Bacillus amyloliquefaciens B10 Alleviates the Immunosuppressive Effects of Deoxynivalenol and Porcine Circovirus Type 2 Infection
Abstract
:1. Introduction
2. Results
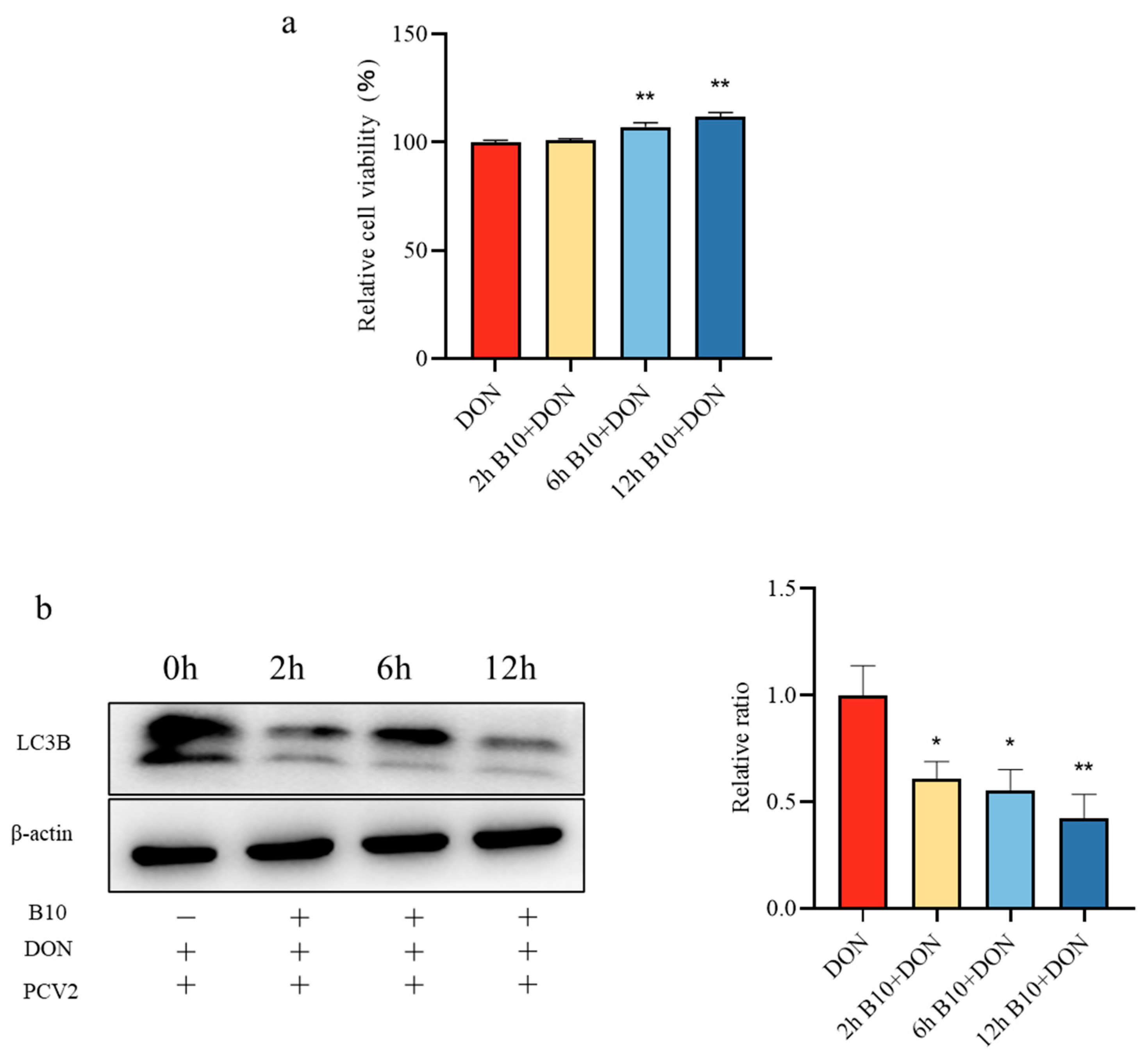
2.1. Cell Viability Assay
2.2. Screening of the Pretreatment Time of Bacillus amyloliquefaciens B10
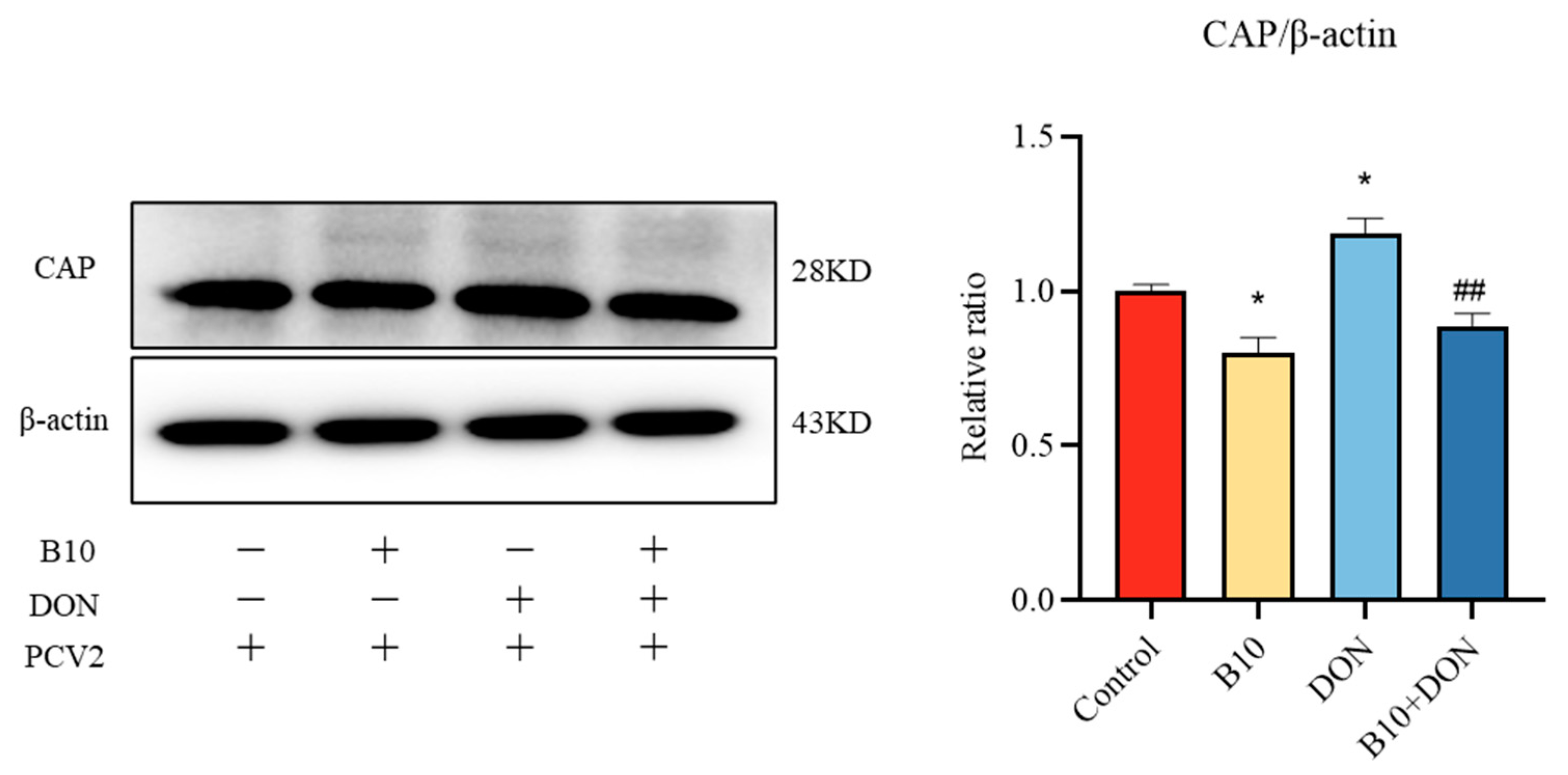
2.3. Effect of Bacillus amyloliquefaciens B10 on DON Promoting PCV2 Infection
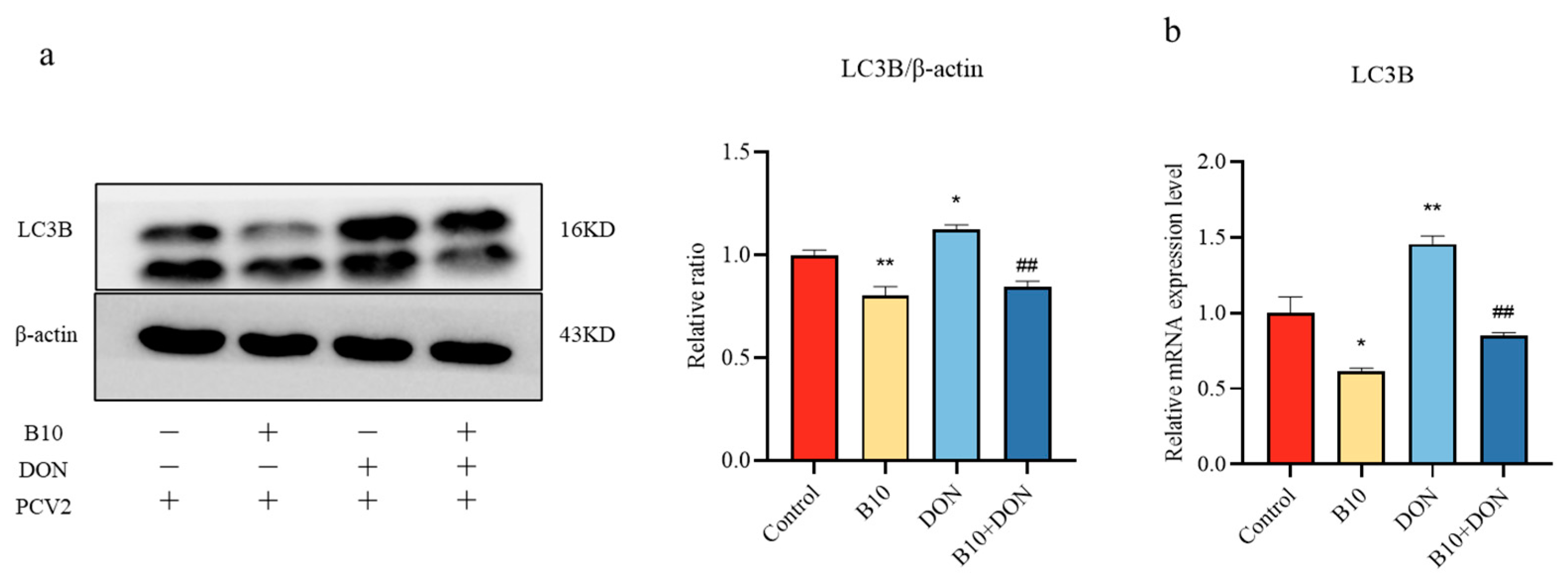
2.4. Effect of DON on the LC3B Protein and Gene after Bacillus amyloliquefaciens B10 Pretreatment
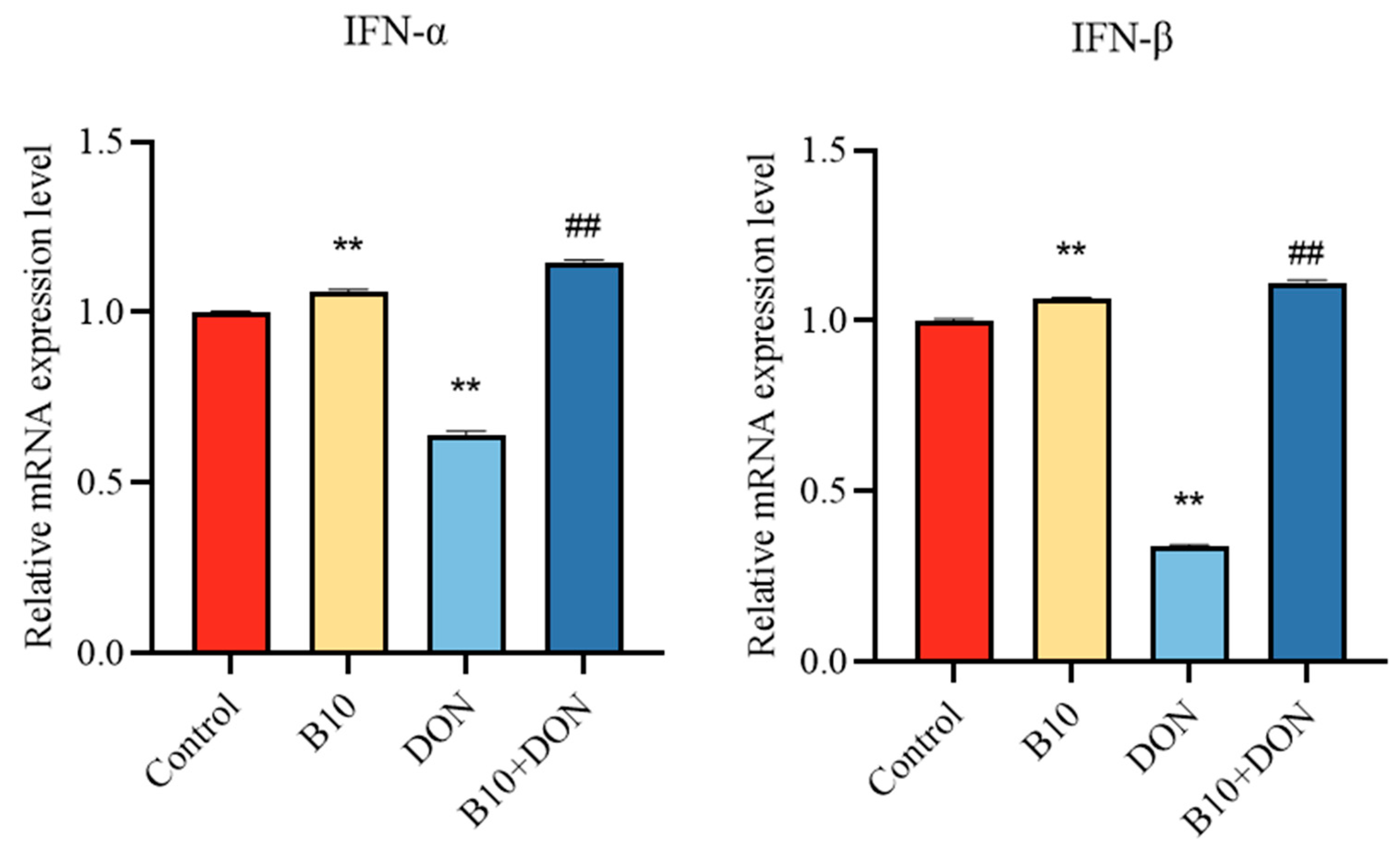
2.5. Type I Interferons IFN-α, IFN-β mRNA Expression after Bacillus amyloliquefaciens B10 Pretreatment
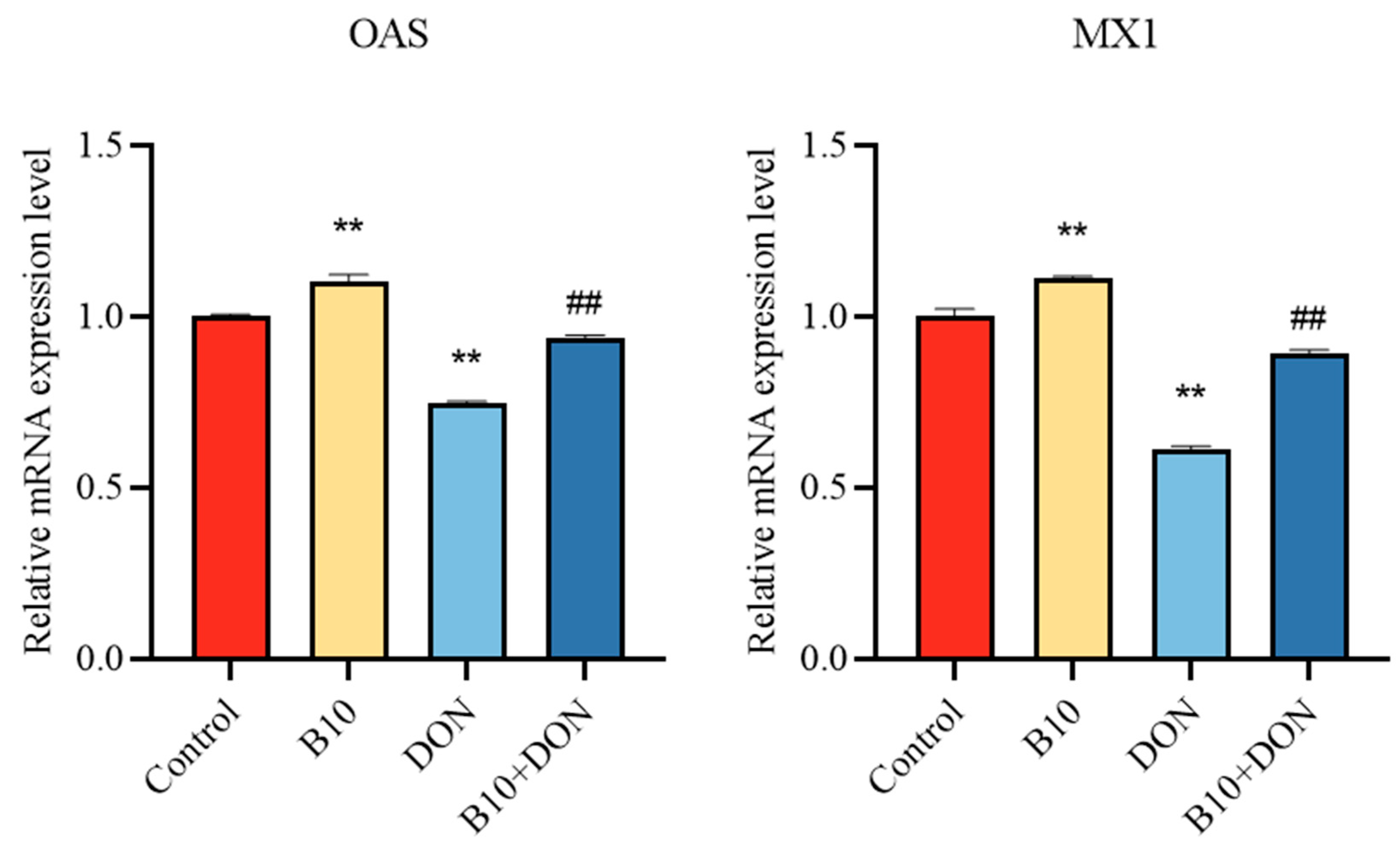
2.6. Expression of the Antiviral Proteins OAS and MX1 mRNA after Bacillus amyloliquefaciens B10 Pretreatment
2.7. Expression of Immune Cytokine IL-10 mRNA after Bacillus amyloliquefaciens B10 Pretreatment
2.8. Bacillus amyloliquefaciens B10 Pretreatment Relieves the Inhibitory Effect of DON on the cGAS–STING Signaling Pathway
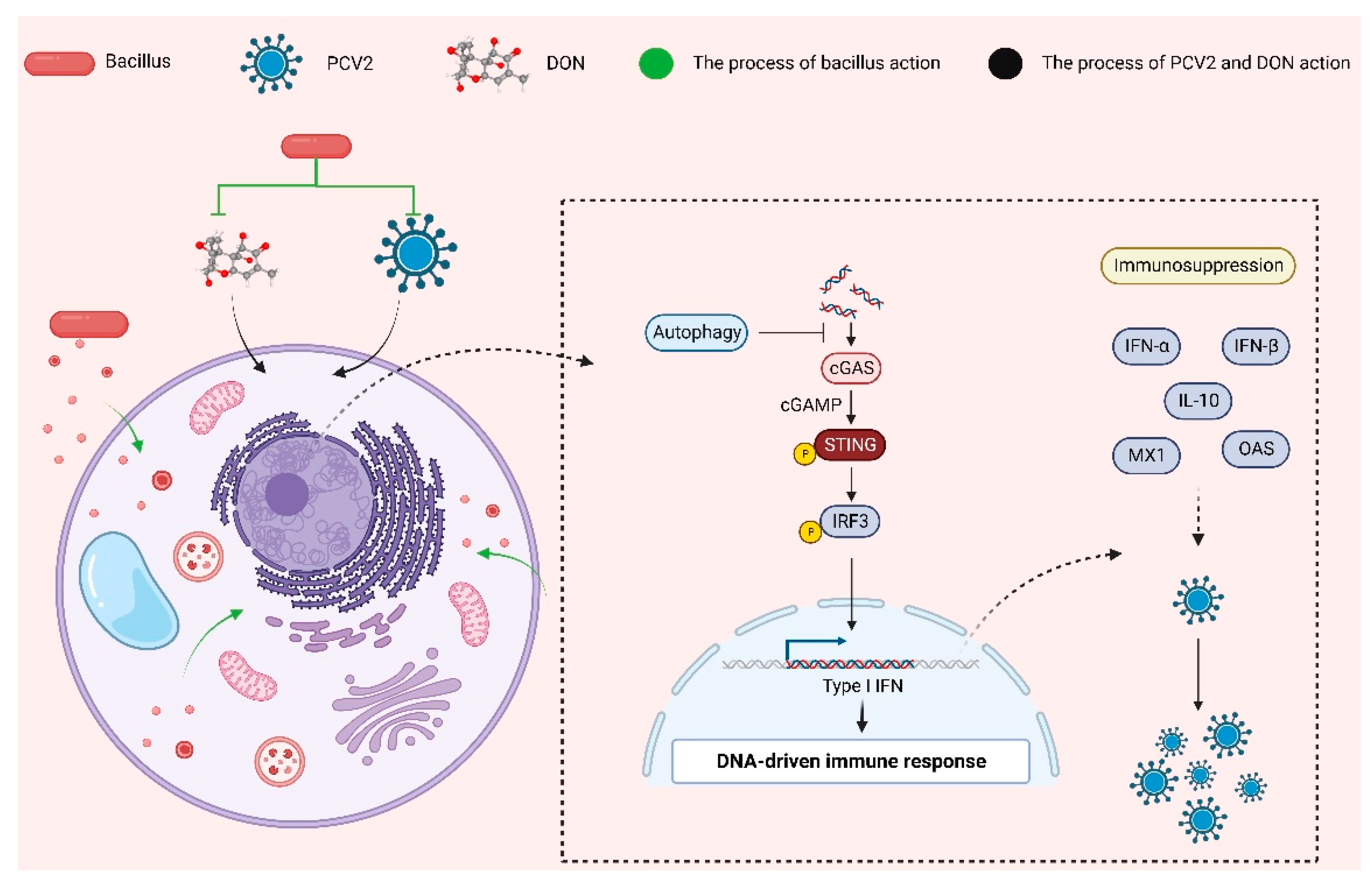
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Virus
5.2. Design of Experiments
5.3. Cell Viability Assay
5.4. qPCR Analysis
5.5. Western Blot
5.6. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Y.; Jiang, J.; Mu, P.; Lin, R.; Wen, J.; Deng, Y. Toxicokinetics and Metabolism of Deoxynivalenol in Animals and Humans. Arch. Toxicol. 2022, 96, 2639–2654. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Zhao, Y.; Kuca, K.; Wang, X.; Oleksak, P.; Chrienova, Z.; Nepovimova, E.; Jaćević, V.; Wu, Q.; Wu, W. Hypoxia, Oxidative Stress, and Immune Evasion: A Trinity of the Trichothecenes T-2 Toxin and Deoxynivalenol (DON). Arch. Toxicol. 2021, 95, 1899–1915. [Google Scholar] [CrossRef] [PubMed]
- Lei, R.; Jiang, N.; Zhang, Q.; Hu, S.; Dennis, B.S.; He, S.; Guo, X. Prevalence of Selenium, T-2 Toxin, and Deoxynivalenol in Kashin-Beck Disease Areas in Qinghai Province, Northwest China. Biol. Trace Elem. Res. 2016, 171, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.-L.; Lu, S.-S.; Wei, W.-K.; Lv, D.-H.; Wen, X.-H.; Zhai, Q.; Chen, Q.-L.; Sun, Y.-W.; Xi, Y. Reservoirs of Porcine Circoviruses: A Mini Review. Front. Vet. Sci. 2019, 6, 319. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Meng, X.-J.; Halbur, P.G. Porcine Circovirus Type 2 Associated Disease: Update on Current Terminology, Clinical Manifestations, Pathogenesis, Diagnosis, and Intervention Strategies. J. Vet. Diagn. Investig. 2007, 19, 591–615. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, R.E.; Meerts, P.; Nauwynck, H.J.; Ellis, J.A.; Pensaert, M.B. Characteristics of Porcine Circovirus-2 Replication in Lymphoid Organs of Pigs Inoculated in Late Gestation or Postnatally and Possible Relation to Clinical and Pathological Outcome of Infection. J. Vet. Diagn. Investig. 2004, 16, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Sewalt, V.; Shanahan, D.; Gregg, L.; La Marta, J.; Carrillo, R. The Generally Recognized as Safe (GRAS) Process for Industrial Microbial Enzymes. Ind. Biotechnol. 2016, 12, 295–302. [Google Scholar] [CrossRef]
- Abriouel, H.; Franz, C.M.A.P.; Ben Omar, N.; Gálvez, A. Diversity and Applications of Bacillus Bacteriocins. FEMS Microbiol. Rev. 2011, 35, 201–232. [Google Scholar] [CrossRef]
- WoldemariamYohannes, K.; Wan, Z.; Yu, Q.; Li, H.; Wei, X.; Liu, Y.; Wang, J.; Sun, B. Prebiotic, Probiotic, Antimicrobial, and Functional Food Applications of Bacillus amyloliquefaciens. J. Agric. Food Chem. 2020, 68, 14709–14727. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Huang, S.-D.; Tu, J.-H.; Yu, J.-S.; Nurlatifah, A.O.; Chiu, W.-C.; Su, Y.-H.; Chang, H.-L.; Putri, D.A.; Cheng, H.-L. Exopolysaccharides of Modulate Glycemic Level in Mice and Promote Glucose Uptake of Cells through the Activation of Akt. Int. J. Biol. Macromol. 2020, 146, 202–211. [Google Scholar] [CrossRef]
- Chang, X.; Wu, Z.; Wu, S.; Dai, Y.; Sun, C. Degradation of Ochratoxin A by Bacillus amyloliquefaciens ASAG1. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 2015, 32, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Siahmoshteh, F.; Siciliano, I.; Banani, H.; Hamidi-Esfahani, Z.; Razzaghi-Abyaneh, M.; Gullino, M.L.; Spadaro, D. Efficacy of Bacillus Subtilis and Bacillus amyloliquefaciens in the Control of Aspergillus Parasiticus Growth and Aflatoxins Production on Pistachio. Int. J. Food Microbiol. 2017, 254, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, H.; Zhu, Z.; Ji, F.; Yin, X.; Hong, Q.; Shi, J. Isolation and Characterization of Bacillus amyloliquefaciens ZDS-1: Exploring the Degradation of Zearalenone by Bacillus spp. Food Control 2016, 68, 244–250. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, T.; Li, P.; Chen, J.; Nepovimova, E.; Long, M.; Wu, W.; Kuca, K. Bacillus amyloliquefaciens B10 Can Alleviate Aflatoxin B1-Induced Kidney Oxidative Stress and Apoptosis in Mice. Ecotoxicol. Environ. Saf. 2021, 218, 112286. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lv, Z.; Chen, J.; Nepovimova, E.; Long, M.; Wu, W.; Kuca, K. Bacillus amyloliquefaciens B10 Can Alleviate Liver Apoptosis and Oxidative Stress Induced by Aflatoxin B1. Food Chem. Toxicol. 2021, 151, 112124. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lv, Z.; Cheng, Z.; Wang, T.; Li, P.; Wu, A.; Nepovimova, E.; Long, M.; Wu, W.; Kuca, K. Bacillus amyloliquefaciens B10 Inhibits Aflatoxin B1-Induced Cecal Inflammation in Mice by Regulating Their Intestinal Flora. Food Chem. Toxicol. 2021, 156, 112438. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, L.; Chen, X.; Du, F.; Shi, H.; Chen, C.; Chen, Z.J. Cyclic GMP-AMP Is an Endogenous Second Messenger in Innate Immune Signaling by Cytosolic DNA. Science 2013, 339, 826–830. [Google Scholar] [CrossRef]
- Decout, A.; Katz, J.D.; Venkatraman, S.; Ablasser, A. The cGAS-STING Pathway as a Therapeutic Target in Inflammatory Diseases. Nat. Rev. Immunol. 2021, 21, 548–569. [Google Scholar] [CrossRef]
- Paul, P.; Münz, C. Autophagy and Mammalian Viruses: Roles in Immune Response, Viral Replication, and Beyond. Adv. Virus Res. 2016, 95, 149–195. [Google Scholar] [CrossRef]
- Nassour, J.; Radford, R.; Correia, A.; Fusté, J.M.; Schoell, B.; Jauch, A.; Shaw, R.J.; Karlseder, J. Autophagic Cell Death Restricts Chromosomal Instability during Replicative Crisis. Nature 2019, 565, 659–663. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, M.; Wang, X.; Bu, Q.; Wang, Q.; Su, W.; Li, L.; Zhou, H.; Lu, L. XBP1 Deficiency Promotes Hepatocyte Pyroptosis by Impairing Mitophagy to Activate mtDNA-cGAS-STING Signaling in Macrophages during Acute Liver Injury. Redox Biol. 2022, 52, 102305. [Google Scholar] [CrossRef] [PubMed]
- Grau-Roma, L.; Stockmarr, A.; Kristensen, C.S.; Enøe, C.; López-Soria, S.; Nofrarías, M.; Bille-Hansen, V.; Hjulsager, C.K.; Sibila, M.; Jorsal, S.E.; et al. Infectious Risk Factors for Individual Postweaning Multisystemic Wasting Syndrome (PMWS) Development in Pigs from Affected Farms in Spain and Denmark. Res. Vet. Sci. 2012, 93, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Milićević, D.; Nastasijevic, I.; Petrovic, Z. Mycotoxin in the Food Supply Chain-Implications for Public Health Program. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2016, 34, 293–319. [Google Scholar] [CrossRef] [PubMed]
- Savard, C.; Provost, C.; Alvarez, F.; Pinilla, V.; Music, N.; Jacques, M.; Gagnon, C.A.; Chorfi, Y. Effect of Deoxynivalenol (DON) Mycotoxin on in Vivo and in Vitro Porcine Circovirus Type 2 Infections. Vet. Microbiol. 2015, 176, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Savard, C.; Pinilla, V.; Provost, C.; Gagnon, C.A.; Chorfi, Y. In Vivo Effect of Deoxynivalenol (DON) Naturally Contaminated Feed on Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Infection. Vet. Microbiol. 2014, 174, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-T.; Lumsden, J.S. Impact of Feed Restriction, Chloroquine and Deoxynivalenol on Viral Haemorrhagic Septicaemia Virus IVb in Fathead Minnow Pimephales Promelas Rafinesque. J. Fish Dis. 2021, 44, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Poelaert, K.C.K.; Van Cleemput, J.; Laval, K.; Favoreel, H.W.; Couck, L.; Van den Broeck, W.; Azab, W.; Nauwynck, H.J. Equine Herpesvirus 1 Bridles T Lymphocytes To Reach Its Target Organs. J. Virol. 2019, 93, e02098-18. [Google Scholar] [CrossRef]
- Li, M.; Harkema, J.R.; Cuff, C.F.; Pestka, J.J. Deoxynivalenol Exacerbates Viral Bronchopneumonia Induced by Respiratory Reovirus Infection. Toxicol. Sci. 2007, 95, 412–426. [Google Scholar] [CrossRef]
- Liu, D.; Ge, L.; Wang, Q.; Su, J.; Chen, X.; Wang, C.; Huang, K. Low-Level Contamination of Deoxynivalenol: A Threat from Environmental Toxins to Porcine Epidemic Diarrhea Virus Infection. Environ. Int. 2020, 143, 105949. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Hu, X.; Liu, W. Current Status of Probiotics as Supplements in the Prevention and Treatment of Infectious Diseases. Front. Cell. Infect. Microbiol. 2022, 12, 789063. [Google Scholar] [CrossRef]
- Rastelli, M.; Cani, P.D.; Knauf, C. The Gut Microbiome Influences Host Endocrine Functions. Endocr. Rev. 2019, 40, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Zhou, Y.; Xu, Y.; Wang, S.; Wang, B.; Zeng, Z.; Wang, Q.; Ye, X.; Jin, L.; Yue, M.; et al. Bacillus amyloliquefaciens SC06 Alleviates the Obesity of Ob/Ob Mice and Improves Their Intestinal Microbiota and Bile Acid Metabolism. Food Funct. 2022, 13, 5381–5395. [Google Scholar] [CrossRef] [PubMed]
- Jebali, R.; Ben Salah-Abbès, J.; Abbès, S.; Hassan, A.M.; Abdel-Aziem, S.H.; El-Nekeety, A.A.; Oueslati, R.; Abdel-Wahhab, M.A. Lactobacillus Plantarum Alleviate Aflatoxins (B1 and M1) Induced Disturbances in the Intestinal Genes Expression and DNA Fragmentation in Mice. Toxicon 2018, 146, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, S.; Rodiles, A.; Hatef, A.; Picchietti, S.; Cossignani, L.; Merrifield, D.L.; Unniappan, S.; Carnevali, O. Influence of Probiotics Administration on Gut Microbiota Core: A Review on the Effects on Appetite Control, Glucose, and Lipid Metabolism. J. Clin. Gastroenterol. 2018, 52 (Suppl. S1), S50–S56. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Christodoulides, S.; Scott, S.M.; Whelan, K. Mechanisms of Action of Probiotics and the Gastrointestinal Microbiota on Gut Motility and Constipation. Adv. Nutr. 2017, 8, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Ashkar, A.A. The Dual Nature of Type I and Type II Interferons. Front. Immunol. 2018, 9, 2061. [Google Scholar] [CrossRef]
- Jiao, R.; Cai, Y.; He, P.; Munir, S.; Li, X.; Wu, Y.; Wang, J.; Xia, M.; He, P.; Wang, G.; et al. Bacillus amyloliquefaciens YN201732 Produces Lipopeptides With Promising Biocontrol Activity Against Fungal Pathogen Erysiphe Cichoracearum. Front. Cell. Infect. Microbiol. 2021, 11, 598999. [Google Scholar] [CrossRef]
- Ma, Z.; Jacobs, S.R.; West, J.A.; Stopford, C.; Zhang, Z.; Davis, Z.; Barber, G.N.; Glaunsinger, B.A.; Dittmer, D.P.; Damania, B. Modulation of the cGAS-STING DNA Sensing Pathway by Gammaherpesviruses. Proc. Natl. Acad. Sci. USA 2015, 112, E4306–E4315. [Google Scholar] [CrossRef]
- Lio, C.-W.J.; McDonald, B.; Takahashi, M.; Dhanwani, R.; Sharma, N.; Huang, J.; Pham, E.; Benedict, C.A.; Sharma, S. cGAS-STING Signaling Regulates Initial Innate Control of Cytomegalovirus Infection. J. Virol. 2016, 90, 7789–7797. [Google Scholar] [CrossRef]
- Wu, J.; Li, W.; Shao, Y.; Avey, D.; Fu, B.; Gillen, J.; Hand, T.; Ma, S.; Liu, X.; Miley, W.; et al. Inhibition of cGAS DNA Sensing by a Herpesvirus Virion Protein. Cell Host Microbe 2015, 18, 333–344. [Google Scholar] [CrossRef]
- Gan, F.; Zhou, Y.; Qian, G.; Huang, D.; Hou, L.; Liu, D.; Chen, X.; Wang, T.; Jiang, P.; Lei, X.; et al. PCV2 Infection Aggravates Ochratoxin A-Induced Nephrotoxicity via Autophagy Involving P38 Signaling Pathway in Vivo and in Vitro. Environ. Pollut. 2018, 238, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lin, J.; Su, J.; Chen, X.; Jiang, P.; Huang, K. Glutamine Deficiency Promotes PCV2 Infection through Induction of Autophagy via Activation of ROS-Mediated JAK2/STAT3 Signaling Pathway. J. Agric. Food Chem. 2018, 66, 11757–11766. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xu, J.; Qian, G.; Hamid, M.; Gan, F.; Chen, X.; Huang, K. Selenizing Astragalus Polysaccharide Attenuates PCV2 Replication Promotion Caused by Oxidative Stress through Autophagy Inhibition via PI3K/AKT Activation. Int. J. Biol. Macromol. 2018, 108, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.L.; Liu, S.Z.; Shang, Z.D.; Liu, Y.J.; Wang, J.; Zhang, W.X.; Dong, B.; Cao, Y.H. Bacillus amyloliquefaciens TL106 Protects Mice against Enterohaemorrhagic Escherichia Coli O157:H7-Induced Intestinal Disease through Improving Immune Response, Intestinal Barrier Function and Gut Microbiota. J. Appl. Microbiol. 2021, 131, 470–484. [Google Scholar] [CrossRef]



| Primer | Primer Sequences | Tm |
|---|---|---|
| GAPDH | F: CGTCAAGCTCATTTCCTGGT R: TGGGATGGAAACTGGAAGTC | 55.2 54.5 |
| LC3 | F: CCGAACCTTCGAACAGAGAG R: AGGCTTGGTTAGCATTGAGC | 55.5 55.6 |
| IFN-α | F: CAACCAGGTCCAGAAGGCTCAAG R: GCTGATCCAGTCCAGTGCAGAAC | 56.5 56.5 |
| IFN-β | F: CAGTATTGATTATCCACGAGA R: TCTGCCCATCAAGTTCCAC | 48.3 55.1 |
| OAS | F: CCTTGCTGCTATTCCGTGCTCTG R: TCCTGTTGTGGCTTAGAGACCTCTC | 56.5 52.0 |
| MX1 | F: CGGCTGTTTACCAAGATGCGAAATG R: TTCACAAACCCTGGCAACTCTCTC | 48.0 50.0 |
| IL-10 | F: AAGCCTTGTCAGAGATGATCCAGT R: CTCCTTGATATCCTCCCCATCACTC | 45.8 52.0 |
| Antibody | Number |
|---|---|
| cGAS Rabbit pAb | A8335 |
| STING Rabbit mAb | A3262 |
| Phospho-STING Rabbit mAb | AP1223 |
| IRF3 Rabbit pAb | A11118 |
| Phospho-IRF3-S386 Rabbit pAb | Ap0857 |
| β-Actin Rabbit pAb | Ac006 |
| Rabbit anti-PCV2 Cap protein pAb | Bs20021R |
| LC3A/B (D3U4C) Rabbit mAb | #12741 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, H.; Qin, Z.; Xu, B.; Long, M.; Wu, Q.; Guo, X.; Zhang, H.; Li, Z.; Wu, W. Bacillus amyloliquefaciens B10 Alleviates the Immunosuppressive Effects of Deoxynivalenol and Porcine Circovirus Type 2 Infection. Toxins 2024, 16, 14. https://doi.org/10.3390/toxins16010014
Xiao H, Qin Z, Xu B, Long M, Wu Q, Guo X, Zhang H, Li Z, Wu W. Bacillus amyloliquefaciens B10 Alleviates the Immunosuppressive Effects of Deoxynivalenol and Porcine Circovirus Type 2 Infection. Toxins. 2024; 16(1):14. https://doi.org/10.3390/toxins16010014
Chicago/Turabian StyleXiao, Huiping, Zihui Qin, Baocai Xu, Miao Long, Qinghua Wu, Xinyi Guo, Huayue Zhang, Zelin Li, and Wenda Wu. 2024. "Bacillus amyloliquefaciens B10 Alleviates the Immunosuppressive Effects of Deoxynivalenol and Porcine Circovirus Type 2 Infection" Toxins 16, no. 1: 14. https://doi.org/10.3390/toxins16010014
APA StyleXiao, H., Qin, Z., Xu, B., Long, M., Wu, Q., Guo, X., Zhang, H., Li, Z., & Wu, W. (2024). Bacillus amyloliquefaciens B10 Alleviates the Immunosuppressive Effects of Deoxynivalenol and Porcine Circovirus Type 2 Infection. Toxins, 16(1), 14. https://doi.org/10.3390/toxins16010014







