Lactobacillus fermentum Alleviates the Colorectal Inflammation Induced by Low-Dose Sub-Chronic Microcystin-LR Exposure
Abstract
:1. Introduction
2. Results
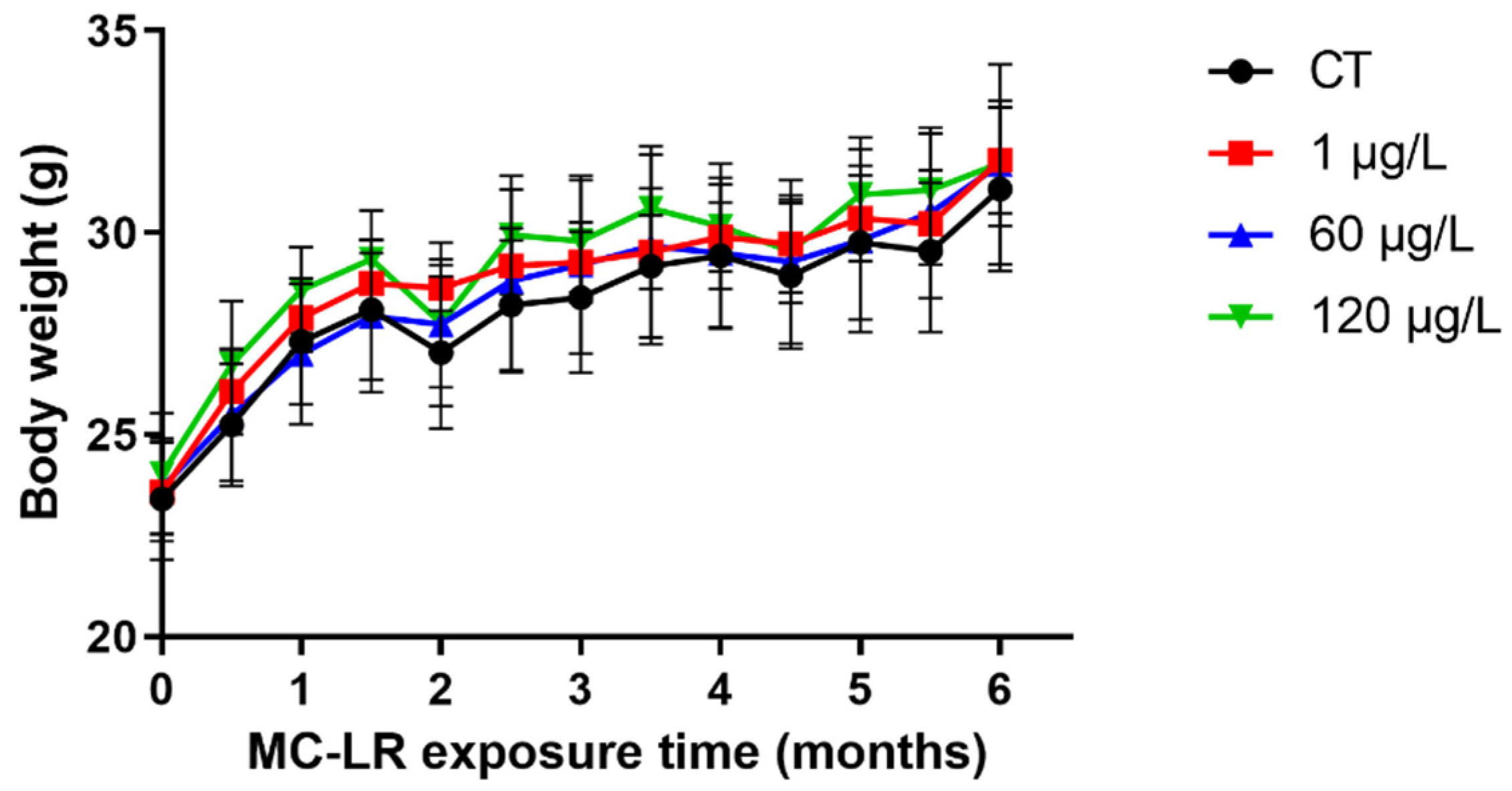
2.1. The Body Weight of MC-LR-Induced Mice
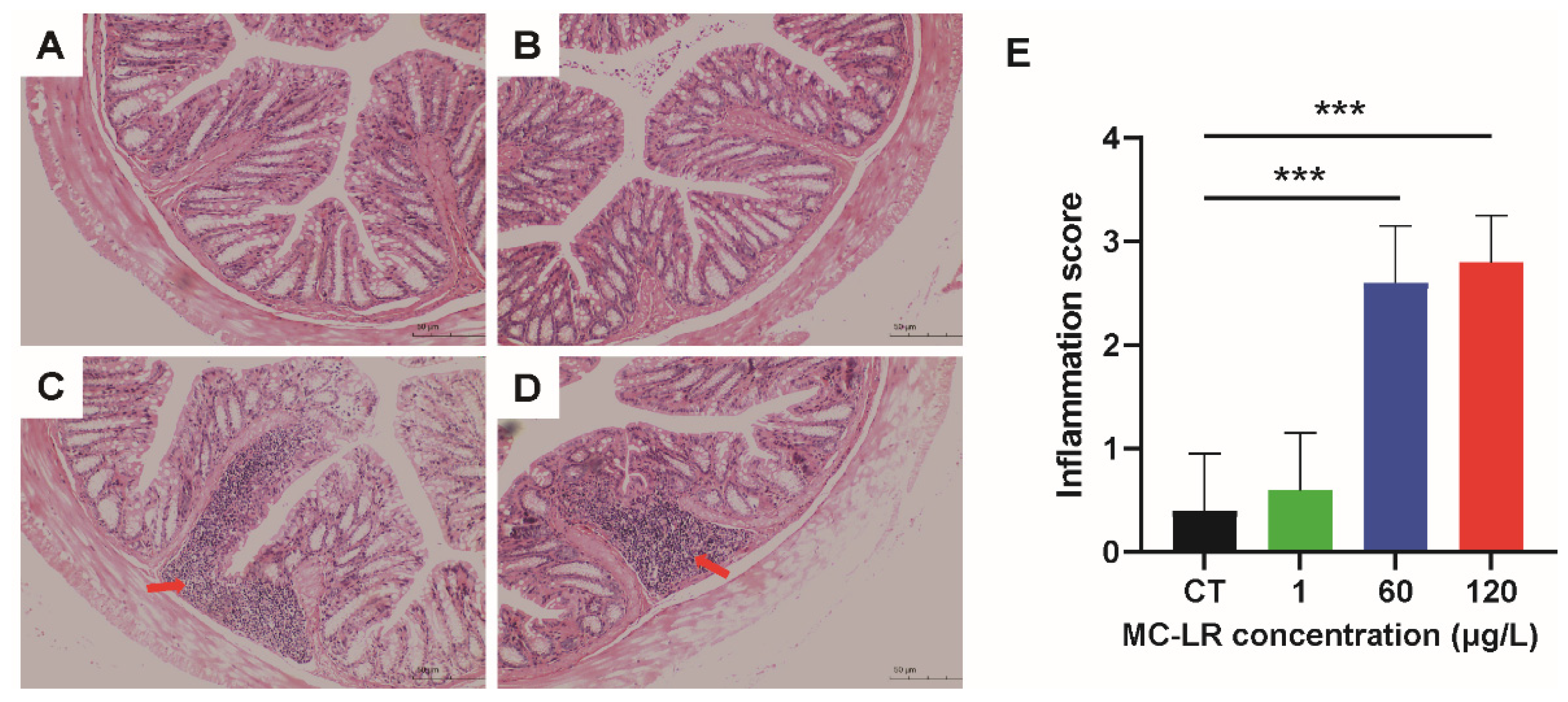
2.2. Histopathology Damage of MC-LR-Induced Mice
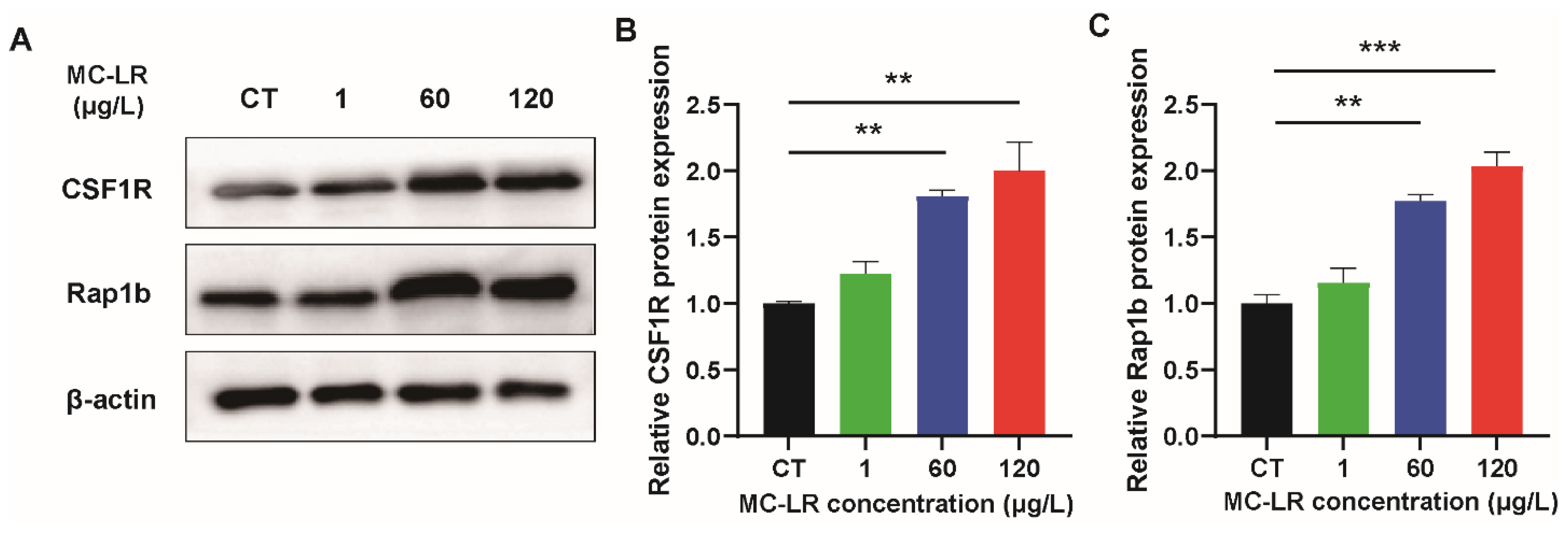
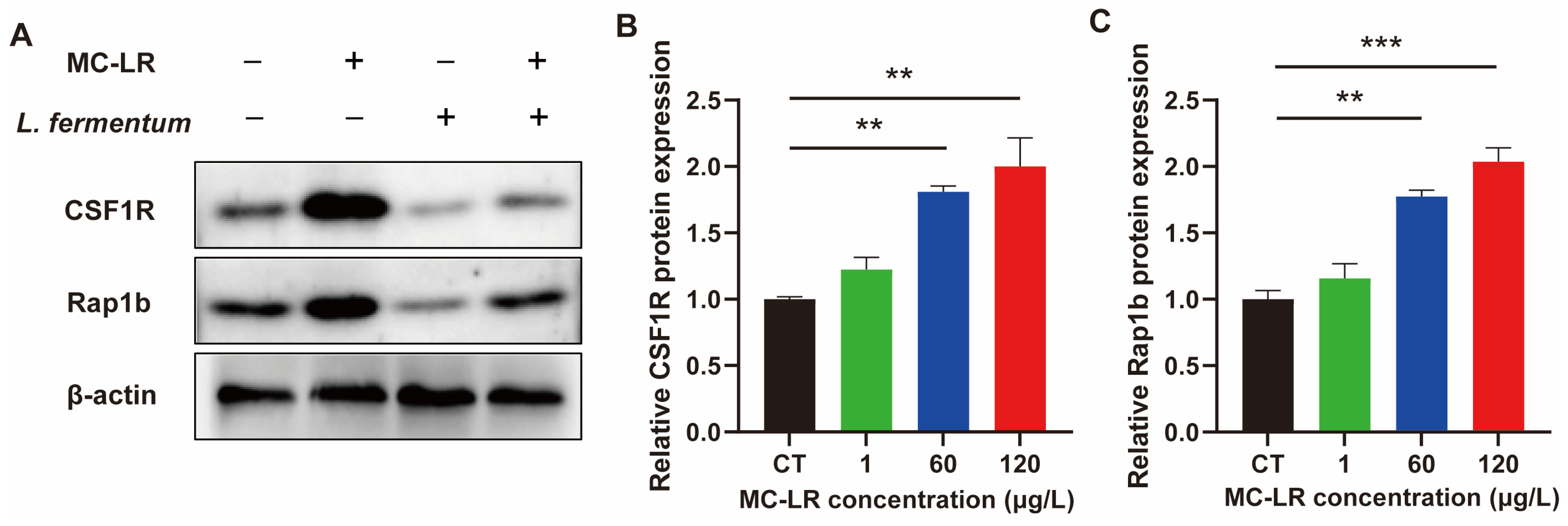
2.3. The Expression of Inflammatory Factors and CSF1R/Rap1b Signaling Pathway-Related Proteins in MC-LR-Induced Mice
2.4. Gut Microbiome Alterations of MC-LR-Induced Mice
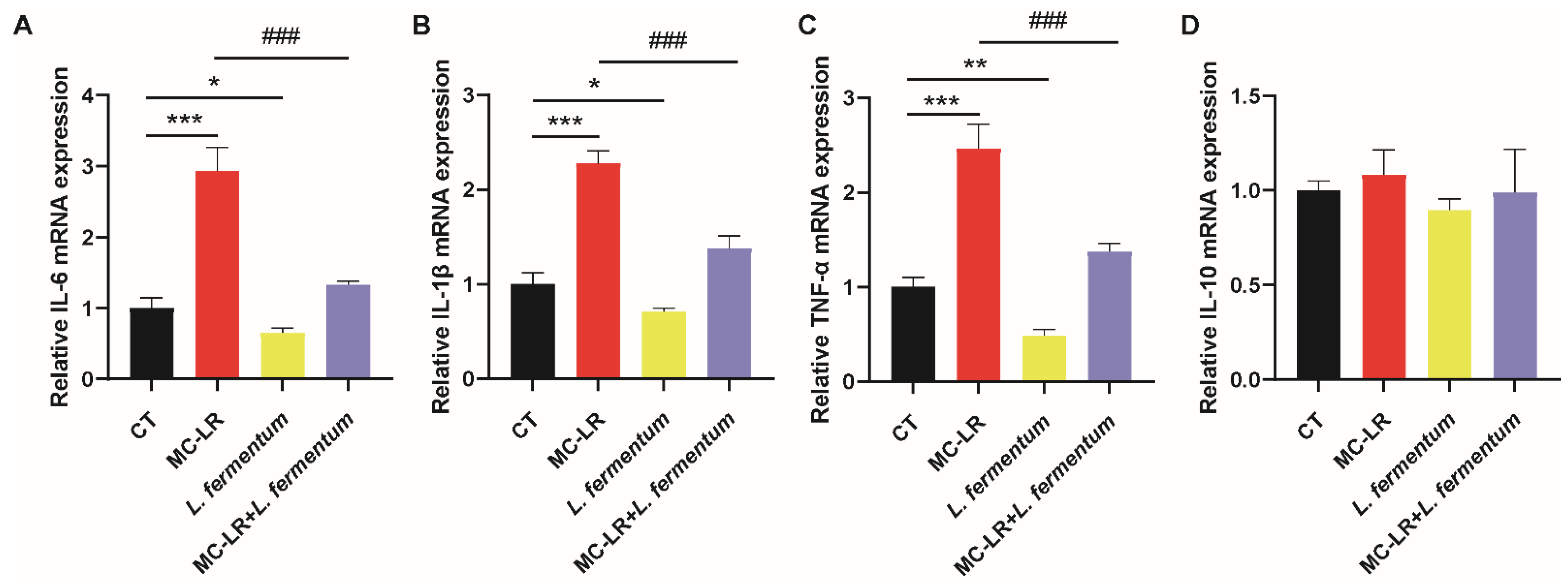
2.5. Effect of Lactobacillus fermentum (L. fermentum) Treatment on the Expression of CSF1R/Rap1b Signaling Pathway-Related Proteins and the Inflammatory Factors in MC-LR-Treated Cell Model
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Reagents
5.2. Design of Animal Experiment
5.3. Histological Analysis
5.4. Metagenomic Sequencing
5.5. Design of Cell Experiment
5.6. Western Blotting (WB)
5.7. qRT-PCR
5.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Deng, S.; Tang, Y.; Liu, Y.; Yang, Y.; Xu, S.; Tang, P.; Lu, Y.; Duan, Y.; Wei, J.; et al. Microcystin-LR Combined with Cadmium Exposures and the Risk of Chronic Kidney Disease: A Case-Control Study in Central China. Environ. Sci. Technol. 2022, 56, 15818–15827. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Massey, I.Y.; Guo, J.; Yang, S.; Pu, Y.; Zeng, W.; Tan, H. Microcystin-LR degradation utilizing a novel effective indigenous bacterial community YFMCD1 from Lake Taihu. J. Toxicol. Environ. Health A 2018, 81, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Pengji, Z.; Zhang, J.; Peng, T.; Luo, J.; Yang, F. Biodegradation of MC-LR and its key bioactive moiety Adda by Sphingopyxis sp. YF1: Comprehensive elucidation of the mechanisms and pathways. Water Res. 2023, 229, 119397. [Google Scholar] [CrossRef]
- Zheng, S.; Yang, Y.; Wen, C.; Liu, W.; Cao, L.; Feng, X.; Chen, J.; Wang, H.; Tang, Y.; Tian, L.; et al. Effects of environmental contaminants in water resources on nonalcoholic fatty liver disease. Environ. Int. 2021, 154, 106555. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.A.; Zhao, Q.; Pu, C.; Qiu, Z.; Zhang, R.; Shu, W. A cross-sectional investigation of chronic exposure to microcystin in relationship to childhood liver damage in the Three Gorges Reservoir Region, China. Environ. Health Perspect. 2011, 119, 1483–1488. [Google Scholar] [CrossRef]
- Lin, H.; Liu, W.; Zeng, H.; Pu, C.; Zhang, R.; Qiu, Z.; Chen, J.A.; Wang, L.; Tan, Y.; Zheng, C.; et al. Determination of Environmental Exposure to Microcystin and Aflatoxin as a Risk for Renal Function Based on 5493 Rural People in Southwest China. Environ. Sci. Technol. 2016, 50, 5346–5356. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Wang, X.; Chen, L.; Liu, W.; Cai, D.; Deng, S.; Chu, H.; Liu, Y.; Feng, X.; et al. Long-term environmental levels of microcystin-LR exposure induces colorectal chronic inflammation, fibrosis and barrier disruption via CSF1R/Rap1b signaling pathway. J. Hazard. Mater. 2022, 440, 129793. [Google Scholar] [CrossRef]
- Du, C.; Zheng, S.; Yang, Y.; Feng, X.; Chen, J.; Tang, Y.; Wang, H.; Yang, F. Chronic exposure to low concentration of MC-LR caused hepatic lipid metabolism disorder. Ecotoxicol. Environ. Saf. 2022, 239, 113649. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, H.; Chen, K. Relationship between microcystin in drinking water and colorectal cancer. Biomed. Environ. Sci. 2002, 15, 166–171. [Google Scholar]
- Sedan, D.; Laguens, M.; Copparoni, G.; Aranda, J.O.; Giannuzzi, L.; Marra, C.A.; Andrinolo, D. Hepatic and intestine alterations in mice after prolonged exposure to low oral doses of Microcystin-LR. Toxicon 2015, 104, 26–33. [Google Scholar] [CrossRef]
- Honda, K.; Littman, D.R. The microbiome in infectious disease and inflammation. Annu. Rev. Immunol. 2012, 30, 759–795. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Tian, X.; Xu, P.; Sun, K.; Ren, N. Acute toxic effects of microcystin-LR on crayfish (Procambarus clarkii): Insights from antioxidant system, histopathology and intestinal flora. Environ. Sci. Pollut. Res. Int. 2023, 30, 56608–56619. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, H.; Wang, C.; Li, S.; Cai, Y. Subchronic Toxicity of Microcystin-LR on Young Frogs (Xenopus laevis) and Their Gut Microbiota. Front. Microbiol. 2022, 13, 895383. [Google Scholar] [CrossRef]
- Ding, W.; Shangguan, Y.; Zhu, Y.; Sultan, Y.; Feng, Y.; Zhang, B.; Liu, Y.; Ma, J.; Li, X. Negative impacts of microcystin-LR and glyphosate on zebrafish intestine: Linked with gut microbiota and microRNAs? Environ. Pollut. 2021, 286, 117685. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Kim, S.K.; Guevarra, R.B.; Kim, Y.T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.H. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef]
- Abraham, B.P.; Quigley, E. Probiotics in Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2017, 46, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yang, W.R.; Wang, X.H.; Li, G.Q.; Xu, L.Q.; Cui, X.; Liu, Y.; Zuo, X.L. Clostridium butyricum alleviates intestinal low-grade inflammation in TNBS-induced irritable bowel syndrome in mice by regulating functional status of lamina propria dendritic cells. World J. Gastroenterol. 2019, 25, 5469–5482. [Google Scholar] [CrossRef]
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O.; Eid, J. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes 2021, 13, 1–28. [Google Scholar] [CrossRef]
- Baradaran, G.S.; Asadzadeh, A.H.; Sorrentino, D.; Shahrokh, S.; Farmani, M.; Ashrafian, F.; Dore, M.P.; Keshavarz, A.R.S.; Mobin, K.S.; Zali, M.R. Probiotic-Induced Tolerogenic Dendritic Cells: A Novel Therapy for Inflammatory Bowel Disease? Int. J. Mol. Sci. 2021, 22, 8274. [Google Scholar] [CrossRef]
- Chen, T.; Wang, R.; Duan, Z.; Yuan, X.; Ding, Y.; Feng, Z.; Bu, F.; Liu, L.; Wang, Q.; Zhou, J.; et al. Akkermansia muciniphila Protects Against Psychological Disorder-Induced Gut Microbiota-Mediated Colonic Mucosal Barrier Damage and Aggravation of Colitis. Front. Cell. Infect. Microbiol. 2021, 11, 723856. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Tian, F.; Zhai, Q.; Yu, R.; Zhang, H.; Gu, Z.; Chen, W. Protective effects of a cocktail of lactic acid bacteria on microcystin-LR-induced hepatotoxicity and oxidative damage in BALB/c mice. RSC Adv. 2017, 7, 20480–20487. [Google Scholar] [CrossRef]
- El-Gamal, M.I.; Al-Ameen, S.K.; Al-Koumi, D.M.; Hamad, M.G.; Jalal, N.A.; Oh, C.H. Recent Advances of Colony-Stimulating Factor-1 Receptor (CSF-1R) Kinase and Its Inhibitors. J. Med. Chem. 2018, 61, 5450–5466. [Google Scholar] [CrossRef] [PubMed]
- Achkova, D.; Maher, J. Role of the colony-stimulating factor (CSF)/CSF-1 receptor axis in cancer. Biochem. Soc. Trans. 2016, 44, 333–341. [Google Scholar] [CrossRef]
- Su, R.C.; Blomquist, T.M.; Kleinhenz, A.L.; Khalaf, F.K.; Dube, P.; Lad, A.; Breidenbach, J.D.; Mohammed, C.J.; Zhang, S.; Baum, C.E.; et al. Exposure to the Harmful Algal Bloom (HAB) Toxin Microcystin-LR (MC-LR) Prolongs and Increases Severity of Dextran Sulfate Sodium (DSS)-Induced Colitis. Toxins 2019, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Su, R.C.; Warner, E.A.; Breidenbach, J.D.; Lad, A.; Blomquist, T.M.; Kleinhenz, A.L.; Modyanov, N.; Malhotra, D.; Kennedy, D.J.; Haller, S.T. CD40 Receptor Knockout Protects against Microcystin-LR (MC-LR) Prolongation and Exacerbation of Dextran Sulfate Sodium (DSS)-Induced Colitis. Biomedicines 2020, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Chen, Y.; Xu, T.; Wang, J.; Li, D.; Han, X. Chronic exposure to microcystin-leucine-arginine promoted proliferation of prostate epithelial cells resulting in benign prostatic hyperplasia. Environ. Pollut. 2018, 242, 1535–1545. [Google Scholar] [CrossRef]
- He, J.; Shu, Y.; Dai, Y.; Gao, Y.; Liu, S.; Wang, W.; Jiang, H.; Zhang, H.; Hong, P.; Wu, H. Microcystin-leucine arginine exposure induced intestinal lipid accumulation and MC-LR efflux disorder in Lithobates catesbeianus tadpoles. Toxicology 2022, 465, 153058. [Google Scholar] [CrossRef]
- Kiu, R.; Hall, L.J. An update on the human and animal enteric pathogen Clostridium perfringens. Emerg. Microbes Infect. 2018, 7, 141. [Google Scholar] [CrossRef]
- Cao, L.; Huang, F.; Massey, I.Y.; Wen, C.; Zheng, S.; Xu, S.; Yang, F. Effects of Microcystin-LR on the Microstructure and Inflammation-Related Factors of Jejunum in Mice. Toxins 2019, 11, 482. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Shu, Y.; Tian, C.; Wang, C.; Fang, T.; Xiao, B.; Wu, X. Effects of chronic exposure to microcystin-LR on life-history traits, intestinal microbiota and transcriptomic responses in Chironomus pallidivittatus. Sci. Total Environ. 2022, 823, 153624. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Xiong, D.; Wang, Y.; Dong, H.; Huang, J.; Zhang, J. Effects of Microcystis aeruginosa and microcystin-LR on intestinal histology, immune response, and microbial community in Litopenaeus vannamei. Environ. Pollut. 2020, 265, 114774. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Pan, R.; Liang, X.; Wu, X.; Wu, Y.; Zhang, H.; Zhao, J.; Chen, W. Perfluorooctanoic acid-induced liver injury is potentially associated with gut microbiota dysbiosis. Chemosphere 2021, 266, 129004. [Google Scholar] [CrossRef]
- Ball, L.M.; Rafter, J.J.; Gustafsson, J.A.; Gustafsson, B.E.; Kohan, M.J.; Lewtas, J. Formation of mutagenic urinary metabolites from 1-nitropyrene in germ-free and conventional rats: Role of the gut flora. Carcinogenesis 1991, 12, 1–5. [Google Scholar] [CrossRef]
- O’Callaghan, J.; O’Toole, P.W. Lactobacillus: Host-microbe relationships. Curr. Top. Microbiol. Immunol. 2013, 358, 119–154. [Google Scholar] [CrossRef]
- Petrova, M.I.; Reid, G.; Vaneechoutte, M.; Lebeer, S. Lactobacillus iners: Friend or Foe? Trends Microbiol. 2017, 25, 182–191. [Google Scholar] [CrossRef]
- Heeney, D.D.; Gareau, M.G.; Marco, M.L. Intestinal Lactobacillus in health and disease, a driver or just along for the ride? Curr. Opin. Biotechnol. 2018, 49, 140–147. [Google Scholar] [CrossRef]
- Kong, Y.; Olejar, K.J.; On, S.; Chelikani, V. The Potential of Lactobacillus spp. for Modulating Oxidative Stress in the Gastrointestinal Tract. Antioxidants 2020, 9, 610. [Google Scholar] [CrossRef]
- Jian, Y.P.; Yang, G.; Zhang, L.H.; Liang, J.Y.; Zhou, H.L.; Wang, Y.S.; Xu, Z.X. Lactobacillus plantarum alleviates irradiation-induced intestinal injury by activation of FXR-FGF15 signaling in intestinal epithelia. J. Cell. Physiol. 2022, 237, 1845–1856. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, D.; Qi, W.; Hong, T.; Xiong, T.; Wu, T.; Geng, F.; Xie, M.; Nie, S. Exopolysaccharides from Lactobacillus plantarum NCU116 Facilitate Intestinal Homeostasis by Modulating Intestinal Epithelial Regeneration and Microbiota. J. Agric. Food Chem. 2021, 69, 7863–7873. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, J.; Sun, R.; Wang, M.; Wang, K.; Li, Y.; Shang, H.; Hou, J.; Jiang, Z. Lactobacillus plantarum 23-1 improves intestinal inflammation and barrier function through the TLR4/NF-kappaB signaling pathway in obese mice. Food Funct. 2022, 13, 5971–5986. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cheng, S.; Huo, J.; Dong, K.; Ding, Y.; Man, C.; Zhang, Y.; Jiang, Y. Lactobacillus plantarum J26 Alleviating Alcohol-Induced Liver Inflammation by Maintaining the Intestinal Barrier and Regulating MAPK Signaling Pathways. Nutrients 2022, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Health Organization. Health criteria and other sup-porting information. In Guidelines for Drinking-Water Quality, 2nd ed.; World Health Organization: Geneva, Switzerland, 2020; Volume 2. [Google Scholar]
- Fawell, J.K.; Mitchell, R.E.; Everett, D.J.; Hill, R.E. The toxicity of cyanobacterial toxins in the mouse: I microcystin-LR. Hum. Exp. Toxicol. 1999, 18, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yan, Y.; Xie, L.; Wang, L.; He, Y.; Wan, X.; Xue, Q. Long-term environmental exposure to microcystins increases the risk of nonalcoholic fatty liver disease in humans: A combined fisher-based investigation and murine model study. Environ. Int. 2020, 138, 105648. [Google Scholar] [CrossRef]
- Li, X.; Sun, H.; Li, B.; Zhang, X.; Cui, J.; Yun, J.; Yang, Y.; Zhang, L.; Meng, Q.; Wu, S.; et al. Probiotics Ameliorate Colon Epithelial Injury Induced by Ambient Ultrafine Particles Exposure. Adv. Sci. 2019, 6, 1900972. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xie, Q.; Etareri, E.S.; Liu, D.; Dong, J.; Ping, L.; Liu, F.; Li, B.; Huo, G. Bifidobacterium dentium N8 with potential probiotic characteristics prevents LPS-induced intestinal barrier injury by alleviating the inflammatory response and regulating the tight junction in Caco-2 cell monolayers. Food Funct. 2021, 12, 7171–7184. [Google Scholar] [CrossRef]


| Genes | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| M-IL-6 | CCACGGCCTTCCCTACTTC | TTGGGAGTGGTATCCTCTGTGA |
| M-TNF-α | CCCACGTCGTAGCAAACCA | ACAAGGTACAACCCATCGGC |
| M-IL-1β | GCACTACAGGCTCCGAGATGAA | GTCGTTGCTTGGTTCTCCTTGT |
| M-IL-10 | AGAGCTGCGGACTGCCTTCA | ACCTGCTCCACTGCCTTGCT |
| M-β-Actin | TCAAGATCATTGCTCCTCCTGAG | ACATCTGCTGGAAGGTGGACA |
| H-IL-6 | CCTTCGGTCCAGTTGCCTTCTC | AGAGGTGAGTGGCTGTCTGTGT |
| H-TNF-α | TGCTCCTCACCCACACCATCA | CCCAAAGTAGACCTGCCCAGAC |
| H-IL-1β | TCTGTACCTGTCCTGCGTGTTG | TCTGCTTGAGAGGTGCTGATGT |
| H-IL-10 | TGTTGCCTGGTCCTCCTGACTG | CGCCTTGATGTCTGGGTCTTGG |
| H-β-Actin | GCACTCTTCCAGCCTTCCTTCC | CCGCCAGACAGCACTGTGTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Wen, C.; Zheng, S.; Song, F.; Liu, Y.; Yao, X.; Tang, Y.; Feng, X.; Chen, J.; Yang, F. Lactobacillus fermentum Alleviates the Colorectal Inflammation Induced by Low-Dose Sub-Chronic Microcystin-LR Exposure. Toxins 2023, 15, 579. https://doi.org/10.3390/toxins15090579
Yang Y, Wen C, Zheng S, Song F, Liu Y, Yao X, Tang Y, Feng X, Chen J, Yang F. Lactobacillus fermentum Alleviates the Colorectal Inflammation Induced by Low-Dose Sub-Chronic Microcystin-LR Exposure. Toxins. 2023; 15(9):579. https://doi.org/10.3390/toxins15090579
Chicago/Turabian StyleYang, Yue, Cong Wen, Shuilin Zheng, Fengmei Song, Ying Liu, Xueqiong Yao, Yan Tang, Xiangling Feng, Jihua Chen, and Fei Yang. 2023. "Lactobacillus fermentum Alleviates the Colorectal Inflammation Induced by Low-Dose Sub-Chronic Microcystin-LR Exposure" Toxins 15, no. 9: 579. https://doi.org/10.3390/toxins15090579
APA StyleYang, Y., Wen, C., Zheng, S., Song, F., Liu, Y., Yao, X., Tang, Y., Feng, X., Chen, J., & Yang, F. (2023). Lactobacillus fermentum Alleviates the Colorectal Inflammation Induced by Low-Dose Sub-Chronic Microcystin-LR Exposure. Toxins, 15(9), 579. https://doi.org/10.3390/toxins15090579






