Abstract
Aflatoxins are potent carcinogenic compounds, mainly produced by fungi species of the genus Aspergillus in the soil. Because of their stability, they are difficult to remove completely, even under extreme conditions. Aflatoxin contamination is one of the main causes of safety in peanuts, maize, wheat and other agricultural products. Aflatoxin contamination originates from the soil. Through the investigation of soil properties and soil microbial distribution, the sources of aflatoxin are identified, aflatoxin contamination is classified and analysed, and post-harvest crop detoxification and corresponding contamination prevention measures are identified. This includes the team’s recent development of the biofungicide ARC-BBBE (Aflatoxin Rhizobia Couple-B. amyloliquefaciens, B. laterosporu, B. mucilaginosus, E. ludwiggi) for field application and nanomaterials for post-production detoxification of cereals and oilseed crops, providing an effective and feasible approach for the prevention and control of aflatoxin contamination. Finally, it is hoped that effective preventive and control measures can be applied to a large number of cereal and oilseed crops.
Key Contribution:
The introduction of biocides into the soil will effectively contain and control the toxic aflatoxin-producing fungi at the source and will continue to improve the soil environment and reduce aflatoxin contamination.
1. Introduction
Aflatoxin is produced by Aspergillus flavus, which is classified as a Group 1 carcinogen by the International Agency for Research on Cancer (IARC). It is one of the most toxic compounds known, acting mainly on human and animal liver tissues, capable of inducing cancer of the liver (primarily), as well as the pancreas, kidney, bladder and other organs. Aflatoxin may also lead to malnutrition, immunosuppression, and other pathologies with mutagenic, hepatotoxic, and nephrotoxic outcomes [1,2]. Aflatoxin mainly contaminates grain and oil crops, feed, nuts, Chinese herbs and other crops, and then contaminates meat, eggs, milk and other by-products after being ingested by animals. The import and export of agricultural and sideline products all over the world have strict limits on aflatoxin, thus restricting industrial development and export trade. Aflatoxin contamination not only causes huge economic losses to food crops, but also has a negative impact on the health of consumers. According to the data from the Food and Agriculture Organization of the United Nations (FAO), about 25% of crops worldwide are contaminated with moulds and their toxins each year, while about 2% of agricultural products lose their value due to excessive toxin contamination [3], such as the 100,000 turkey deaths that first occurred in the 1960s in the UK, and the high annual economic losses caused by aflatoxin contamination in peanuts in Georgia, USA [4].
There have been many cases of human and animal mass poisonings caused by aflatoxin contamination of agricultural products and foodstuffs all over the world. Aflatoxin is highly toxic to the liver and central nervous system of humans and animals. It can cause acute poisoning or even death in humans and animals when ingested in large amounts at one time and can be teratogenic, mutagenic, and carcinogenic when ingested in small doses over a long period of time [5,6]. According to the IARC, about 500 million people in the developing world alone are still at risk of aflatoxin exposure [7]. The European Union, one of the economies with the best food safety management systems today [8], has strict limits for fungi toxin contamination in food and feed, and China also has strict limits for aflatoxin B1 in food (Table 1). The Chinese GB 2761-2017 “National Standard for Food Safety Limits for Mycotoxins in Food” requires that the maximum limits for aflatoxin B1 (AFB1) in different cereal products range between 5–20 µg/kg, while the maximum limit for AFB1 and aflatoxin M1 (AFM1) in special dietary foods is 0.5 µg/kg and should not be detected in infant diets [9].

Table 1.
Acceptable limits of aflatoxin in crops in several countries.
Therefore, research on the prevention, control and detoxification of aflatoxins in food and feed has become one of the most important aspects of food safety and has attracted widespread attention. In order to prevent and control aflatoxin contamination from the source of crop production, improve the quality and safety of agricultural products in China, and ensure consumer safety and healthy development of the agricultural industry, the source, nature and contamination pathways of aflatoxin, and the current effective methods to deal with aflatoxin in crop production were summarized in this paper. It is expected that the emerging new technologies for aflatoxins control in soils will be widely used in crop production.
2. Aspergillus flavus in Agricultural Soils
There are an estimated 7000 species of fungi that inhabit the soil [10]. Luo et al. [11] studied the rhizosphere soil fungi community composition of camellia and explored the correlation between rhizosphere soil fungi and soil environmental factors, concluding that camellia diseases could be prevented by regulating soil environmental factors. Wu et al. [12] studied the fungi community structure in the rhizosphere soil of Rehmannia varieties and found that changes in the number of some common fungi pathogens such as A. flavus and Aspergillus niger might be the cause of soilborne diseases in the soil, which suggests that the Rehmannia root system had a certain plastic ability to the number, composition and species of fungi in rhizosphere soil.
So far, there are few reports on soil fungi of grain and oil crops. Due to the limitation of separation and detection technology in soil, only species suitable for artificial environments can be isolated from soil, so it cannot fully reflect the real soil colony environment. Fungi isolated from soil can be cultured. Only propagules capable of growing and sporing on the isolated medium used can be detected, and only about 17% of known fungi species can be successfully grown in the culture at present [13].
A single fungus may produce multiple mycotoxins, and a toxin may also be produced by multiple fungi; there are over 150 species of fungi that can produce one or more of 300 potential mycotoxins. As fungal growth is geographically specific, the predominant mycotoxins vary from region to region, e.g., in subtropical and tropical regions, agricultural products and feed are mainly contaminated with aflatoxins and certain ochratoxins. A. flavus was first used by LINK in 1809 as a generic term for saprophytic moulds in soils [14]. It has a wide range of hosts and has been reported in agriculture on maize, rice, wheat, cottonseed, peanuts and nuts, with peanuts and maize being the most affected [15,16]. The aflatoxin-producing fungi in the soil are diverse, and the distribution characteristics of different toxin-producing A. flavus occur differently. So far, the infestation pathways, effects and field distribution characteristics of A. flavus as the source of aflatoxin production in soil have not been systematically studied.
Aspergillus flavus is widely present in soil. According to the data from FAO, A. flavus is one of the most important contaminating fungi of cereals worldwide [4]. The optimum growth temperature for A. flavus ranges from 12 °C to 34 °C, while the optimum toxicity-producing temperature ranges from 20 °C to 30 °C, within 45 °C latitude [17,18]. A. flavus is mostly distributed in the soils of the Yangtze River basin and has the greatest risk of contamination [19]. Studies on the distribution of soil microbial flora and toxin contamination have also been carried out all over the world. Soil type is also related to aflatoxin pollution to some extent. According to different soil, the distribution of microbial flora is different, and the degree of mycotoxin pollution is also different, so targeted prevention and control measures can be taken. Wei et al. [20] found the existence of non-toxic and toxin-producing A. flavus in peanut soil. Based on the distribution characteristics of the strains, the risk of toxin contamination in different producing areas of China was evaluated. Zhang et al. [21] studied the genetic characteristics of A. flavus in peanut soil, providing a technical basis for the later screening of non-toxic strains and the development of aflatoxin biocontrol fungi.
In recent years, only the isolation and screening of aflatoxin in peanut root soil has been reported. Yang et al. [22] predicted the aflatoxin contamination of postpartum peanuts based on the number of aflatoxin colonies in the soil of four peanut-producing areas in China, so as to ensure the prevention and control of aflatoxin in peanuts in the later period. Zhang et al. [19], Zhu et al. [23] studied the distribution, toxin production and aflatoxin infection of A. flavus in the soil in the main peanut-producing areas of China, which provided a theoretical basis for the establishment of a model for the prevention and control of aflatoxin in China. According to the analysis of aflatoxin and its virulence in 11 producing areas of China, the Yangtze River Basin has the largest distribution of aflatoxin and the greatest risk of aflatoxin pollution. Because of the unique climatic conditions and geographical environment of the Yangtze River Basin, Hubei province has also become the largest peanut production area in China. Zhu et al. [24] also studied the distribution and toxic characteristics of aflatoxin in the soil of typical peanut growing areas in Hubei Province, providing a theoretical basis for the establishment of the early warning and prevention model of aflatoxin pollution of peanuts in Hubei Province. Zhang et al. [25] first discussed the relationship between soil types and A. flavus colonies in the peanut production area of Xiangyang, Hubei province. This work suggested that the number of A. flavus groups in the clay loam was higher and the virulence was higher than that in the sandy loam, while the sandy loam had a smaller distribution density and infection risk of A. flavus under appropriate irrigation conditions. The results of this study have important guiding significance for field fertilization, irrigation, A. flavus control and other agronomic management in the local peanut planting process.
3. Aflatoxin Contamination
So far, the content of mycotoxin detected in the soil is all in the μg range. For example, the maximum content for zearalenone is 72.1 μg/kg, for deoxynivalenol is 32.1 μg/kg, for ochratoxin A is 23.7 μg/kg, for nivalenol is 6.7 μg/kg, and for aflatoxin is 5.5 μg/kg. The retention of mycotoxins in soil is affected by soil type. Clay soil is easy to absorb toxin compounds, but sandy soil has the potential to leachate compounds [26]. In 1980, C14 was used to label aflatoxins to analyze the decomposition rate of aflatoxins in soil. Since microbial degradation function existed in soil, AFB1 could not be detected after 77 days [27]. Hence, the pollution risk of aflatoxin in soil was low, and its main risk was in the storage period after harvest. In 1997, the first report on Aspergillus oryzae detected in water storage tanks showed that although it was not drinking water, there was still a risk of potential mycotoxin contamination [28]. Although mycotoxins in freshwater samples have been increasingly reported, no reports of mycotoxins in detected sediments were found. Accinelli et al. [29] studied aflatoxin residues in soil and corn crops and proposed that AFB1 could degrade quickly in a 28.8 °C soil environment (half-life is 5 days), and AFB1 was mainly produced by the residues of corn crops on the soil surface. Corn residues may be an important source of aflatoxin pollution in soil. Therefore, if maize returning to the field in late harvest is effectively controlled, the aflatoxin pollution will be greatly reduced. In general, soil and sediment are still under-represented in the study of mycotoxin potential for environmental contamination.
Aflatoxins are mainly produced by toxic fungi such as A. flavus, as well as Aspergillus parasiticus and Aspergillus nomius. Aflatoxin-contaminated soil and agricultural products, especially grain and oil crops and nuts, are at the greatest risk of contamination. Khan et al. [30] believed that soil was the main source of aflatoxin contamination of crops. Tran-dinh et al. [31] studied A. flavus in Vietnamese soil and found that all the isolated A. flavus came from cultivated soil. Aflatoxin pollution is mainly concentrated in A. flavus (Table 2), which comes from the soil. Soil fungi are an important part of soil microorganisms. Horn et al. [32] proposed that climate and crop composition affect colony density and aflatoxin toxicity. A. flavus exists in soil in the form of conidia, sclerotia and mycelia, as the main inoculum for direct infection of peanuts or above-ground crops. Peanut is a crop with a lot of aflatoxin infection since the peanut shells are in direct contact with the soil. Aflatoxin pollution in peanuts mainly comes from the soil Aspergillus. In the study of the rhizosphere soil, through the dynamic analysis of the soil Aspergillus, it is of great significance to discuss the prenatal prevention and control of aflatoxin pollution.
Many studies have shown that soil is the main source of aflatoxin pollution in most rhizosphere crops [33,34,35], and the direct contact between soil and plant roots and the exchange of nutrients have a great impact on the occurrence of aflatoxin pollution in crops [36]. There are many research studies on soil microbial flora, but the investigation of A. flavus in soil is less. It was also reported that different soil types and A. flavus had different distributions, and the virulence of the strain was also very different, thus affecting the aflatoxin pollution of crops [37,38]. Coupling studies can change the population structure through crop rotation and management methods [39]. Horn and Dorner [40] studied the A. flavus strains in the soil of peanut planting in some areas where cotton is widely grown in the United States. In some studies, it has been possible to control aflatoxin contamination by adjusting crop rotations and changing soil temperatures.

Table 2.
Sources and contamination of toxins in food crops.
Table 2.
Sources and contamination of toxins in food crops.
| Toxin | Fungus | Susceptible Crop(s) |
|---|---|---|
| aflatoxins | Aspergillus spp. | peanut, soybean, maize, etc. [41] |
| zearalenones | Fusarium spp. | wheat, oats, etc. [42] |
| ochratoxins | Aspergillus spp. Penicillium spp. | wheat, maize, rice, soybean, etc. [43] |
| trichothecenes | Fusarium spp. | wheat, oats, maize, etc. [44] |
| fumonisins | Fusarium spp. | maize [45] |
Aflatoxin is a secondary metabolite produced by multiple Aspergillus species. It is colourless, odourless, and extremely toxic. It has been well studied that its chemical structure includes coumarin and difuran rings, and it has many derivatives and isomers that have been well studied [46]. B aflatoxins are so named because they fluoresce blue, while G aflatoxins fluoresce green when exposed to long-wave UV light (365 nm) [4]. Only 50% of A. flavus strains produce aflatoxins and B aflatoxins only, whereas almost all A. parasiticus strains produce both group B and G aflatoxins (Figure 1) [47]. The main forms of aflatoxins present in crops are AFB1, AFG1, AFB2 and AFG2, with toxicity being AFB1 > AFG1 > AFB2 > AFG2. Among them, AFB1 has the most stable structure and AFB1 is classified as a class I carcinogen [22]. International European standards have limits of AFB1 ≤ 2 µg/kg and total aflatoxin (AFT) must not exceed 4 µg/kg [48]. According to Chinese standard GB2761-2017 [9] “Food Safety National Standard Food Mycotoxin Limits”, the maximum contain limitation for AFB1 is 20 µg/kg in peanuts, corn and their products, 10 µg/kg in rice and oils, and 5 µg/kg in grain, beans, fermented foods and condiments, etc.
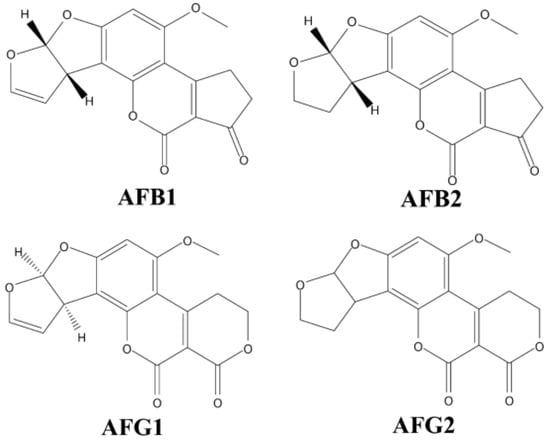
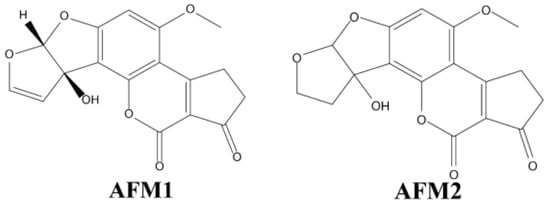
Figure 1.
Chemical structures for six main types of aflatoxin.
According to the relationship between virulence and the size of its sclerotia, A. flavus can also be divided into L and S types. The S-type strains produced numerous small (<400 μm) sclerotia, while the L-type strains produced fewer, larger sclerotia [49]. Most of the L types are non-aflatoxigenic, while most of the S-type strains are highly toxic [50,51]. Crop rotation and soil temperature can also affect the distribution of fungi community structure. Ramon et al. [52] found that the number of A. flavus and the proportion of S-type strains increased with soil temperature. Therefore, we can control A. flavus pollution by changing soil temperatures and crop rotation.
4. Aflatoxin Pollution Prevention and Control Measures
Aflatoxin contamination of crops predominantly comes from the soil and is not uncontrollable. Relevant prevention and control measures have also been studied and reported in the past two years. One approach involves a post-harvest perspective, whereby rapid detoxification and use of the product reduce the loss of marketable agricultural products. This would use physicochemical and biological methods to degrade or adsorb aflatoxin (Table S1), such as the adsorption method, radiation method, ultraviolet method, fumigation method and/or microbial enzyme degradation method [53]. These methods are relatively simple, quick, easily replicated and efficient; however, there is a risk of waste should the detoxification be unsuccessful. An alternative perspective, pre-harvest prevention, reduces aflatoxin pollution through (1) improvement of the soil microenvironment, thereby reducing the distribution of aflatoxin-producing strains, or (2) establishment of a mechanism of control in advance of planting aflatoxin-susceptible crops. Pre-harvest control is achieved through the use of biological agents acting on the soil, thereby changing the proportion of microbial strains in the soil. For example, adding ARC-BBBE biofungicide to the soil can reduce the distribution of aflatoxin colonies in peanut soil, thus reducing the total aflatoxin content of peanuts after production [51]. Biological control methods are better for maintaining the original raw material’s nutritional value and are mild, irreversible and economically viable. However, the living organisms used as agents can be influenced by the environment and have the potential to alter the soil environment in unwanted ways. A third perspective involves the establishment of an early warning model to be used by growers of crops affected by aflatoxin contamination ahead of planting, so that growers know if there is a potential risk of aflatoxin contamination. The modelling system is safe and effective in the long-term; however, there are limitations, such as being restricted by regions with geographical differences. Currently, there are very few aflatoxin modelling systems in use [54,55].
4.1. Aflatoxin Prevention and Control Using Biological Agents
Some soil biological control agents use competitive growth, or the secretion of secondary metabolites, to inhibit growth and/or toxin production by A. flavus. Examples of effective microorganisms include fungi such as Aspergillus niger and non-aflatoxigenic A. flavus, as well as lactic acid bacteria (Lactobacillus spp.) [56].
4.1.1. Non-Aflatoxigenic Aspergillus Strains
Dorner et al. [57] started to study the feasibility of non-toxic producing fungi for the control of aflatoxin contamination in peanut cultivation in 1992 with satisfactory results. Researchers such as Horn [58], Cotty [59] and Abbas [60] investigated the effectiveness of different non-aflatoxigenic A. flavus strains as formulations for the biological control of aflatoxin in peanut, cotton and maize fields, respectively. In these studies, the mechanism of control reportedly used by non-aflatoxigenic A. flavus strains to inhibit the growth of toxin-producing Aspergillus strains was competitive exclusion. Mark et al. [61] have found that field inoculation with inhibitory strains can reduce the probability of A. flavus contamination in both pre- and post-harvest. In the field, spore preparation of 11.2–22.4 kg/hm2 can inhibit aflatoxin in peanut crops by up to 90%, and this inhibition effect can still be sustained. In this method, non-aflatoxigenic A. flavus strains are used to inhibit the growth of toxin-producing A. flavus strains in soil, while non-aflatoxigenic strains in crops have a certain protective effect on crops after harvest [62]. Liu et al. [63], Xing et al. [64] and Zhang et al. [65] studied several strains of non-aflatoxigenic A. flavus and their roles in aflatoxin degradation, and the inhibition levels in the laboratory reached 98%. No finished formulations have yet been applied to field soils, and research has mainly focused on the screening and optimisation of biocontrol fungi in the laboratory and the investigation of control mechanisms.
4.1.2. Yeasts
In 2022, Natarajan et al. [66] isolated 45 strains of yeast from the soil to inhibit the growth of A. flavus, and the inhibition rate reached 99% in the laboratory, but it was not applied in the field. Biological control of yeasts is widely used for post-production detoxification, using their adsorption capacity to remove aflatoxins from food, and there are no live strain preparations that have been applied in actual field trials [67].
4.1.3. Bacteria
In 1985, Coallier-Ascah et al. [68] inoculated Lactococcus lactis into a culture of aflatoxigenic A. flavus spores and did not detect aflatoxin after shaking bed incubation. In 2008, Petchkongkaew et al. [69] used Bacillus subtilis and Lactobacillus licheniformis to inhibit the growth and toxin production of aflatoxin and both achieved good control results. In recent years, Zhou et al. [51] studied ARC-BBBE biological bacteriological agents and applied three species of Bacillus to the rhizosphere soil. During three years of field demonstration in major peanut-producing areas in China, the abundance of toxic A. flavus in soil decreased by 66.5%, the detection rate of aflatoxin in post-harvest peanuts was also greatly reduced, and nodulation with nitrogen fixation of root system was discovered unexpectedly. Large field demonstration trials have achieved substantial yield increases.
4.2. New Material Detoxification Methods
With the rapid development of materials, biology, environment and energy science, it is the direction of future efforts to find a low-cost, fast, safe, efficient and stable green technology for aflatoxin detoxification in grain and oil by combining the above technologies effectively. Nanomaterial scales are equivalent to 10 to 100 atoms packed tightly together. It has been reported that nanomaterials can be used for the elimination of aflatoxins [70]. By modifying nanomaterials with surface modification, nanomaterials with specific adsorption of aflatoxins can be utilized effectively. Liang et al. [71] analysed the feasibility of the magnetic nanoparticles selective adsorption method and tested it for the detoxification of aflatoxin in peanut oil. In the later stage, Mao et al. [70,72,73] studied the semiconductor material g-C3N4, which could be degraded to carbon dioxide and water after the adsorption of AFB1 and 2 h of sunlight irradiation. They also prepared z-composites at a later stage and designed effective photocatalysts to reduce secondary contamination by aflatoxin toxicity tests on cells.
4.3. Early Warning Models
The crop aflatoxin production is influenced directly by environmental temperature and humidity changes at the planting, harvesting, storage, transportation and processing stages. To reduce the risk of aflatoxin pollution, early warning models are often established according to the relationships between environmental temperature, humidity changes and aflatoxin production. As early as 1990, Thai et al. [74] studied the process dynamics of aflatoxin pollution under drought conditions and established the relationship model between soil temperature and aflatoxin, but it has not been applied in practice. In 1998, the CROPGRO-peanut model was released in the United States, which comprehensively introduced the relationship between environmental parameters and the growth of A. flavus [75]. It was also applied to the risk warning of aflatoxin in Niger and was also well applied to the prediction of aflatoxin content in Mali [76].
Li et al. [77] established Boltzmann and logistic models to explore the relationship between temperature and humidity during storage and aflatoxin, effectively preventing contamination. Jiang et al. [78] put forward recommended measures for the whole-process prevention and control of aflatoxins based on GMP standards by pre-harvest investigations of the peanut varieties, soil, planting methods, pest control and field irrigation before flower production, as well as the harvesting equipment and post-harvest considerations like receiving time, drying and cleaning, transportation conditions and storage environment. Zhang et al. [79] analysed the causes of product hazards, critical control points and control measures from three aspects, and used HACCP to study the whole process control of aflatoxin production in exported peanuts. Wu et al. [80] studied the pre-harvest, post-harvest and whole-process early warning methods of aflatoxin, which collects data in different locations and uses different links to build models, and is also the development direction of aflatoxin early warning technology in the future.
The existing research shows that it is feasible to carry out the whole early warning of peanut aflatoxin. However, different regions of our country, different climates, impact factors and key control points are different. It is a long-term and efficient method to control aflatoxin pollution by systematically studying the critical control points of aflatoxin pollution in different producing areas and different links, establishing an early warning model, and early detection and early prevention.
5. Conclusions and Prospects
The strong toxicity and carcinogenicity of aflatoxin is a serious threat to human health and food safety. It is important to find a green, environmentally friendly and efficient means to effectively prevent and control aflatoxin contamination. In the long term, to ensure the quality and safety of agricultural products, it is also necessary to establish a full range of aflatoxin contamination early warning technology to control all aspects of the crop, to achieve the controllability of aflatoxin contamination, to achieve the full range of prevention and control from farm to fork, and to prevent and control mycotoxin contamination of agricultural product quality and safety. However, the model for China is established late, which needed to be based on different countries, different regions, different links, and different crops. The key control points are different, and the operability is more difficult. Although there are many ways for aflatoxin controlling, the biological control method has great advantages in terms of nutrients not being destroyed and not causing a lot of pollution. For example, the method for the introduction of biofungal agents in the soil not only effectively decreases the abundance of toxic aflatoxin-producing fungi, but also maintains the original quality of agricultural products. Moreover, it has more advantages of high safety, high efficiency and long persistence.
At present, a lot of research has been done on aflatoxin contamination control measures. Among them, the study of the interaction pattern between soil, plant and inter-root microorganisms provides new hints for the innovation of biological control methods of aflatoxin contamination in soil. The new direction of biological control measures for A. flavus in soil includes studying the distribution of microflora in different soil environments and resolving the interactions among inter-root microorganisms. Through risk assessment and early warning of contamination risk for crops in different growing regions, more data will be obtained to support the precise biological control of aflatoxin contamination in crops, thus improving the applicability of aflatoxin control mechanisms and reducing the losses caused by aflatoxin contamination.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxins15080475/s1, Table S1: types of post-harvest aflatoxin detoxification. References [81,82,83,84,85,86,87,88] are cited in Supplementary Materials.
Author Contributions
Conceptualization, X.W.; methodology, D.W.; formal analysis, X.W. and Q.Z.; investigation, X.W., D.W., S.Z., M.Z., Q.Y., Q.Z., P.F. and J.D.; resources, Q.Z.; writing original draft, X.W.; writing—review and editing, D.W. and Q.Z.; project administration, D.W. and P.F.; funding acquisition, D.W. and Q.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the Key Project of the National Natural Science Foundation of China (32030085); the Key Laboratory of Biotoxin Detection, Ministry of Agriculture and Rural Affairs, SWDSJC2023001; the Key Project of Hongshan Laboratory of Hubei Province, No. 2021HSZD015; the Natural Science Foundation of Hubei Province, No. 2022cfD094; and Xiangyang Youth Talents Development Project (DPXYYT2018-d).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request from the authors.
Acknowledgments
Thanks to the open project of the Key Laboratory of Biotoxin Detection, Ministry of Agriculture and Rural Affairs (SWDSJC2023001) and the Key Project of the National Natural Science Foundation of China (32030085) for funding this project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, Y.J.; Liu, F.J.; Zhou, X.; Liu, M.R.; Zang, H.R.; Liu, X.; Shan, A.S.; Feng, X.J. Alleviation of Oral Exposure to Aflatoxin B1-Induced Renal Dysfunction, Oxidative Stress, and Cell Apoptosis in Mice Kidney by Curcumin. Antioxidants 2022, 11, 1082. [Google Scholar] [CrossRef]
- Ajmal, M.; Bedale, W.; Akram, A.; Yu, J.-H. Comprehensive Review of Aflatoxin Contamination, Impact on Health and Food Security, and Management Strategies in Pakistan. Toxins 2022, 14, 845. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A. Mycotoxins: Risks in Plant, Animal and Human Systems; Council for Agricultural: Ames, IA, USA, 2003; pp. 48–50. Available online: http://www.researchgate.net/publication/305113928_Mycotoxins_Risks_in_plant_animal_and_human_systems (accessed on 1 January 2003).
- Klich, M.A. Aspergillus flavus: The major producer of aflatoxin. Mol. Plant Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Huang, T.R.; Su, J.J.; Liang, F.; Wei, Z.L.; Liang, Y.Q.; Luo, H.T.; Kuang, S.Y.; Qian, G.S.; Sun, G.J.; et al. Hepatocellular Carcinoma and Aflatoxin Exposure in Zhuqing Village, Fusui County, People’s Republic of China. Cancer Epidemiol. Biomark. Prev. 2001, 10, 143–146. [Google Scholar]
- Wild, C.P.; Hall, A.J. Primary prevention of hepatocellular carcinoma in developing countries. Mutat. Res. Rev. Mut. Res. 2000, 462, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.E.; Prendergast, A.J.; Turner, P.C.; Humphrey, J.H.; Stoltzfus, R.J. Aflatoxin Exposure During Pregnancy, Maternal Anemia, and Adverse Birth Outcomes. Am. J. Trop. Med. Hyg. 2017, 96, 770–776. [Google Scholar] [CrossRef]
- Bei, J.; Sun, L.; Yang, Y.; Yin, J. EU Rapid Alert System for Food and Feed Notifications 2018 Situation analysis. J. Food Saf. Food Qual. 2019, 10, 4781–4787. [Google Scholar]
- GB 2761-2017; Food Safety National Standard-Limit of Mycotoxins in Food. National Health and Family Planning Commission of the People’s Republic of China. State Administration of Food and Drug Administration: Beijing, China, 2017.
- Hawksworth, D.L. The fungal dimension of biodiversity: Magnitude, significance, and conservation. Mycol. Res. 1991, 95, 641–655. [Google Scholar] [CrossRef]
- Luo, X.; Wu, Y.K.; Zhang, N.N.; Xu, J.; Yang, Z.H. Composition and Diversity of Fungal Community in Rhizosphere Soil of Camellia oleifera. J. Agric. Sci. Technol. 2023, 25, 199. [Google Scholar] [CrossRef]
- Wu, T.J.; Du, Q.; Xie, X.L. Study on Fungal Community Structure of Rhizosphere Soil of Different Rehmannia glutinosa Species. J. Anhui Agricul. Sci. 2021, 49, 159–163, 177. [Google Scholar]
- Bridge, P.; Spooner, B. Soil fungi: Diversity and detection. Plant Soil 2001, 232, 147–154. [Google Scholar] [CrossRef]
- Zhao, Z.H. Advances of Research on Mycotoxins in Agricultural Products and Feed. J. Beijing Technol. Bus. Univ. Nat. Sci. Ed. 2012, 30, 8–11. [Google Scholar] [CrossRef]
- Amaike, S.; Keller, N.P. Aspergillus flavus. Annu. Rev. Phytopathol. 2011, 49, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, M.T.; Pasqualotto, A.C.; Warn, P.A.; Bowyer, P.; Denning, D.W. Aspergillus flavus: Human pathogen, allergen and mycotoxin producer. Microbiology 2007, 153, 1677–1692. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, Y.L.; Zhang, H.Y.; Zhang, C.X.; Yang, B.L.; Huang, S.J.; Liu, Y. Factors that affect the formation of mycotoxins: A literature review. Mycosystema 2020, 39, 477–491. [Google Scholar]
- Li, D.; Qing, L.; Wang, S.H.; Yuan, J. Research progress on the omics of secondary metabolism of Aspergillus flavus. Mycosystema 2020, 39, 509–520. [Google Scholar]
- Zhang, X. Study on Distribution, Virulence and Infection of Aspergillus flavus in Typical Peanut Producing Areas of China. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2019. Available online: https://www.ckcest.cn/default/es3/detail/1003/dw_thesis_copy/a7b600017950a8c5e0379d6644288c52 (accessed on 1 May 2019).
- Wei, D.D.; Zhou, L.; Zhang, C.S.; Liu, Y. Atoxigenic Aspergillus flavus Competitively Reduce Aflatoxin Production by the Toxigenic Aspergillus flavus; Chinese Academy of Agricultural Sciences: Beijing, China, 2014; Available online: http://www.researchgate.net/publication/286173904_Atoxigenic_Aspergillus_flavus_competitively_reduce_aflatoxin_production_by_the_toxigenic_Aspergillus_flavus (accessed on 1 June 2014).
- Zhang, C.S. Study on Distribution, Toxicity and Genetic Diversity of Aspergillus flavus in Peanut Soil in Four Ecological Regions of China; Chinese Academy of Agricultural Sciences: Beijing, China, 2013. [Google Scholar]
- Yang, B.L.; Geng, H.R.; Wang, G.; Zhang, C.X.; Li, L.; Nie, C.R.; Xing, F.G.; Liu, Y. Study on the Correlation between the distribution of soil Aspergillus flavus in Peanuts and the contamination of aflatoxin in postpartum Peanuts. J. Nucl. Agric. 2021, 35, 863–869. [Google Scholar]
- Zhu, T.T. Distribution, Virulence and Contamination of Aflatoxin fungi. In Peanut Soil; Chinese Academy of Agricultural Sciences: Beijing, China, 2018. [Google Scholar]
- Zhu, T.T.; Chen, L.; Yue, X.F.; Bai, Y.Z.; Ding, X.X.; Li, P.W.; Zhang, Q.; Zhang, W. Distribution, Aflatoxin production of Aspergillus flavus in soils of typical peanut planting areas in Hubei Province. Chin. J. Oil Crop. Sci. 2019, 41, 255–260. [Google Scholar]
- Zhang, S.J.; Wang, X.; Wang, D.; Chu, Q.M.; Zhang, Q.; Yue, X.F.; Zhu, M.J.; Dong, J.; Li, L.; Jiang, X.G.; et al. Discovery of the Relationship between Distribution and Aflatoxin Production Capacity of Aspergillus species and Soil Types in Peanut Planting Areas. Toxins 2022, 14, 425. [Google Scholar] [CrossRef]
- Juraschek, L.M.; Kappenberg, A.; Amelung, W. Mycotoxins in soil and environment. Sci. Total Environ. 2021, 814, 152425. [Google Scholar] [CrossRef]
- Angle, J.S.; Wagner, G.H. Decomposition of Aflatoxin in Soil. Soil Sci. Soc. Am. J. 1980, 44, 1237–1240. [Google Scholar] [CrossRef]
- Paterson, R.R.; Kelley, J.; Gallagher, M. Natural occurrence of aflatoxins and Aspergillus flavus (Link) in water. Lett. Appl. Microbiol. 2010, 25, 435–436. [Google Scholar] [CrossRef]
- Accinelli, C.; Abbas, H.K.; Zablotowicz, R.M.; Wilkinson, J.R. Aspergillus flavus aflatoxin occurrence and expression of aflatoxin biosynthesis genes in soil. Can. J. Microbiol. 2008, 54, 371–379. [Google Scholar] [CrossRef]
- Khan, R.; Ghazali, F.M.; Mahyudin, N.A.; Samsudin, N.I.P. Biocontrol of Aflatoxins Using Non-Aflatoxigenic Aspergillus flavus: A Literature Review. J. Fungi 2021, 7, 381. [Google Scholar] [CrossRef]
- Tran-Dinh, N.; Kennedy, I.; Bui, T.; Carter, D. Survey of Vietnamese Peanuts, Corn and Soil for the Presence of Aspergillus flavus and Aspergillus parasiticus. Mycopathologia 2009, 168, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Horn, B.W. Ecology and Population Biology of Aflatoxigenic Fungi in Soil. J. Toxicol. Toxin Rev. 2003, 22, 351–379. [Google Scholar] [CrossRef]
- Hideharu, S.; Akikazu, S. Gas Plasma Sterilization in Microbiology; Caister Academic: Tokyo, Japan, 2016; pp. 91–121. [Google Scholar]
- Laroussi, M.; Leipold, F. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int. J. Mass Spectrom. 2004, 233, 81–86. [Google Scholar] [CrossRef]
- Sakudo, A.; Toyokawa, Y.; Misawa, T.; Imanishi, Y. Degradation and detoxification of aflatoxin B1 using nitrogen gas plasma generated by a static induction thyristor as a pulsed power supply. Food Control 2017, 73, 619–626. [Google Scholar] [CrossRef]
- Laroussi, M.; Kong, M.G.; Morfill, G.; Stolz, W. Plasma Medicine: Applications of Low-Temperature Gas Plasmas in Medicine and Biology; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar] [CrossRef]
- Gai, Y.X.; Zhao, M.M.; Cui, C.; Kong, L.H.; Wu, X. Effects of different pretreatment methods on the content of aflatoxin in peanut meal enzymolysis solution. Food Ferment. Ind. 2007, 33, 18–21. [Google Scholar]
- Qi, D.S.; Liu, F.; Yu, Y.H.; He, W.L.; Tu, H.R. Adsorption of Aflatoxin B1 by montmorillonite and modified Montmorillonite. J. Anim. Sci. Vet. Med. 2003, 34, 620–622. [Google Scholar]
- Angle, J.S.; Dunn, K.A.; Wagner, G.H. Effect of Cultural Practices on the Soil Population of Aspergillus flavus and Aspergillus parasiticus. Soil Sci. Soc. Am. J. 1982, 46, 301–304. [Google Scholar] [CrossRef]
- Horn, B.W.; Dorner, J.W. Soil populations of Aspergillus species from section Flavi along a transect through peanut-growing regions of the United States. Mycologia 1998, 90, 767–776. [Google Scholar] [CrossRef]
- Tabata, S.; Kamimura, H.; Ibe, A.; Hashimoto, H.; Iida, M.; Tamura, Y.; Nishima, T. Aflatoxin Contamination in Foods and Foodstuffs in Tokyo: 1986-1990. J. AOAC Int. 1993, 76, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Schuhmacher, R.; Buttinger, G.; Krska, R. Rapid simultaneous determination of major type A- and B-trichothecenes as well as zearalenone in maize by high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2005, 1062, 209–216. [Google Scholar] [CrossRef]
- Madhyastha, S.M.; Marquardt, R.R.; Frohlich, A.A.; Platford, G.; Abramson, D. Effects of different cereal and oilseed substrates on the growth and production of toxins by Aspergillus alutaceus and Penicillium verrucosum. J. Agric. Food Chem. 1990, 38, 1506–1510. [Google Scholar] [CrossRef]
- Sudakin, D.L. Trichothecenes in the environment: Relevance to human health. Toxicol. Lett. 2003, 143, 97–107. [Google Scholar] [CrossRef]
- Thiel, P.G.; Marasas, W.F.O.; Sydenham, E.W.; Shephard, G.S.; Gelderblom, W.C.A. The implications of naturally occurring levels of fumonisins in corn for human and animal health. Mycopathologia 1992, 117, 3–9. [Google Scholar] [CrossRef]
- Abdel, H.H.; Palmery, M.; Leone, M.G.; Saso, L.; Silvestrini, B. Relaxant Effects of Aflatoxins on Isolated Guinea Pig Trachea. Toxicol. Sci. 2000, 55, 162–170. [Google Scholar] [CrossRef]
- Xu, J.; Luo, X.Y. Molecular biology of aflatoxin biosynthesis. Health Hyg. Res. 2003, 32, 628–631, 636. [Google Scholar]
- Wang, S.X.; Lu, S.S.; Zhang, Y.; Xie, G.; Wang, H.O. Current situation and progress of the revision of mycotoxin detection standards at home and abroad. Sci. Technol. Food Ind. 2011, 408, 412–416. [Google Scholar]
- Cotty, P.J. Aflatoxin-producing potential of communities of Aspergillus section Flavi from cotton producing areas in the United States. Mycol. Res. 1997, 101, 698–704. [Google Scholar] [CrossRef]
- Gao, J.X.; Gao, P.; Liu, Z.X. Analysis of virus-producing characteristics of Aspergillus flavus in soil-crop system. Bull. Sci. Tech. Consult. 2007, 33, 18–21. [Google Scholar]
- Zhou, Y.; Yue, X.F.; Tang, X.Q.; Yan, H.L.; Zhang, Q.; Li, P.W. A preliminary study on the coupling effect of aflatoxin green control and super-nodulation. Chin. J. Oil Crop. Sci. 2021, 43, 947. [Google Scholar] [CrossRef]
- Jaime Garcia, R.; Cotty, P.J. Crop rotation and soil temperature influence the community structure of Aspergillus flavus in soil. Soil Biol. Biochem. 2010, 42, 1842–1847. [Google Scholar] [CrossRef]
- Xing, F.G.; Li, X.; Zhang, C.X. Biosynthesis Mechanisms and Control Strategies of Aflatoxin. J. Food Sci. Technol. 2021, 39, 13–26, 64. [Google Scholar] [CrossRef]
- Battilani, P.; Camardo Leggieri, M.; Rossi, V.; Giorni, P. AFLA-maize, a mechanistic model for Aspergillus flavus infection and aflatoxin B1 contamination in maize. Comput. Electron. Agric. 2013, 94, 38–46. [Google Scholar] [CrossRef]
- Liu, N.J.; Liu, C.; Dudaš, T.N.; Loc, M.Č.; Bagi, F.F.; Fels-Klerx, H.J. Improved Aflatoxins and Fumonisins Forecasting Models for Maize (PREMA and PREFUM), Using Combined Mechanistic and Bayesian Network Modeling—Serbia as a Case Study. Front. Microbiol. 2021, 12, 643604. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Gong, X.M.; Dong, J.; Sun, J. Research progress of biological method to control aflatoxin B1. Food Feed Ind. 2011, 11, 22–23. [Google Scholar]
- Dorner, J.W.; Cole, R.J.; Blankenship, P.D. Use of a Biocompetitive Agent to Control Preharvest Aflatoxin in Drought Stressed Peanuts. J. Food Prot. 1992, 55, 888–892. [Google Scholar] [CrossRef]
- Horn, B.W.; Dorner, J.W. Effect of nontoxigenic Aspergillus flavus and A. parasiticus on aflatoxin contamination of wounded peanut seeds inoculated with agricultural soil containing natural fungal populations. Biocont. Sci. Technol. 2009, 19, 249–262. [Google Scholar] [CrossRef]
- Cotty, P.J. Influence of Field Application of an Atoxigenic Strain of Aspergillus flavus on the Populations of A. flavus Infecting Cotton Bolls and on the Aflatoxin Content of Cottonseed. Phytopathology 1994, 84, 1270. [Google Scholar] [CrossRef]
- Abbas, H.K.; Wilkinson, J.R.; Zablotowicz, R.M.; Accinelli, C.; Abel, C.A.; Bruns, H.A.; Weaver, M.A. Ecology of Aspergillus flavus, regulation of aflatoxin production, and management strategies to reduce aflatoxin contamination of corn. Toxin Rev. 2009, 28, 142–153. [Google Scholar] [CrossRef]
- Weaver, M.A.; Abbas, H.K.; Brewer, M.J.; Pruter, L.S.; Little, N.S. Integration of biological control and transgenic insect protection for mitigation of mycotoxins in corn. Crop. Prot. 2017, 98, 108–115. [Google Scholar] [CrossRef]
- Cotty, P.J. Effect of atoxigenic strains of Aspergillus flavus on aflatoxin contamination of developing cottonseed. Plant Dis. 1990, 74, 233–235. [Google Scholar] [CrossRef]
- Liu, Y.T.; Xing, F.G. A Strain of Non-Toxic Aspergillus flavus and Its Application in the Degradation of Aflatoxin. CN 107245453 B, 9 June 2020. [Google Scholar]
- Xing, F.G.; Liu, Y. Degradation of Aflatoxin by Non-Toxic Aspergillus flavus. CN 107279686A, 24 October 2017. [Google Scholar]
- Zhang, J.C.; Zhang, C.S.; Sun, J. An Antagonist of Toxic Aflatoxin-Producing Aspergillus, Its Preparation Method and Application. CN 110122508B, 12 January 2021. [Google Scholar]
- Natarajan, S.; Balachandar, D.; Senthil, N.; Velazhahan, R.; Paranidharan, V. Volatiles of antagonistic soil yeasts inhibit growth and aflatoxin production of Aspergillus flavus. Microbiol. Res. 2022, 263, 127–150. [Google Scholar] [CrossRef]
- Corassin, C.H.; Bovo, F.; Rosim, R.E.; Oliveira, C.A.F. Efficiency of Saccharomyces cerevisiae and lactic acid bacteria strains to bind aflatoxin M1 in UHT skim milk. Food Control. 2013, 31, 80–83. [Google Scholar] [CrossRef]
- Coallier, A.J.; Idziak, E.S. Interaction between Streptococcus lactis and Aspergillus flavus on production of aflatoxin. Appl. Environ. Microbiol. 1985, 49, 163–167. [Google Scholar] [CrossRef]
- Petchkongkaew, A.; Taillandier, P.; Gasaluck, P.; Lebrihi, A. Isolation of Bacillus spp. from Thai fermented soybean (Thua-nao): Screening for aflatoxin B1 and ochratoxin A detoxification. J. Appl. Microbiol. 2008, 104, 1495–1502. [Google Scholar] [CrossRef]
- Mao, J.; Li, P.W.; Wang, J.M.; Wang, H.T.; Zhang, Q.; Zhang, L.X.; Li, H.; Zhang, W.; Peng, T.Y. Insights into photocatalytic inactivation mechanism of the hypertoxic site in aflatoxin B1 over clew-like WO3 decorated with CdS nanoparticles. Appl. Catal. B 2019, 248, 477–486. [Google Scholar] [CrossRef]
- Liang, L.L.; Li, B.; Chen, L. Research progress of aflatoxin detoxification transformation. Food Oil 2004, 3, 39–40. [Google Scholar]
- Mao, J.; Zhang, L.X.; Wang, H.T.; Zhang, Q.; Zhang, W.; Li, P.W. Facile fabrication of nanosized graphitic carbon nitride sheets with efficient charge separation for mitigation of toxic pollutant. Chem. Eng. J. 2018, 342, 30–40. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, Q.; Li, P.W.; Zhang, L.X.; Zhang, W. Geometric architecture design of ternary composites based on dispersive WO3 nanowires for enhanced visible-light-driven activity of refractory pollutant degradation. Chem. Eng. J. 2018, 334, 2568–2578. [Google Scholar] [CrossRef]
- Thai, C.N.; Blankenship, P.D.; Cole, R.J.; Sanders, T.H.; Domer, J.W. Relationship between aflatoxin production and soil temperature for peanuts under drought stress. Trans. ASAE 1990, 33, 324–329. [Google Scholar] [CrossRef]
- Boote, K.J.; Jones, J.W.; Hoogenboom, G. Simulation of crop growth: CROPGRO model. Int. J. Remote Sens. 1998, 18, 651–692. [Google Scholar]
- Boken, V.K.; Hoogenboom, G.; Williams, J.H.; Diarra, B.; Dione, S.; Easson, G.L. Monitoring peanut contamination in Mali (Africa) using AVHRR satellite data and a crop simulation model. Int. J. Remote Sens. 2008, 29, 117–129. [Google Scholar] [CrossRef]
- Li, R.F.; Han, B.Z.; Chen, J.Y. Establishment of growth prediction model of Aspergillus flavus and change of toxin content. Food Res. Devel. 2007, 12, 129–132. [Google Scholar]
- Jiang, Z.L.; Men, A.J.; Zhang, Y.B. Aflatoxin Pollution and Control in Peanuts; Standards Press of China: Beijing, China, 2015. [Google Scholar]
- Zhang, C.X.; Li, J.J. Application of HACCP in Controlling Aflatoxin Contamination of Peanut; China National Times: Beijing, China, 2005. [Google Scholar]
- Wu, L.X.; Du, X.H.; Ding, X.X.; Zhou, H.Y.; Chen, L.; Li, P.W. Advances on early warning techniques of peanut aflatoxins. J. China Pet. Resour. 2016, 38, 120–126. [Google Scholar]
- Zhang, J.Y.; Xu, S.R. Aflatoxin Toxicity and Detoxification measures. Food Nutr. China 2001, 5, 47–48. Available online: http://vp1.a.s99s.top/article/detail.aspx?id=5709131 (accessed on 1 May 2001).
- Liu, Y. Study on the Adsorption Characteristics and Effects of Mycotoxin Adsorbent on Aflatoxin B1; Sichuan University of Agriculture: Chengdu, China, 2011. [Google Scholar]
- Zhang, X.Q.; Li, F.Q. Selection and Application of Myctoxin Adsorbents. China Poult. 2007, 29, 47–48. [Google Scholar]
- Raksha Rao, K.; Vipin, A.V.; Hariprasad, P.; Anu Appaiah, K.A.; Venkateswaran, G. Biological detoxification of Aflatoxin B1 by Bacillus licheniformis CFR1. Food Control 2017, 71, 234–241. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Xing, F.G.; Wei, Y.M. Study on the Adsorption of Aflatoxin B1 by Probiotic bacteria. In Proceedings of the 2nd Symposium on Probiotics and Health and Applied Technology Exchange, Nanning, China, 2 January 2010. [Google Scholar]
- Luo, X.H.; Wang, R.; Wang, L.; Li, Y.F.; Wang, Y.; Chen, Z.X. Detoxification of aflatoxin in corn flour by ozone. J. Sci. Food Agric. 2014, 94, 2253–2258. [Google Scholar] [CrossRef]
- Chen, Z.J.; Liu, Y.; Xing, F.G.; Liang, J.P.; Liu, C. Optimization of conditions for the degradation of Aflatoxin B1 in maize by ammonia fumigation. Food Sci. 2010, 31, 33–37. [Google Scholar]
- Liang, D.D. Study on the Inhibition of Growth and Toxicity Production of Aspergillus flavus in Corn by Three Plant Essential Oils; Chinese Academy of Agricultural Sciences: Beijing, China, 2015. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).