.; Konno, K. Novel neuroprotective peptides in the venom of the solitary scoliid wasp Scolia decorata ventralis. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, e20200171. [Google Scholar] [CrossRef]
- Alberto-Silva, C.; Portaro, F.C.V.; Kodama, R.T.; Pantaleão, H.Q.; Inagaki, H.; Nihei, K.; Konno, K. Comprehensive analysis and biological characterization of venom components from solitary scoliid wasp Campsomeriella annulata annulata. Toxins 2021, 13, 885. [Google Scholar] [CrossRef]
- Konno, K.; Palma, M.S.; Hitara, I.Y.; Juliano, M.A.; Juliano, L.; Yasuhara, T. Identification of bradykinins in solitary wasp venoms. Toxicon 2002, 40, 309–312. [Google Scholar] [CrossRef]
- Nakajima, T.; Yasuhara, T.; Yoshida, N.; Takemoto, Y.; Shinonaga, S.; Kano, R.; Yoshida, H. The pattern analysis of biologically active amines in some Hymenopteran venoms by high performance liquid chromatography. Med. Entomol. Zool. 1983, 34, 61–71. [Google Scholar] [CrossRef]
- de Souza, J.M.; Goncalves, B.D.C.; Gomez, M.V.; Vieira, L.B.; Ribeiro1, F.M. Animal toxins as therapeutic tools to treat neurodegenerative diseases. Front. Pharmacol. 2018, 9, 145. [Google Scholar] [CrossRef]
- Oliveira, A.L.; Viegas, M.F.; da Silva, S.L.; Soares, A.M.; Ramos, M.J.; Fernandes, P.A. The chemistry of snake venom and its medicinal potential. Nat. Rev. Chem. 2022, 6, 451. [Google Scholar] [CrossRef]
- Walczak-Nowicka, Ł.J.; Herbet, M. Acetylcholinesterase inhibitors in the treatment of neurodegenerative diseases and the role of acetylcholinesterase in their pathogenesis. Int. J. Mol. Sci. 2021, 22, 9290. [Google Scholar] [CrossRef]
- Prasasty, V.; Radifar, M.; Istyastono, E. Natural peptides in drug discovery targeting acetylcholinesterase. Molecules 2018, 23, 2344. [Google Scholar] [CrossRef]
- Teixeira, J.; de Castro, A.; Soares, F.; da Cunha, E.; Ramalho, T. Future therapeutic perspectives into the Alzheimer’s disease targeting the oxidative stress hypothesis. Molecules 2019, 24, 4410. [Google Scholar] [CrossRef]
- Isaac, R.E.; Bland, N.D.; Alan, D.; Shirras, A.D. Neuropeptidases and the metabolic inactivation of insect neuropeptides. Gen. Comp. Endocrinol. 2009, 162, 8–17. [Google Scholar] [CrossRef]
- Malheiros, F.B.M.; Vicente, M.E.; Morales, A.G.; Alberto-Silva, C. Efficiency of the removal of tetraethyl pyrophosphate (TEPP) pesticide in water: Use of cork granules as a natural adsorbent on acetylcholinesterase activity in neuronal PC12 cell. J. Environ. Sci. Health B 2022, 57, 554–560. [Google Scholar] [CrossRef]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb. Protoc. 2016. [Google Scholar] [CrossRef]
- Stockert, J.C.; Blázquez-Castro, A.; Cañete, M.; Horobin, R.W.; Villanueva, Á. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem. 2012, 114, 785–796. [Google Scholar] [CrossRef]
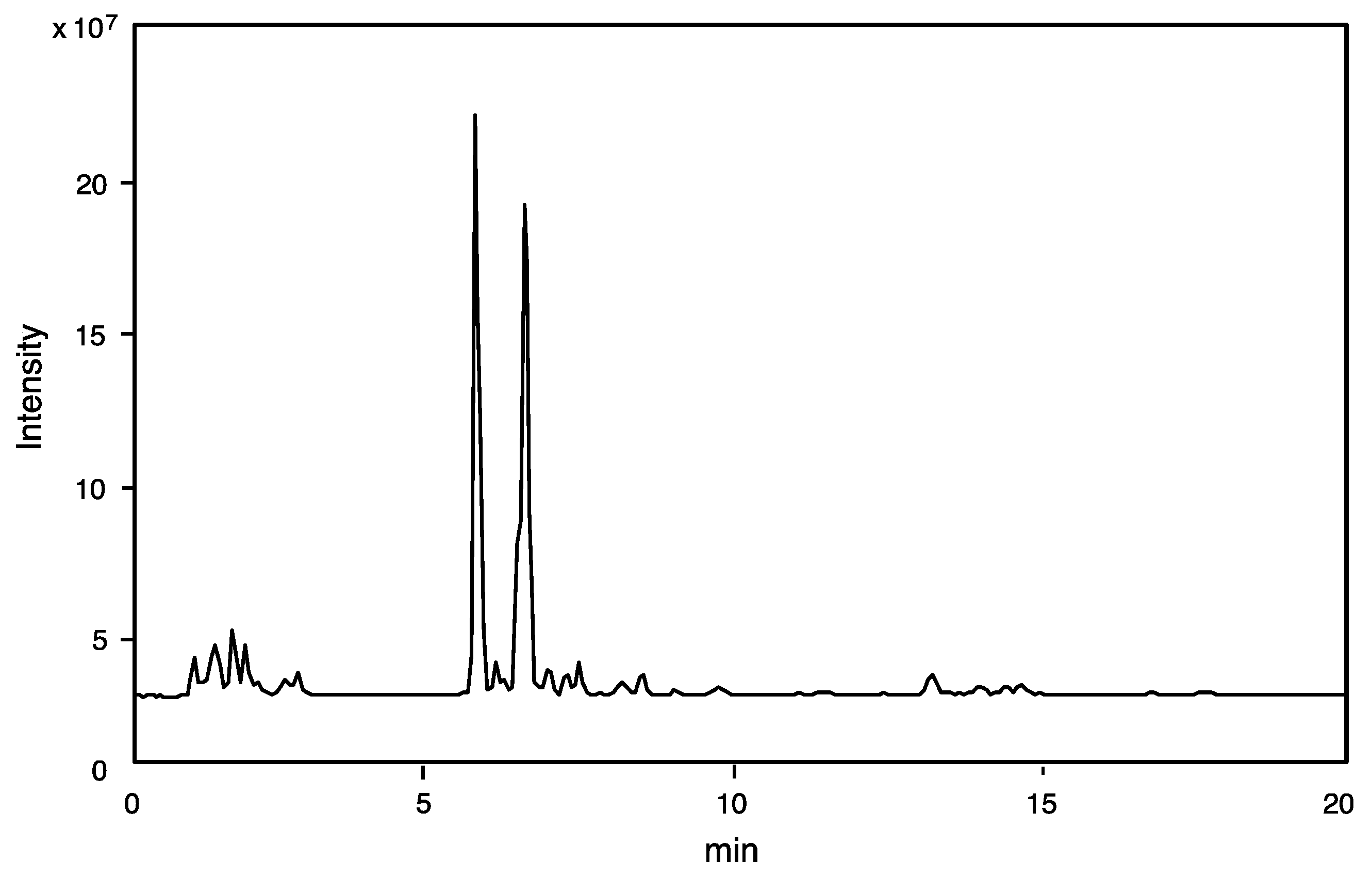


| Fr. No. | Retention Time (min) | (M + H)+ m/z |
|---|---|---|
| 1 | 0.9–1.5 | 90.053, 106.050, 112.087, 116.070, 118.086, 120.065, 133.061, 134.045, 148.069, 154.086, 156.076, 175.119, 258.110 |
| 2 | 1.5–2.0 | 132.102, 150.058, 182.081, 268.104, 284.099, 348.069, 349.054, 364.065, 664.109 |
| 3 | 2.0–2.3 | 269.088, 1270.626 |
| 4 | 2.3–3.0 | 166.086, 609.359, 666.381, 1053.557, 1082.546, 1238.636 |
| 5 | 3.0–4.0 | 1237.782, 2693.467 |
| 6 | 4.0–5.0 | 205.097, 604.344, 962.551, 1091.567, 1306.624 |
| 7 | 5.0–5.4 | 806.403, 1004.587, 1393.829 |
| 8 | 5.4–5.8 | 676.377, 1003.596, 1203.717, 1302.786, 1465.848 |
| 9 | 5.8–6.2 | 1580.874, 1682.892, 2006.099 |
| 10 | 6.2–6.6 | 813.448, 1004.585, 1103.652, 1155.655, 1266.718, 1545.804 |
| 11 | 6.6–7.0 | 997.532, 1230.679, 1337.754, 1395.759,1492.811, 2033.062 |
| 12 | 7.0–7.4 | 1034.511, 1346.671, 1368.653 |
| 13 | 7.4–7.9 | 1095.686, 1110.615, 1139.648, 1572.767, 1607.836 |
| 14 | 7.9–8.2 | — |
| 15 | 8.2–9.0 | 1282.551 |
| RT (min) | Intensity × 104 | [M + H]+ m/z | Elemental Composition | Iminium Ion m/z | Elemental Composition | Compound |
|---|---|---|---|---|---|---|
| 1.07 | 10 | 156.076 | C6H10N3O2 | — | Histidine | |
| 35 | 175.119 | C6H15N4O2 | — | Arginine | ||
| 1.28 | 2 | 90.055 | C3H7NO2 | — | Alanine | |
| 4 | 106.050 | C3H8NO3 | — | Serine | ||
| 180 | 116.070 | C5H10NO2 | 70.065 | C4H8N | Proline | |
| 20 | 118.086 | C5H12NO2 | 72.081 | C4H10N | Valine | |
| 6 | 120.065 | C4H10NO3 | — | Threonine | ||
| 4 | 133.061 | C4H9N2O3 | — | Asparagine | ||
| 9 | 134.045 | C4H8NO4 | — | Aspartic acid | ||
| 100 | 148.060 | C5H10NO4 | 102.050 | C4H8NO2 | Glutamic acid | |
| 1.55 | 3 | 150.058 | C5H12NO2S | — | Methionine | |
| 1.69 | 30 | 132.102 | C6H14NO2 | 86.096 | C5H12N | L/I * |
| 1.76 | 14 | 182.081 | C9H12NO3 | — | Tyrosine | |
| 2.56 | 4 | 166.086 | C9H12NO2 | 120.081 | C8H10N | Phenylalanine |
| 4.33 | 1 | 205.097 | C11H13N2O2 | — | Tryptophan |
| RT (min) | Intensity × 104 | [M + H]+ m/z | Elemental Composition | Deammonia m/z | Elemental Composition | Compound |
|---|---|---|---|---|---|---|
| 1.07 | 12 | 112.087 | C5H10N3 | 95.060 | C5H7N2 | Histamine |
| 1.34 | 42 | 154.086 | C8H12NO2 | 137.059 | C8H9O2 | Dopamine |
| RT (min) | Intensity × 104 | [M + H]+ m/z | Elemental Composition | Compound |
|---|---|---|---|---|
| 1.28 | 30 | 258.110 | C10H16N3O5 | Thymidine |
| 1.63 | 52 | 348.069 | C10H15N5O7P | AMP (Adenosine monophosphate) * |
| 2 | 664.109 | C21H28N7O14P2 | NAD (Nicotinamide adenine dinucleotide) * | |
| 1.69 | 150 | 268.104 | C10H14N5O4 | Adenosine |
| 1.76 | 5 | 364.065 | C10H15N5O8P | GMP (Guanosine monophosphate) * |
| 1.98 | 9 | 284.099 | C10H14N5O5 | Guanosine |
| 20 | 349.054 | C10H14N4O8P | IMP (Inosine monophosphate) * | |
| 2.05 | 14 | 269.088 | C10H13N4O5 | Inosine |
| Fr | RT | Intensity × 103 | Precursor Ion m/z (Charge) | Molecular Mass (M + H)+ | Sequence |
|---|---|---|---|---|---|
| 4 | 2.51 | 210 | 305.183 (2+) | 609.359 | YVTVK |
| 2.68 | 77 | 361.521 (3+) | 1082.546 | SKPSWHRDA-NH2 | |
| 2.73 | 360 | 413.550 (3+) | 1238.636 | GVSKPSWHRDA-NH2 | |
| 2.88 | 51 | 333.695 (2+) | 666.381 | YVTVKG | |
| 2.95 | 23 | 351.858 (3+) | 1053.557 | GVSKPSWHR | |
| 7 | 4.49 | 36 | 302.676 (2+) | 604.344 | FNPKV |
| 8 | 5.45 | 18 | 338.692 (2+) | 676.377 | GFSPLR |
| 5.61 | 183 | 434.934 (3+) | 1302.786 | VTVKGFSPLRKA | |
| 5.65 | 7400 | 489.288 (3+) | 1465.848 | YVTVKGFSPLRKA | |
| 5.66 | 660 | 401.911 (3+) | 1203.717 | TVKGFSPLRKA | |
| 9 | 5.95 | 163 | 527.629 (3+) | 1580.873 | DYVTVKGFSPLRKA |
| 6.06 | 36 | 561.636 (3+) | 1682.892 | pQLFTKPSGNEGLRPR | |
| 10 | 6.27 | 300 | 578.332 (2+) | 1155.655 | SLGGGVGGLGGLGR-NH2 |
| 6.34 | 40 | 407.228 (2+) | 813.448 | YVTVKGF | |
| 6.49 | 13,600 | 422.911 (3+) | 1266.718 | YVTVKGFSPLR | |
| 11 | 6.63 | 150 | 499.270 (2+) | 997.532 | YVTVKGFSP |
| 6.66 | 18 | 465.925 (3+) | 1395.759 | YVTVKGFSPLRE | |
| 6.77 | 28 | 446.590 (3+) | 1337.754 | AYVTVKGFSPLR | |
| 6.84 | 660 | 498.276 (3+) | 1492.811 | YVTVKGFSPLREP | |
| 13 | 7.49 | 136 | 536.617 (3+) | 1607.836 | DYVTVKGFSPLREP |
| 7.66 | 25 | 548.347 (2+) | 1095.686 | PKLLQSLNAL-NH2 | |
| 7.81 | 18 | 555.812 (2+) | 1110.615 | YVTVKGFSPLR | |
| 15 | 8.37 | 790 | 820.441 (2+) | 1639.873 | pQLFTKPSGNEGLRLP |
| 8.39 | 112 | 630.789 (3+) | 1260.569 | pQDDLSDFNPKV | |
| 16 | 9.37 | 23 | 629.814 (2+) | 1258.620 | pQDVDHVFLRF |
| RT | Intensity × 103 | (M + H)+ | Sequence |
|---|---|---|---|
| Scoliidines (Bradykinin-related peptides) | |||
| 2.51 | 210 | 609.359 | YVTVK |
| 2.88 | 51 | 666.381 | YVTVKG |
| 6.34 | 40 | 813.448 | YVTVKGF |
| 6.63 | 150 | 997.532 | YVTVKGFSP |
| 7.81 | 18 | 1110.615 | YVTVKGFSPL |
| 5.45 | 18 | 676.377 | GFSPLR |
| 6.49 | 13,600 | 1266.718 | YVTVKGFSPLR (γ-scoliidine) |
| 6.77 | 28 | 1337.754 | AYVTVKGFSPLR |
| 5.66 | 660 | 1203.717 | TVKGFSPLRKA |
| 5.61 | 183 | 1302.786 | VTVKGFSPLRKA |
| 5.65 | 7400 | 1465.848 | YVTVKGFSPLRKA |
| 5.95 | 163 | 1580.873 | DYVTVKGFSPLRKA (β-scoliidine) |
| 6.66 | 18 | 1395.759 | YVTVKGFSPLRE |
| 6.84 | 660 | 1492.811 | YVTVKGFSPLREP (δ-scoliidine) |
| 7.49 | 136 | 1607.836 | DYVTVKGFSPLREP (ε-scoliidine) |
| Miscellaneous | |||
| 2.95 | 23 | 1053.557 | GVSKPSWHR |
| 2.73 | 360 | 1238.636 | GVSKPSWHRDA-NH2 |
| 2.68 | 77 | 1082.546 | SKPSWHRDA-NH2 |
| 8.37 | 790 | 1639.873 | pQLFTKPSGNEGLRLP |
| 6.06 | 36 | 1682.892 | pQLFTKPSGNEGLRPR |
| 7.66 | 25 | 1095.686 | PKLLQSLNAL-NH2 |
| 6.27 | 300 | 1155.655 | SLGGGVGGLGGLRG-NH2 |
| Peptide | Sequence | References |
|---|---|---|
| Bradykinin (BK) | RPPGFSPFR | [8,27] |
| Thr6-Bradykinin (Thr6-BK) | RPPGFTPFR | [18,19,27] |
| Megascoliakinin | RPPGFTPFRKA | [20] |
| α-Campsomerin | PRLRRLTGLSPLR | [26] |
| β-Campsomerin | PRLRRLTGLSPLRAP | [26] |
| α-Scoliidine | DYVTVKGFSPLR | [25] |
| β-Scoliidine | DYVTVKGFSPLRKA | [25] This work |
| γ-Scoliidine | YVTVKGFSPLR | This work |
| δ-Scoliidine | YVTVKGFSPLREP | This work |
| ε-Scoliidine | DYVTVKGFSPLREP | This work |
| Inhibition (%) | Cleavage (%) | |||
|---|---|---|---|---|
| Peptide | NEP | ACE | NEP | ACE |
| bradykinin | - | - | 100 | 100 |
| β-scoliidine | <0.01 | <0.01 | <0.01 | <0.01 |
| γ-scoliidine | <0.01 | <0.01 | <0.01 | <0.01 |
| δ-scoliidine | <0.01 | <0.01 | <0.01 | <0.01 |
| ε-scoliidine | <0.01 | <0.01 | <0.01 | <0.01 |
| Peptides | ProbScore | Prediction |
|---|---|---|
| β-scoliidine | 0.26 | Non-hemolytic |
| γ-scoliidine | 0.37 | Non-hemolytic |
| δ-scoliidine | 0.03 | Non-hemolytic |
| ε-scoliidine | 0.00 | Non-hemolytic |
| Indolicidin | 0.94 | Hemolytic |
| Peptides | ProbScore | Prediction |
|---|---|---|
| β-scoliidine | −0.40 | Non-toxic |
| γ-scoliidine | −0.15 | Non-toxic |
| δ-scoliidine | −0.25 | Non-toxic |
| ε-scoliidine | −0.25 | Non-toxic |
| Mµ-conotoxin SxIIIA | 2.64 | Toxic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alberto-Silva, C.; Vieira Portaro, F.C.; Kodama, R.T.; Gomes, L.; da Silva, B.R.; da Cunha e Silva, F.A.; Nihei, K.-i.; Konno, K. Scoliidines: Neuroprotective Peptides in Solitary Scoliid Wasp Venoms. Toxins 2024, 16, 446. https://doi.org/10.3390/toxins16100446
Alberto-Silva C, Vieira Portaro FC, Kodama RT, Gomes L, da Silva BR, da Cunha e Silva FA, Nihei K-i, Konno K. Scoliidines: Neuroprotective Peptides in Solitary Scoliid Wasp Venoms. Toxins. 2024; 16(10):446. https://doi.org/10.3390/toxins16100446
Chicago/Turabian StyleAlberto-Silva, Carlos, Fernanda Calheta Vieira Portaro, Roberto Tadashi Kodama, Lais Gomes, Brenda Rufino da Silva, Felipe Assumpção da Cunha e Silva, Ken-ichi Nihei, and Katsuhiro Konno. 2024. "Scoliidines: Neuroprotective Peptides in Solitary Scoliid Wasp Venoms" Toxins 16, no. 10: 446. https://doi.org/10.3390/toxins16100446
APA StyleAlberto-Silva, C., Vieira Portaro, F. C., Kodama, R. T., Gomes, L., da Silva, B. R., da Cunha e Silva, F. A., Nihei, K.-i., & Konno, K. (2024). Scoliidines: Neuroprotective Peptides in Solitary Scoliid Wasp Venoms. Toxins, 16(10), 446. https://doi.org/10.3390/toxins16100446






