Evaluation of the Bioaccessible Fraction of T-2 Toxin from Cereals and Its Effect on the Viability of Caco-2 Cells Exposed to Tyrosol
Abstract
1. Introduction
2. Results and Discussion
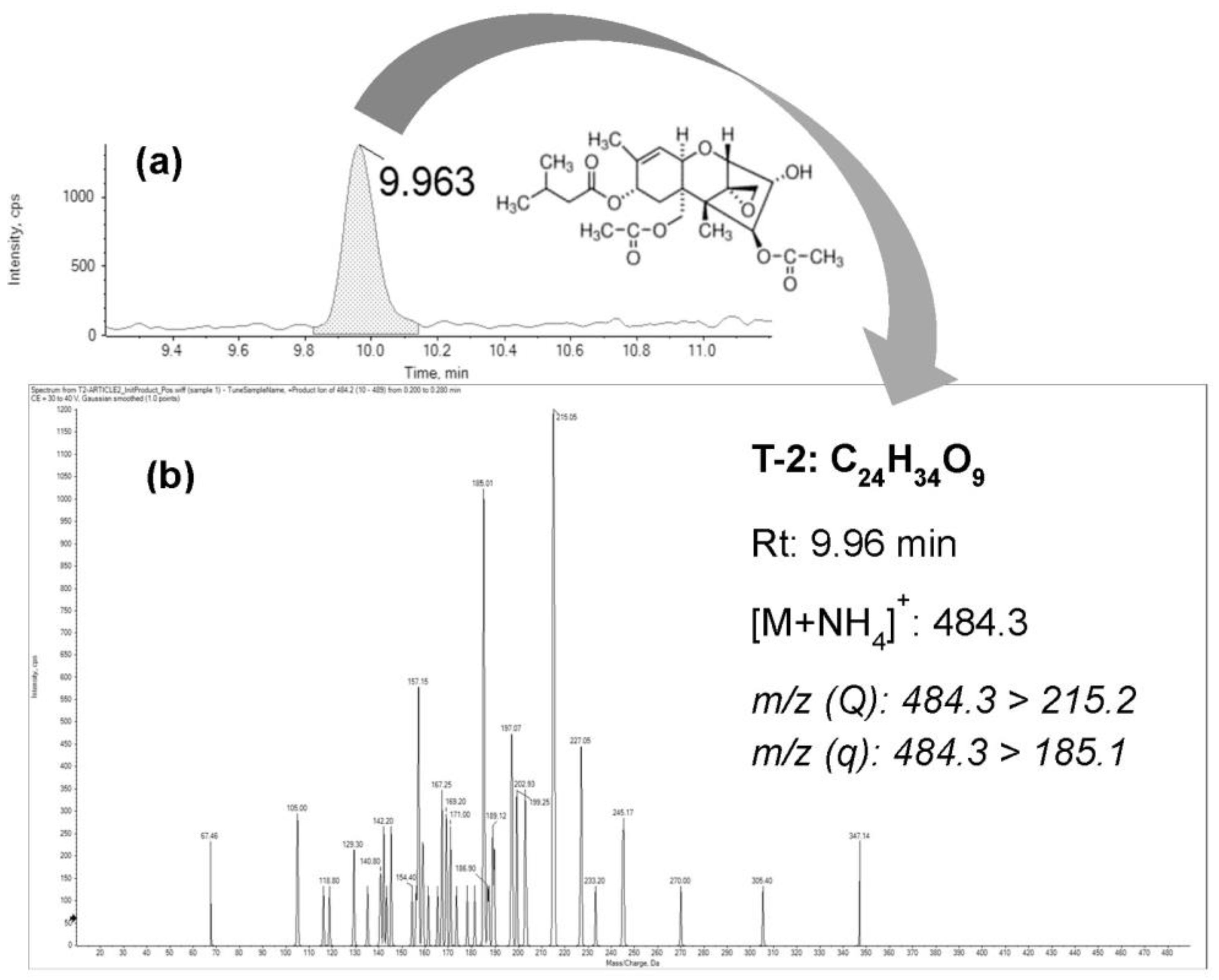
2.1. Bioaccesibility Fraction of T-2
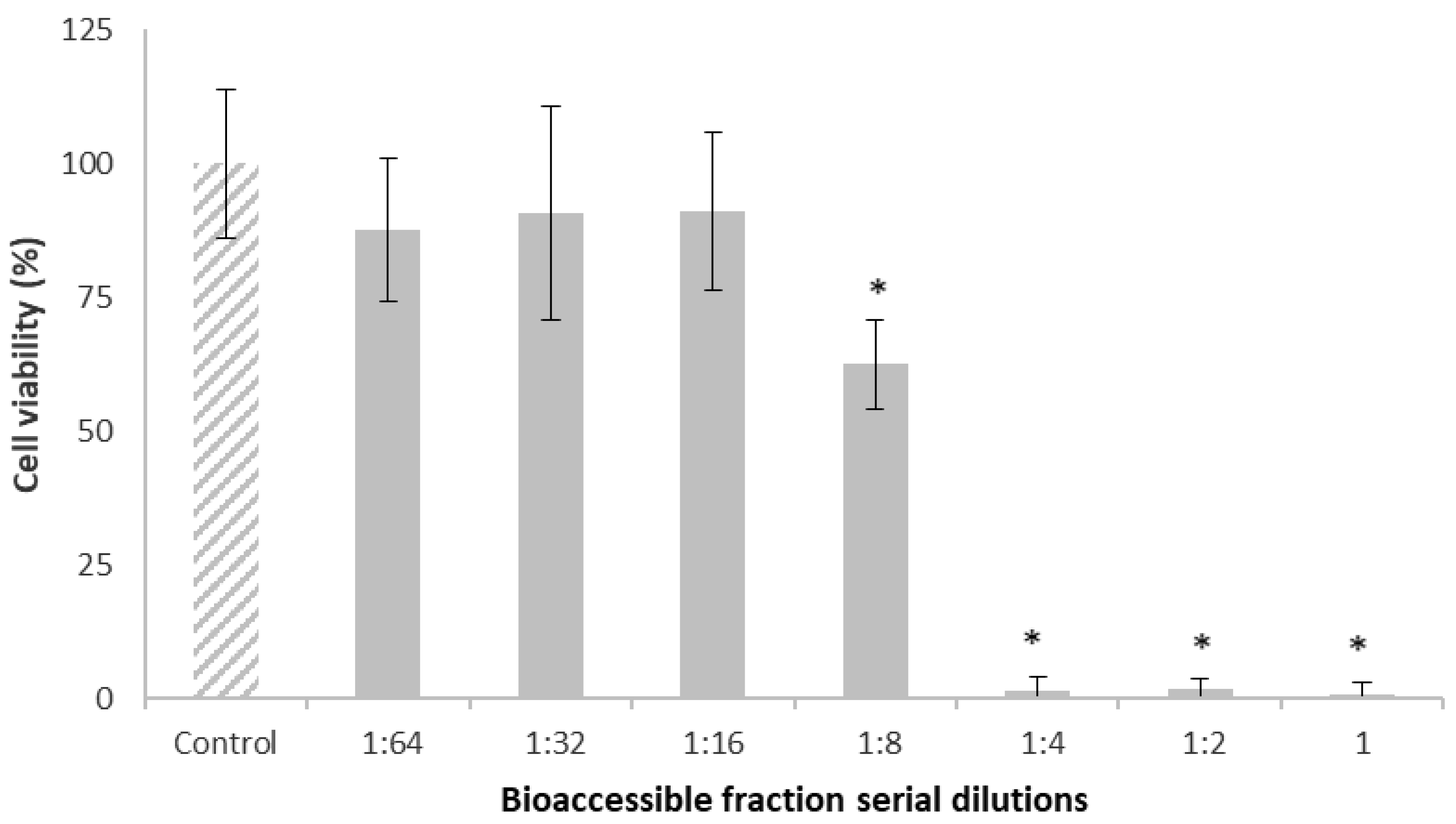
2.2. Effect of the Bioaccessible Fraction on Cell Viability
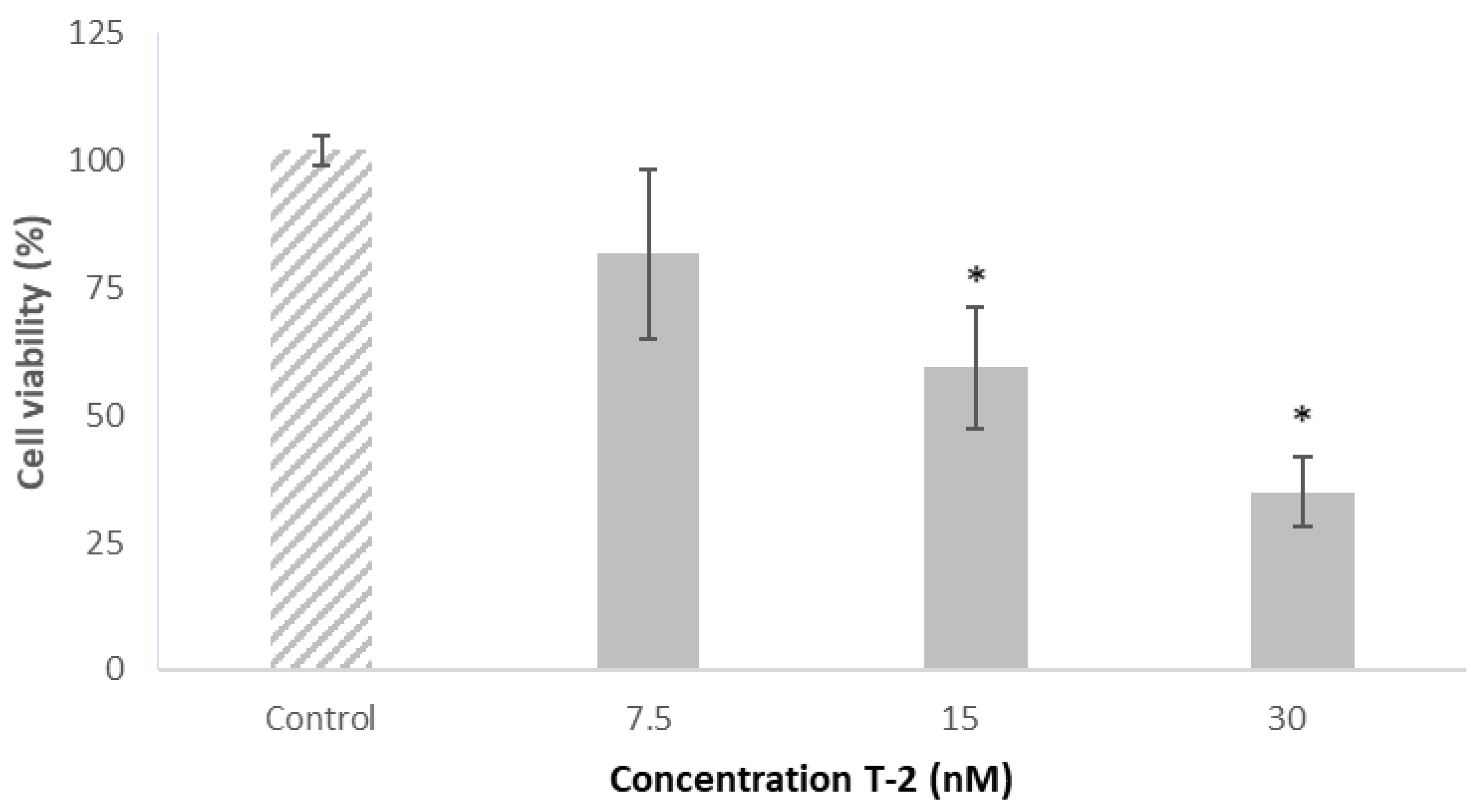
2.3. Effect of T-2 on Caco-2 Cells’ Viability
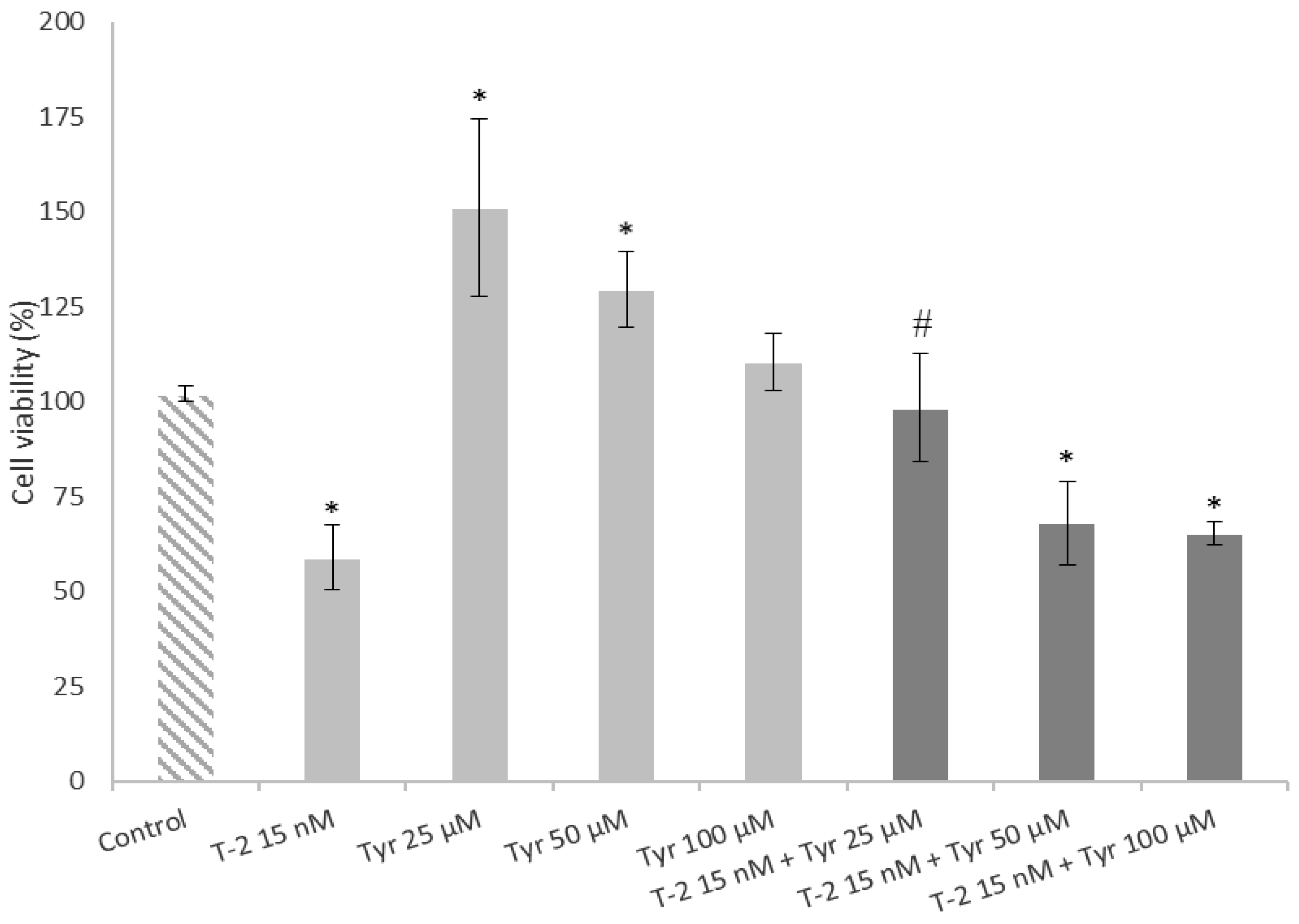
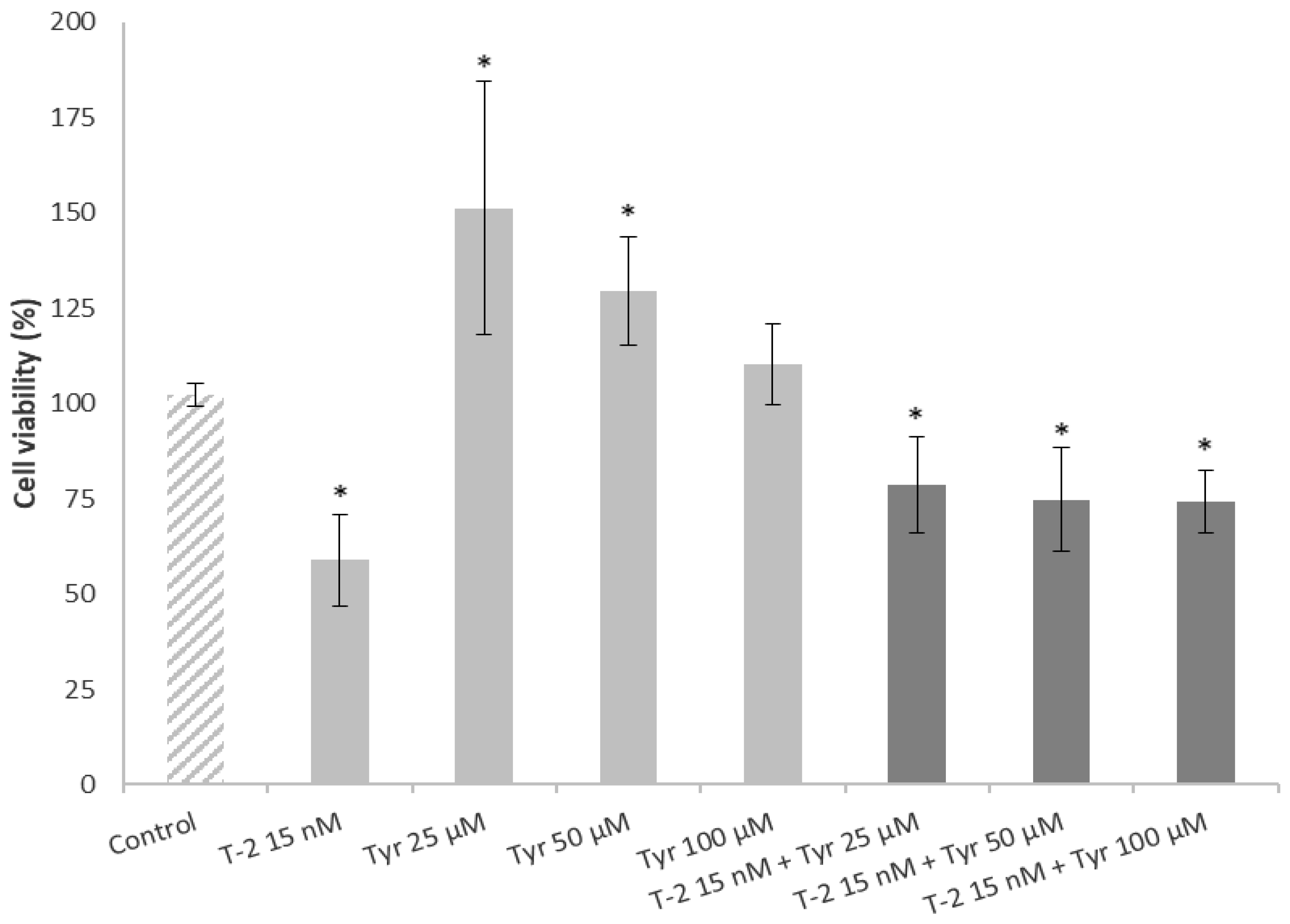
2.4. Effects of a Simultaneous Treatment with T-2 and Tyrosol on Caco-2 Cell Viability
2.5. Effects of a Pre-Treatment on the Viability of Caco-2 Cell Exposed to T-2 and Tyrosol
3. Conclusions
4. Materials and Methods
4.1. Reagents
4.2. Sample Preparation Procedure
4.3. In Vitro Digestion Procedure
4.4. Extraction Procedure
4.5. UHPLC–MS/MS Analysis
4.6. Cell Culture and Treatment
4.7. Determination of Cell Viability by the MTT Assay
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amjad, A.; Javaid, N.; Ijaz, A.; Rahman, I.; Fayyaz, A.; Javaid, M.; Khalid, L.; Sabir, A.; Farooq, F.; Gillani, Z. Health endorsing perspective of cereals and its by products: A systematic review. Biosci. Res. 2021, 18, 2015–2021. [Google Scholar]
- Sarmast, E.; Fallah, A.A.; Jafari, T.; Khaneghah, A.M. Occurrence and fate of mycotoxins in cereals and cereal-based products: A narrative review of systematic reviews and meta-analyses studies. Curr. Opin. Food Sci. 2021, 39, 68–75. [Google Scholar] [CrossRef]
- Liu, L.; Xie, M.; Wei, D. Biological detoxification of mycotoxins: Current status and future advances. Int. J. Mol. Sci. 2022, 23, 1064. [Google Scholar] [CrossRef] [PubMed]
- RASFF (Rapid Alert for Food and Feed). RASFF Annual Report 2021. Available online: https://food.ec.europa.eu/system/files/2022-07/acn_annual-report_2021-final.pdf (accessed on 29 September 2022).
- EFSA (European Food Safety Authority). Scientific report on Human and animals dietary exposure to T-2 and HT-2 toxin. EFSA J. 2017, 15, 4972. [Google Scholar]
- Yang, L.; Tu, D.; Wang, N.; Deng, Z.; Zhan, Y.; Liu, W.; Hu, Y.; Liu, T.; Tan, L.; Li, Y.; et al. The protective effects of DL-Selenomethionine against T-2/HT-2 toxins-induced cytotoxicity and oxidative stress in broiler hepatocytes. Toxicol. In Vitro 2019, 54, 137–146. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Zhao, Y.; Kuca, K.; Wang, X.; Oleksak, P.; Chrienova, Z.; Nepovimova, E.; Jaćević, V.; Wu, Q.; Wu, W. Hypoxia, oxidative stress, and immune evasion: A trinity of the trichothecenes T-2 toxin and deoxynivalenol (DON). Arch. Toxicol. 2021, 95, 1899–1915. [Google Scholar] [CrossRef]
- Commission Recommendation 2013/165/EU of 27 March 2013 on the Presence of T-2 and HT-2 Toxin in Cereals and Cereal Products Text with EEA Relevance. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:091:0012:0015:EN:PDF (accessed on 3 July 2023).
- Katsiki, N.; Pérez-Martínez, P.; Lopez-Miranda, J. Olive oil intake and cardiovascular disease prevention: “Seek and you shall find”. Curr. Cardiol. Rep. 2021, 23, 64. [Google Scholar] [CrossRef]
- Masella, R.; Di Benedetto, R.; Varì, R.; Filesi, C.; Giovannini, C. Novel mechanisms of natural antioxidant compounds in biological systems: Involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005, 16, 577–586. [Google Scholar] [CrossRef]
- Kimothi, S.; Dhaliwal, Y. Nutritional and Health Promoting Attribute of Kidney Beans (Phaseolus vulgaris L.): A Review. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1201–1209. [Google Scholar] [CrossRef]
- Taroncher, M.; Vila-Donat, P.; Tolosa, J.; Ruiz, M.J.; Rodríguez-Carrasco, Y. Biological activity and toxicity of plant nutraceuticals: An overview. Curr. Opin. Food Sci. 2021, 42, 113–118. [Google Scholar] [CrossRef]
- Chiesi, C.; Fernandez-Blanco, C.; Cossignani, L.; Font, G.; Ruiz, M.J. Alternariol-induced cytotoxicity in Caco-2 cells. Protective effect of the phenolic fraction from virgin olive oil. Toxicon 2015, 93, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Millman, J.F.; Okamoto, S.; Teruya, T.; Uema, T.; Ikematsu, S.; Shimabukuro, M.; Masuzaki, H. Extra-virgin olive oil and the gut-brain axis: Influence on gut microbiota, mucosal immunity, and cardiometabolic and cognitive health. Nutr. Rev. 2021, 79, 1362–1374. [Google Scholar] [CrossRef] [PubMed]
- Reboredo-Rodríguez, P.; Olmo-García, L.; Figueiredo-González, M.; González-Barreiro, C.; Carrasco-Pancorbo, A.; Cancho-Grande, B. Application of the INFOGEST standardized method to assess the digestive stability and bioaccessibility of phenolic compounds from Galician extra-virgin olive oil. J. Agric. Food Chem. 2021, 69, 11592–11605. [Google Scholar] [CrossRef] [PubMed]
- Bertero, A.; Fossati, P.; Tedesco, D.E.A.; Caloni, F. Beauvericin and enniatins: In vitro intestinal effects. Toxins 2020, 12, 686. [Google Scholar] [CrossRef]
- González-Arias, C.A.; Marín, S.; Sanchis, V.; Ramos, A.J. Mycotoxin bioaccessibility/absorption assessment using in vitro digestion models: A review. World Mycotoxin J. 2013, 6, 167–184. [Google Scholar] [CrossRef]
- Sharath Babu, G.R.; Ilaiyaraja, N.; Khanum, F.; Anand, T. Cytoprotective propensity of green tea polyphenols against citrinin-induced skeletal-myotube damage in C2C12 cells. Cytotechnology 2017, 69, 681–697. [Google Scholar] [CrossRef]
- Tran, V.N.; Viktorová, J.; Ruml, T. Mycotoxins: Biotransformation and bioavailability assessment using caco-2 cell monolayer. Toxins 2020, 12, 628. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, B.; Pfeiffer, E.; Metzler, M. Absorption and metabolism of the mycotoxins alternariol and alternariol-9-methyl ether in Caco-2 cells in vitro. Mycotoxin Res. 2009, 25, 149–157. [Google Scholar] [CrossRef]
- Escrivá, L.; Agahi, F.; Vila-Donat, P.; Mañes, J.; Meca, G.; Manyes, L. Bioaccessibility study of aflatoxin B1 and ochratoxin A in bread enriched with fermented milk whey and/or pumpkin. Toxins 2022, 14, 6. [Google Scholar] [CrossRef]
- De Angelis, E.; Monaci, L.; Mackie, A.; Salt, L.; Visconti, A. Reprint of “Bioaccessibility of T-2 and HT-2 toxins in mycotoxin contaminated bread models submitted to in vitro human digestion”. Innov. Food Sci. Emerg. Technol. 2014, 25, 88–96. [Google Scholar] [CrossRef]
- Raiola, A.; Meca, G.; Manes, J.; Ritieni, A. Bioaccessibility of deoxynivalenol and its natural co-occurrence with ochratoxin A and aflatoxin B1 in Italian commercial pasta. Food Chem. Toxicol. 2012, 50, 280–287. [Google Scholar] [CrossRef]
- Kabak, B.; Brandon, E.F.; Var, I.; Blokland, M.; Sips, A.J. Effects of probiotic bacteria on the bioaccessibility of aflatoxin B1 and ochratoxin A using an in vitro digestion model under fed conditions. J. Environ. Sci. Health B 2009, 44, 472–480. [Google Scholar] [CrossRef]
- Nielsen, C.; Casteel, M.; Didier, A.; Dietrich, R.; Märtlbauer, E. Trichothecene-induced cytotoxicity on human cell lines. Mycotoxin Res. 2009, 25, 77–84. [Google Scholar] [CrossRef]
- Bouaziz, C.; Abid-Essefi, S.; Bouslimi, A.; El Golli, E.; Bacha, H. Cytotoxicity and related effects of T-2 toxin on cultured Vero cells. Toxicon 2006, 48, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Alonso, C.; Taroncher, M.; Castaldo, L.; Izzo, L.; Rodríguez-Carrasco, Y.; Ritieni, A.; Ruiz, M.J. Effect of Phenolic Extract from Red Beans (Phaseolus vulgaris L.) on T-2 Toxin-Induced Cytotoxicity in HepG2 Cells. Foods 2022, 11, 1033. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.; Müller, W.E.; Eckert, G.P. Cytoprotective effects of olive mill wastewater extract and its main constituent hydroxytyrosol in PC12 cells. Pharmacol. Res. 2010, 62, 322–327. [Google Scholar] [CrossRef]
- Kössler, S.; Nofziger, C.; Jakab, M.; Dossena, S.; Paulmichl, M. Curcumin affects cell survival and cell volume regulation in human renal and intestinal cells. Toxicology 2012, 292, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assuncao, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carriere, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Llorens, P.; Pietrzak-Fiećko, R.; Moltó, J.C.; Mañes, J.; Juan, C. Development of an Extraction Method of Aflatoxins and Ochratoxin A from Oral, Gastric and Intestinal Phases of Digested Bread by In Vitro Model. Toxins 2022, 14, 38. [Google Scholar] [CrossRef]
- Ruiz, M.J.; Festila, L.E.; Fernández, M. Comparison of basal cytotoxicity of seven carbamates in CHO-K1 cells. Toxicol. Environ. Chem. 2006, 88, 345–354. [Google Scholar] [CrossRef]

| Digestion Phase | Sample | Released T-2 (%) (Mean ± SD; n = 3) |
|---|---|---|
| Oral Phase | Control | - |
| Fortified 1 | 25 ± 8 | |
| Gastric Phase | Control | - |
| Fortified 1 | 58 ± 7 | |
| Intestinal Phase | Control | - |
| Fortified 1 | 51 ± 4 |
| Mycotoxin | Rt (min) | Precursor Ion | Product Ion | CE (eV) | DP (V) | EP (V) | CXP (V) |
|---|---|---|---|---|---|---|---|
| T-2 | 9.96 | 484.3 | 215.5 | 29 | 56 | 10 | 18 |
| 9.96 | 484.3 | 185.1 | 31 | 56 | 10 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Alonso, C.; Taroncher, M.; Rodríguez-Carrasco, Y.; Ruiz, M.-J. Evaluation of the Bioaccessible Fraction of T-2 Toxin from Cereals and Its Effect on the Viability of Caco-2 Cells Exposed to Tyrosol. Toxins 2023, 15, 493. https://doi.org/10.3390/toxins15080493
Martínez-Alonso C, Taroncher M, Rodríguez-Carrasco Y, Ruiz M-J. Evaluation of the Bioaccessible Fraction of T-2 Toxin from Cereals and Its Effect on the Viability of Caco-2 Cells Exposed to Tyrosol. Toxins. 2023; 15(8):493. https://doi.org/10.3390/toxins15080493
Chicago/Turabian StyleMartínez-Alonso, Carmen, Mercedes Taroncher, Yelko Rodríguez-Carrasco, and María-José Ruiz. 2023. "Evaluation of the Bioaccessible Fraction of T-2 Toxin from Cereals and Its Effect on the Viability of Caco-2 Cells Exposed to Tyrosol" Toxins 15, no. 8: 493. https://doi.org/10.3390/toxins15080493
APA StyleMartínez-Alonso, C., Taroncher, M., Rodríguez-Carrasco, Y., & Ruiz, M.-J. (2023). Evaluation of the Bioaccessible Fraction of T-2 Toxin from Cereals and Its Effect on the Viability of Caco-2 Cells Exposed to Tyrosol. Toxins, 15(8), 493. https://doi.org/10.3390/toxins15080493






