1. Introduction
PRO are oligo- or polymers of monomeric flavan-3-ols produced as an end-product of the flavonoid biosynthetic pathway, which are widely distributed in flowers, nuts, fruits, bark, and seeds of various plants [
1,
2]. The PRO degree of polymerization can range between 3 and 11 [
1]. The polymerization of flavan-3-ols in PRO are linked by C-C and C-O-C bonds [
3,
4]. Grape seeds are one of the most abundant sources of PRO [
5]. Antioxidant activity is the primary biological characteristic of PRO, which is 50 times that of vitamin E and 20 times that of vitamin C [
6]. Besides antioxidant capacity, PRO also has been proven to possess extensive beneficial health properties, such as antibacterial, anti-inflammatory, anti-diabetic, and anti-obesity in animals and human studies [
7,
8,
9]. For example, diet supplementation with PRO attenuated trinitro-benzene-sulfonic acid-induced recurrent ulcerative colitis in rats by downregulating the expression levels of tumor necrosis factor-alpha, Phosphorylated inhibitor kappa B kinase, and the translocation of nuclear factor kappa-B [
10]. Studies showed that PRO ameliorated peripheral insulin resistance and disorders of lipid metabolism in vivo via inhibiting adipogenesis and improving mitochondrial function [
11,
12]. Moreover, PRO has also been implicated in regulating the growth of intestinal epithelial mucosa by up-regulating the tight-junction protein (TJP) expression levels (e.g., ZO-1, occludin) [
13,
14].
Herbs and spices have been used for medicinal purposes since the earliest civilizations to prevent or cure many diseases, and a quarter of the world’s medicines are prepared from plants [
15,
16]. However, research reports showed phytochemicals or plant extracts are toxic to animals above a certain dose, such as having carcinogenic, nephrotoxic, hepatotoxic, and gastrointestinal effects [
17]. The PRO dose in previous studies was very low (250 mg/kg or 400 mg/kg feed), and the impact of high-dose PRO on animals’ health is still unclear, especially in young pigs [
18,
19]. Therefore, it is pretty essential to evaluate the safety of plant extracts PRO. Moreover, previous studies mainly focused on the antioxidant and anti-inflammatory abilities of PRO, with limited research on its functional characteristics, such as intestinal barrier function and digestive absorption capacity in pigs. Therefore, this study uses weaned piglets as the research model to explore the functional characterization and safety evaluation of PRO.
3. Discussion
In the present study, the maximum dose of PRO added to the feed of weaned piglets was 1200 mg/kg, which is approximately 48 times the optimal dose level (25 mg/kg) in some recent animal nutrition studies [
20,
21]. Numerous studies have indicated that dietary PRO plays a crucial part in beneficial effects for health issues including a powerful antioxidant capacity and possessing immunomodulatory activity in humans and animals. For example, a study in broiler chicks showed that PRO can significantly improve their ADG and antioxidant capacity [
22]. Moreover, PRO as a type of phenolic compound is capable of reducing the colonization of a broad spectrum of Gram-negative and Gram-positive bacteria on the intestinal mucosa at a dose of 60 mg/L or 30 mg/L, which may alleviate the inflammation and injury of the intestinal barrier [
23,
24]. In this study, we observed that the growth performance was improved in 30 or 300 mg/kg PRO-supplemented piglets, as indicated by a decrease in F:G and diarrhea rate. The results were consistent with a previous study that dietary supplementation with 250 mg/kg PRO decreased the diarrhea rate and improved growth performance by reducing intestinal permeability by a urinary lactulose to manmitol ratio test in weaning rats [
18]. Moreover, some similar research also reported that feeding polyphenol-rich plant extracts from grape or hop could decrease the F:G in growing pigs [
25].
Weaning is a critical developmental window in a neonatal porcine. At this stage, Changes in feeding patterns and withdrawal of maternal passive immune protection may increase the susceptibility of piglets to pathogens such as
Escherichia coli, which could result in inflammation and damage to the intestinal epithelium [
26,
27]. The integrity of the intestinal villus-crypt structure has been considered as a symbolic indicator to evaluate the intestinal epithelium function, such as intestinal barrier integrity, nutrient digestion, and absorption capacity of the small intestine [
28,
29]. In our present study, dietary PRO supplementation not only exerted a positive effect on intestinal morphology, which resulted in an increase in duodenal and jejunal VH, but also increased the digestibilities of DM, GE, EE, and ASH contrast with the CON group. The improvement in growth performance may be attributed to increased nutrient digestibility and improved intestinal morphology [
30]. Similarly, previous studies indicated that dietary polyphenol-rich grape products were beneficial in increasing the small intestinal VH: CD in chicks and piglets [
31,
32,
33]. Moreover, a study on rats also showed that supplementation with PRO could improve the intestinal barrier function the same as ZnO by improving the expression of intestinal mucosal tight junction proteins [
18]. Based on those findings, our studies suggested that dietary PRO supplementation may alleviate intestinal damage caused by weaning stress by improving intestinal morphology.
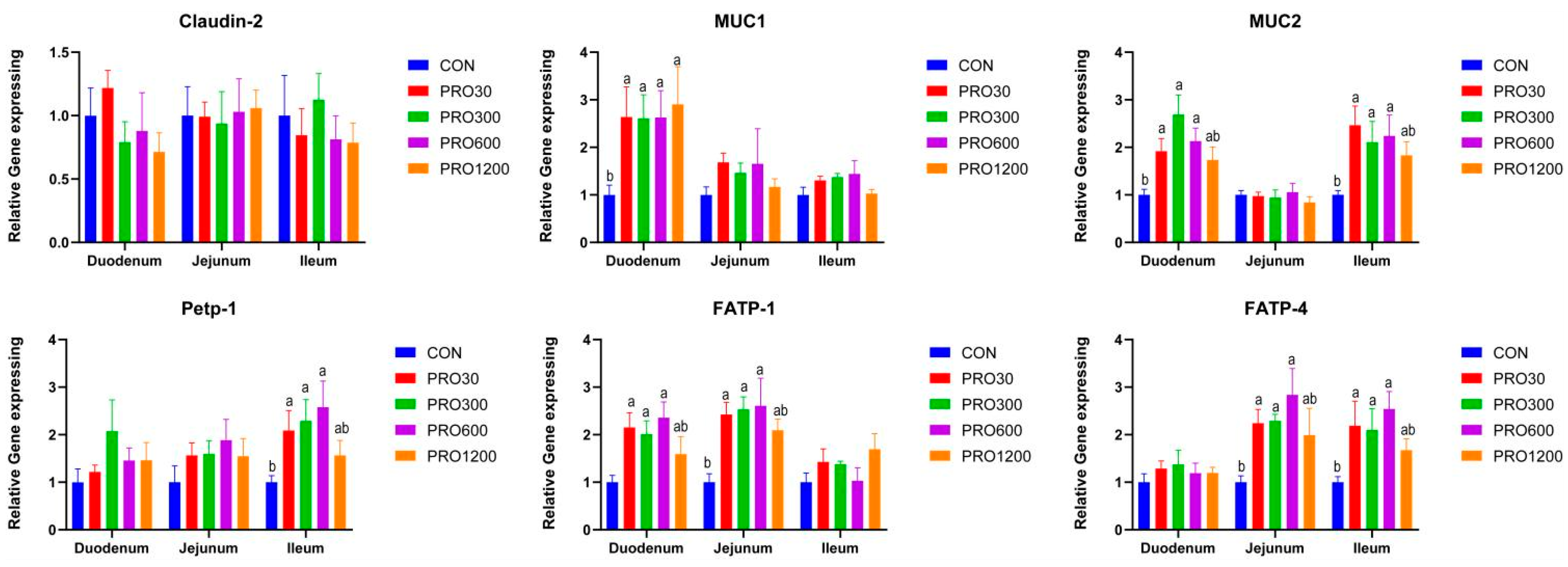
The intestinal epithelial barrier is composed of the mucus layer, intestinal epithelial cells, and their tight junctions [
34]. Under normal conditions, the intestinal tract is protected by a mucus layer with secreted (e.g.,
MUC2,
MUC5B, and
MUC19) and cell-surface mucins (e.g.,
MUC1,
MUC12, and
MUC20) from pathogenic microbes and toxin-induced damage [
35]. Once the mucus layer in the gut is damaged, the intestinal epithelium surface will be exposed to a multitude of noxious numerous macromolecules and microorganisms, which may lead to a continuous immune reaction. We found that PRO significantly improved the expression level of
MUC1 in duodenum and
MUC2 in duodenum and ileum, which was similar to a previous report using a rat model [
13,
14].
MUC2 plays a critical role in intestinal epithelial protection, the highly glycosylated cysteine residues located in the N and C termini of
MUC2 enhance the hydrophilicity of mucins and are beneficial for the lubrication of the intestinal mucosa [
36]. Moreover, commensal bacteria can occupy microbial binding sites by binding to the intestinal mucosa through oligosaccharide linkage structures on
MUC2, which protects against pathogen invasion [
37]. Interestingly, dietary supplementation of PRO improved the expression levels of
FATP-1 in duodenum and
FATP-4 in jejunal and ileum. The ileal
Petp-1 expression level was also elevated upon PRO feeding.
FATP-1 and
FATP-4 are major members of the fatty acid transporter family that are involved in the cellular utilization and storage of long chain fatty acids and the regulation of the level of fatty acyl-Coenzyme A’s [
38,
39]. PepT1 is responsible for nutrient-derived di- and tripeptide transport [
40]. These findings suggested that dietary PRO is beneficial in improving intestinal epithelial functions for weaned pigs, which may contribute to growth performance.
Those results of serum clinical chemistry and hematological parameters were similar to those of previous studies in weaned pigs, and hardly any differences were found between the PRO groups and the CON group at all stages [
41,
42,
43]. Serum ALT was increased in pigs fed 30 and 300 mg/kg PRO compared with that in 600 and 1200 mg/kg PRO in the later period. For ALT, a biomarker of hepatic inflammation, every treatment value was within previous limits observed in weaned pigs [
44,
45,
46], indicating that the numerical difference between groups is not biologically meaningful. Hemoglobin concentration, whose clinical significance is essentially the same as that of hematocrit value, was inversely associated with lung disease [
47]. In this study, the hemoglobin concentration and Hematocrit value were higher in the 30 mg/kg PRO supplementation group than in the CON group, but this difference disappeared after the start of the experiment, and all concentrations were within the normal biological range for the weaned pigs [
48]. Therefore, those findings were not considered to be related to the diets. Interestingly, with the 30 mg/kg PRO exposure, the serum TC concentration of weaned pigs showed a reduction trend. A study in rats demonstrated that supplemented 25 mg/kg PRO could decrease VLDL concentration through interference with genes involved in VLDL assembly (e.g., Microsomal Triglyceride Transfer Protein and diacylglycerol O-acyltransferase 2) [
49]. Moreover, other studies also showed that PRO ameliorated peripheral insulin resistance and disorders of lipid metabolism in vivo via inhibiting adipogenesis and improving mitochondrial function [
11,
12]. These results suggest that PRO supplementation is beneficial to improve lipid metabolism in animals.
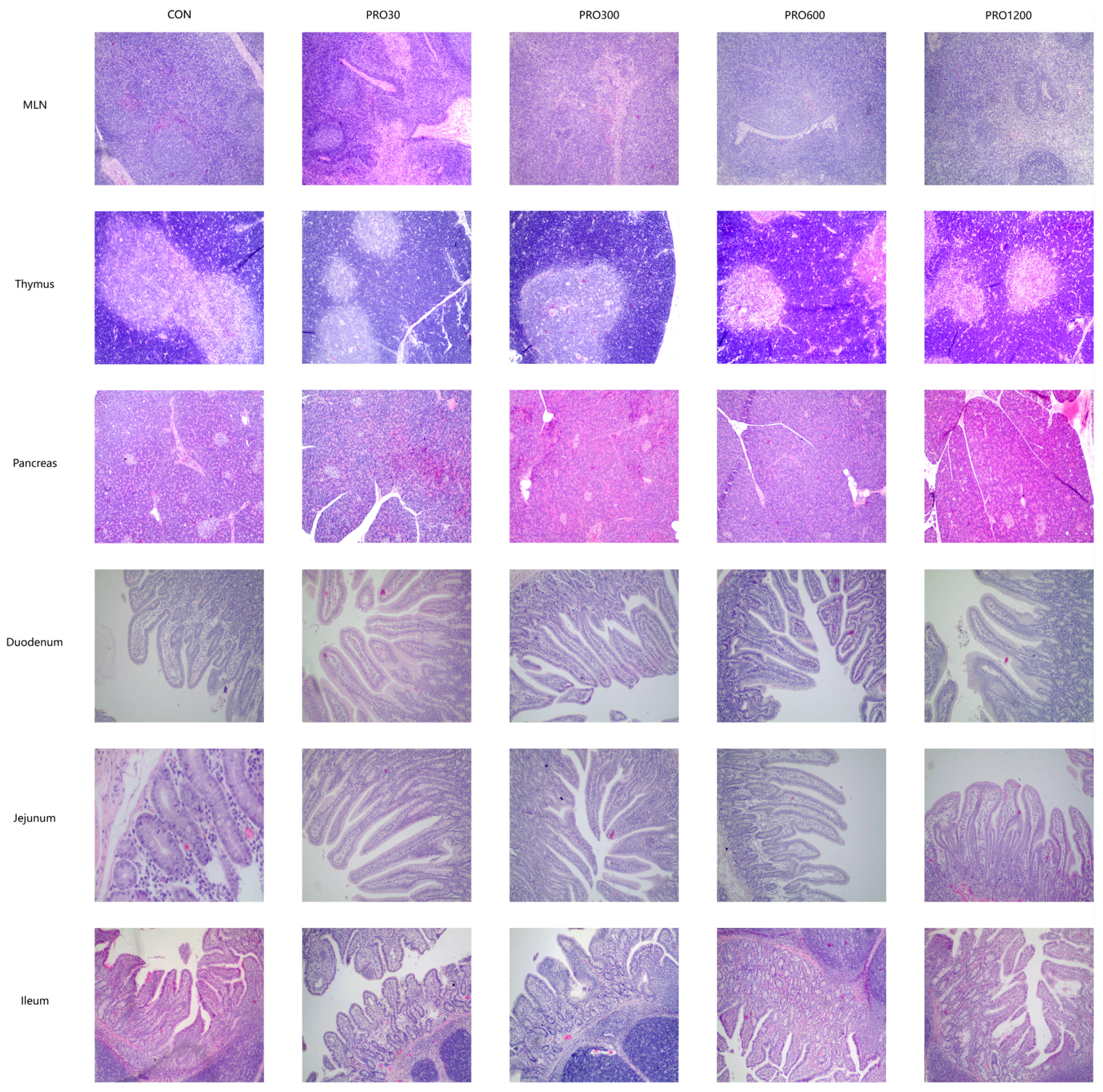
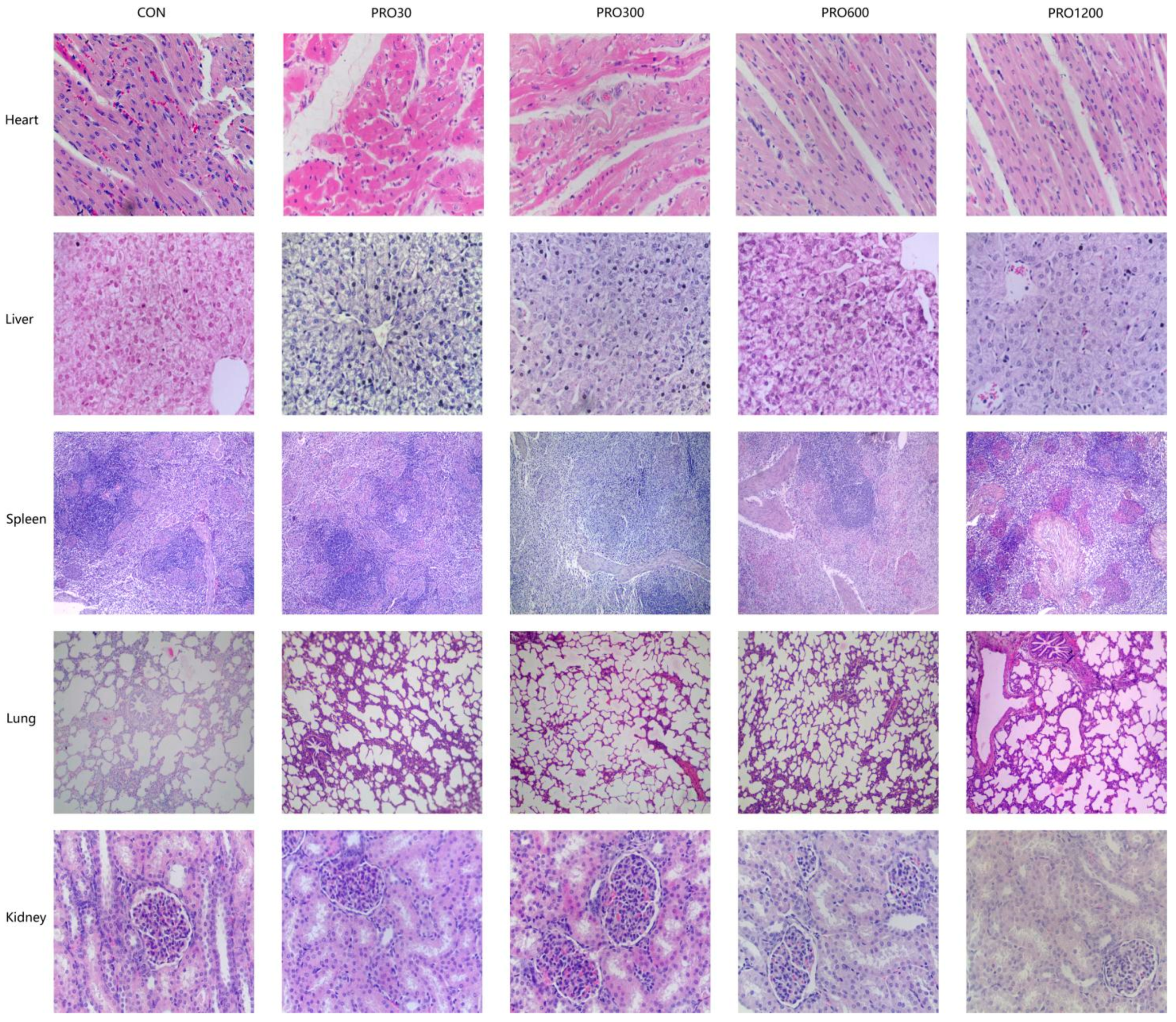
The internal body organ weight is a crucial parameter in assessing the safety of drugs [
50,
51]. Our results found that the PRO supplementation from 0 mg/kg to 1200 mg/kg in diets did not affect the relative weights of organs (e.g., heart, lungs, spleen, liver, and kidney). Moreover, histopathological examination of various internal vital organ sections of all pigs were also shown to be normal and showed no evidence of gross abnormalities. Therefore, it could be concluded that the addition of 48 times the optimal dose level of PRO to pig feed is safe and produces no adverse effects on histological, clinical chemistry, or hematological indicators in pigs.
5. Materials and Methods
The animal experiment in this study was approved by the Animal Care and Use Committee of Sichuan Agricultural University (Chengdu, China, No. 20220716). PRO (Cat no. DAT00140, with a purity of PRO ≥ 95%, of which Oligomeric PRO ≥ 85%, catechine ≥ 1.52%, epicatechine ≥ 2.41%) was purchased from Guilin Feng peng Biological Technology Co., Ltd. (Guangxi, China).
5.1. Experimental Design, Diet, and Animal Housing
Thirty male weaned pigs (Duroc × Landrace × Yorkshire, average body weight 7.86 ± 0.09 kg) at 28 days of age were divided into five groups and fed with a basal diet containing different levels of PRO (0, 30, 300, 600, and 1200 mg/kg) for 42 days. The diet (
Table 7) was formulated based on National Research Council 2012 [
52]. Pigs were raised alone in metabolic cages (1.05 m
2) at appropriate temperature (26 ± 3 °C) and humidity (60 ± 5%) with ad-libitum access to water and foods, which were in a closed system. Before the formal experiment, there was a three-day adaptation period and the piglets had already been vaccinated against swine fever, blue ear, and pseudorabies at an appropriate time.
5.2. Dose Selection and Homogenization of PRO
Based on guidelines for evaluating the safety of livestock and poultry target animals to feed additives carried out by the ministry of Agriculture and Rural Affairs, the evaluation test should include at least three groups, including the control group, effective dose group, and multiple dose group (the multiple dose group is generally 10 times the effective dose). In certain production situations, the supplementation of additives may be increased, and the maximum dose for long-term toxicity testing is generally 30 to 50 times the optimal dose [
53,
54]. In order to provide a higher safe dose range for the product, we have chosen 48 times the optimal dose as the maximum dose. The homogenization of PRO is divided into two steps. Firstly, a certain amount of PRO was diluted by the carrier (extruded corn) to obtain the primary premix. Then, the primary premix is mixed with other raw ingredients powders to obtain a diet.
5.3. Sample Collection
Following defecation, fresh feces from each pig were collected into their individual valve bags, and next, 10 mL of diluted H
2SO
4 solution was injected into 0.1 kg of feces to fix excreta nitrogen during day 39–42 of the trial. On day 1, 22, and 43 in the morning, blood samples were taken from each pig by venipuncture, and then centrifuged at 3500×
g at 4 °C (15 min) after 30 min. Then serums were obtained and stored at −20 °C for subsequent analysis. Then, pigs were slaughtered by electrical stunning to collect the remaining samples. After draining of the blood from the carotid artery, pigs were dissected to separate the organs and entire intestine. The small intestinal mucosal tissues were collected by scraping the mucosal layer with a glass slide and then stored at −80 °C [
55]. Organs were taken from each pig for weighing and calculating the relative indices (liver, kidney, spleen, heart, and lungs) followed by removing any superficial fat or blood, and similar areas on each organ were collected to fix in paraformaldehyde for histology measurements (heart, liver, kidney, spleen, lungs, pancreas, thymus, mesenteric lymph nodes, stomach, duodenum, jejunum, ileum, and cecum).
5.4. Growth Performance and Diarrhea Rate Evaluation
The average daily feed intake (ADFI) was recorded daily and the body weight was recorded at the initial (IBW) and final (FBW) part of this study to calculate the feed/gain ratio (F:G) and average daily gain (ADG). Piglets with watery or sparse feces are considered as having diarrhea and the diarrhea rate is calculated by the previous formula [
56].
5.5. Apparent Total Tract Nutrient Digestibility Analysis
The diet and fecal samples were used for the nutrient digestibility analysis after thawing, homogenizing, and freeze-drying, such as DM, CP, EE, and Ash [
25]. The Cr
2O
3 was used as an external indicator, and all the procedures are according to the AOAC standard [
57]. The GE was detected through the adiabatic calorimeter (LECO, St. Joseph, MI, USA). All contents were calculated by the following formula: 100 − 100 × (digesta nutrient × diet Cr
2O
3)/(diet nutrient × digesta Cr
2O
3) [
58].
5.6. Hematology and Clinical Chemistry Analysis
Serum and whole blood were sent promptly to Ya‘an People’s Hospital (Yaan, China) for hematological parameters analysis. Hematology biochemical measured by automatic biochemical analyzer (7150, HITACHI, Tokyo, Japan) include aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood glucose (GLU), blood urea nitrogen (BUN), creatinine (CREA), alkaline phosphatase (AKP), triglycerides (TG), total cholesterol (TC), albumin (ALB), globulin (GLO), total protein (TP), and total bilirubin (TBIL). Routine blood indexes included hemoglobin (HGB), white blood cell count (WBC), red blood cell count (RBC), platelet count (PLT), Hematocrit value (HCT), neutrophil count, and lymphocyte count and were measured by hematology analyzer (KX–21, SYSMEX, Wuxi, China).
5.7. Intestinal Morphologic Analysis
The small intestinal segments from the fixtive were dewaxed by graded anhydrous ethanol, dyed by hematoxylin and eosin (H&E), and sealed by a neutral resin size as described by Li [
41]. Then, sections of the small intestinal samples were examined for crypt depth (CD), villus height (VH), and the ratio of VH to CD (V/C) by image processing and analysis system (Image-Pro Plus 6.0, Media Cybernetics, Rockville, MD, USA).
5.8. Histopathology Evaluations and Relative Weights of Organ
At terminal necropsy, organ weights (heart, lungs, spleen, liver, and kidney) were recorded and the relative weights of organs were calculated. Freshly harvested samples (heart, liver, kidney, spleen, lungs, pancreas, thymus, mesenteric lymph nodes, stomach, duodenum, jejunum, ileum, and cecum) on a standard selection were fixed in 4% paraformaldehyde and delivered to the Histopathology Laboratory (College of Veterinary Medicine, Southwest University of Science and Technology) where they were stained and made into sections as per the above methods of small intestinal sections [
41,
44]. Under a light microscope, the veterinary pathologist examined the sections for the determination of abnormalities. All relative weights of organs were calculated by following formula: A1/A2 [
59]. A1: organ weight (g); A2: final live body weight (kg).
5.9. RNA Isolation, Reverse Transcription, and Real-Time Quantitative PCR
The RNA of small intestinal mucosal samples from each pig was extracted by Trizol (TAKARA, Gunma, Japan). Then, the RNA extracted from the intestinal mucosa was reverse transcribed into cDNA by PrimeScript RT Reagent Kit with gDNA Eraser (TaKaRa Biotechnology Co., Ltd., Dalian, China). PCR assays were conducted using Premix Ex TaqTM kits (Takara Biotechnology Co., Ltd., Dalian, China). The procedure of q-PCR is as follows: initial denaturation at 95 °C (30 s), followed by 40 cycles between 95 °C (5 s) and 60 °C (30 s). All procedures are based on previous methods [
36]. The primers information is shown in
Supplementary Material Table S1. The mRNA levels of important genes of pattern recognition receptor-related intestinal barrier genes, such as mucin 1, 2 (
MUC-1,
MUC-2),
Claudin-2, and nutrient transporters, such as Oligopeptide Transporter 1 (
Petp-1), fatty acid transport protein-1, 4 (
FATP-1,
FATP-4) were quantified by real-time PCR and the relative expression of each gene was calculated using the 2
−ΔΔCt method [
60].
5.10. Statistical Analysis
The results were shown as the means and structural equation model (SEM). Data were subjected to one-way analysis of variance (ANOVA) followed by Tukey’s multiple-range test to determine significant differences among the groups at p < 0.05 with SPSS 27.0 (IBM, Chicago, IL, USA).