Characterization of Particle-Size-Based Homogeneity and Mycotoxin Distribution Using Laser Diffraction Particle Size Analysis
Abstract
1. Introduction
- Develop a sizing measurement procedure for representative mycotoxin matrices using laser diffraction spectroscopy.
- Determine whether laser diffraction particle size analysis could provide results of the particle size distribution for homogeneity assessment in a practical and time-efficient manner.
- Confirm that particle size-based homogeneity assessment is consistent with ISO Guide 35.
2. Results and Discussion
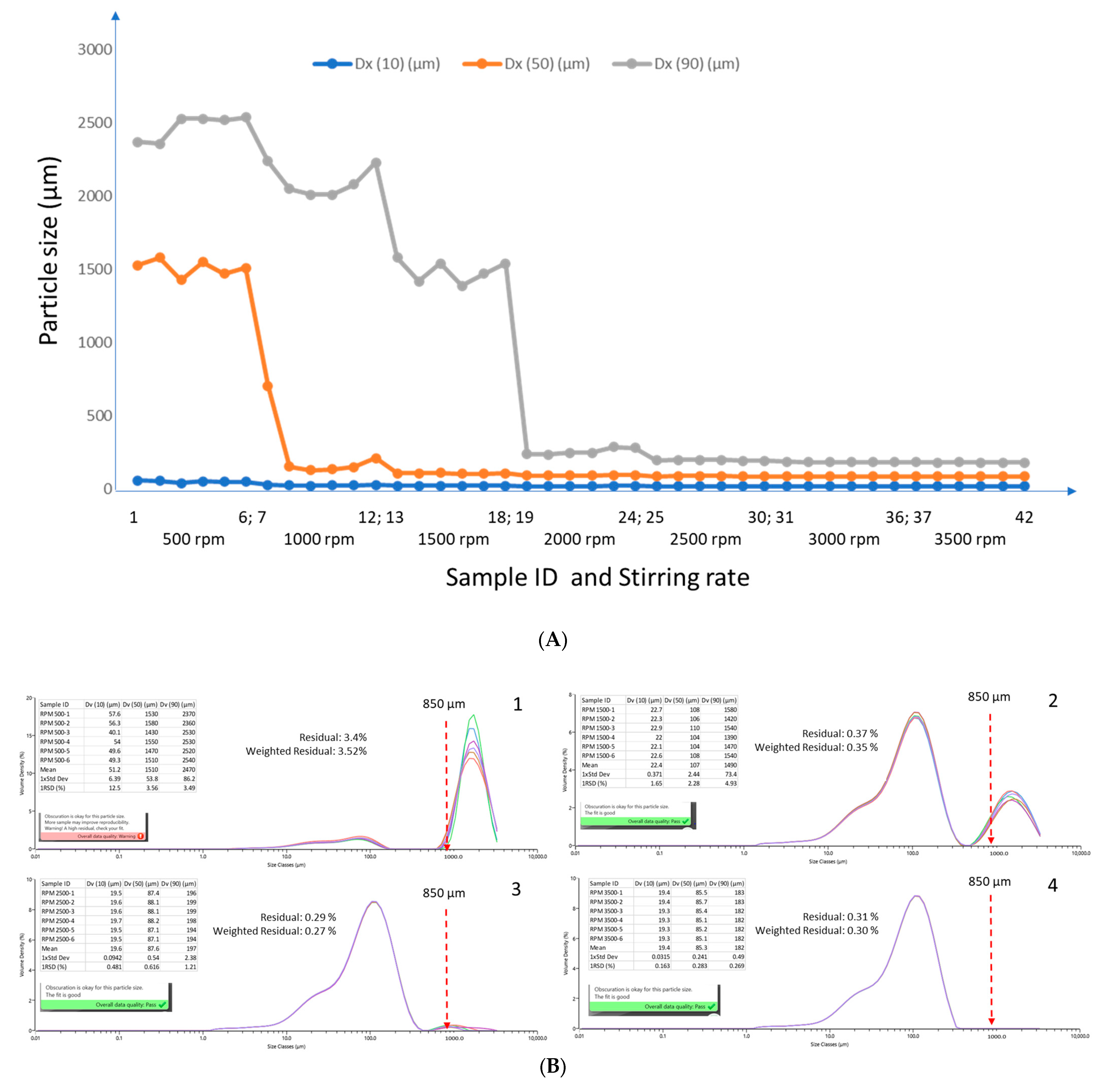
2.1. Effects of Dispersion Parameters: Dispersant and Stirring Rate
2.2. Effects of Optical Parameters: Refractive Index, Absorption Index, and Obscuration
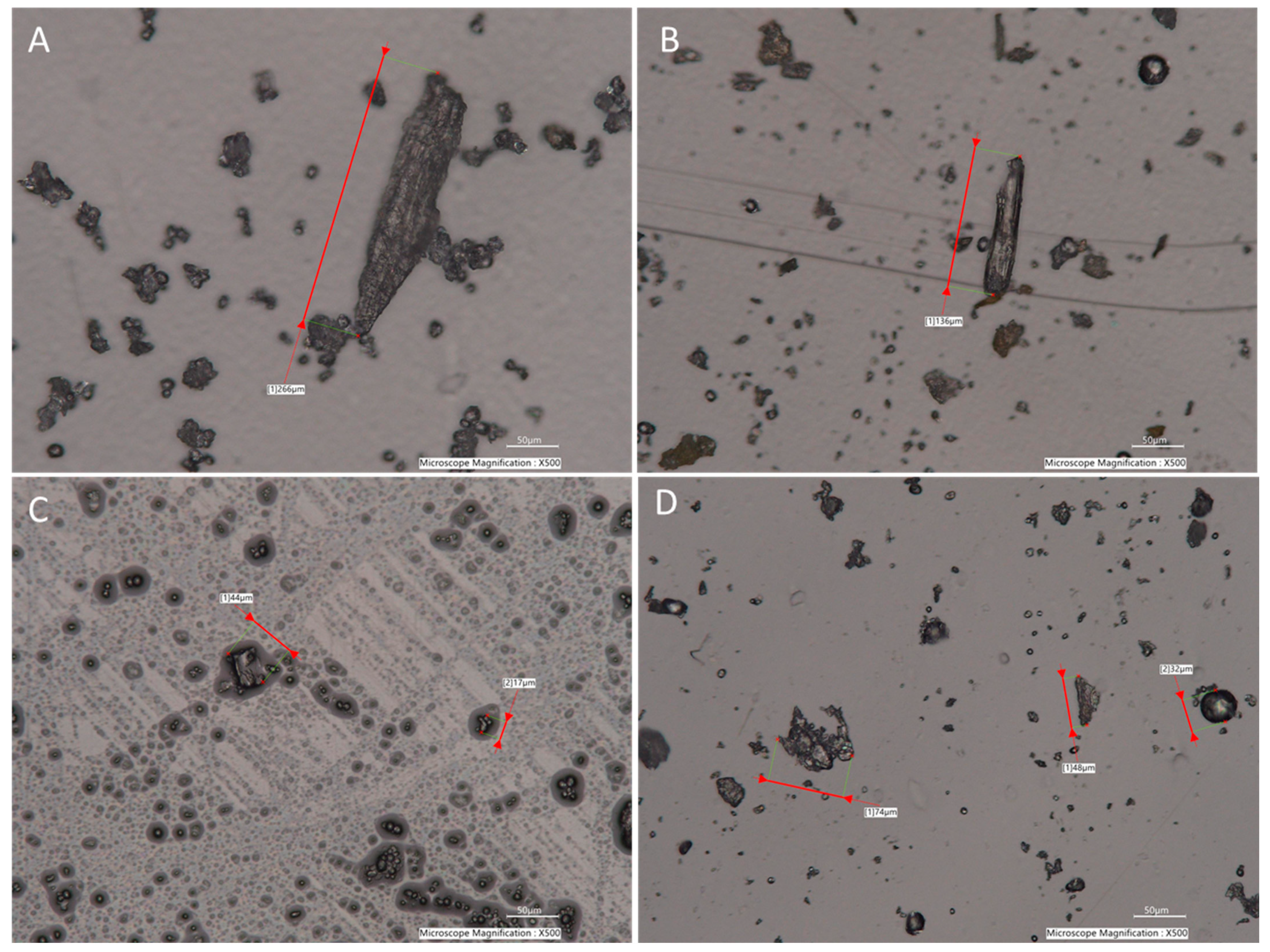
2.3. Particle Size Distribution of Corn, Compound Feed, Peanut Butter, and Wheat Flour
2.4. Confirmation of Homogeneity Using ISO Guide 35
3. Conclusions
4. Materials and Methods
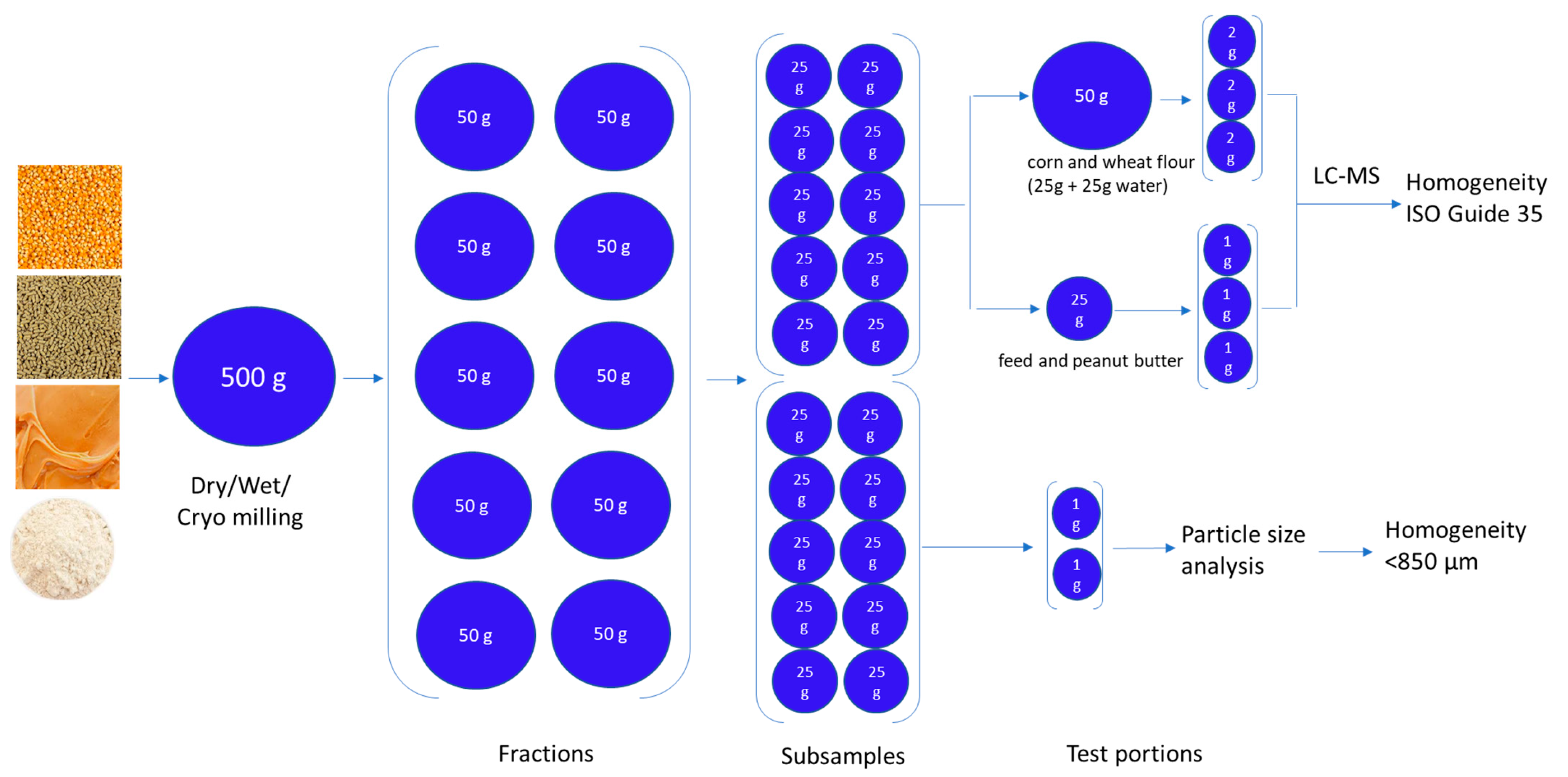
4.1. Sample Comminution and Subsampling
4.2. Particle Size Analysis
4.3. Confirmation of Homogeneity Using ISO GUIDE 35
4.4. LC-MS Analysis
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, A.D.; Whitaker, T.B.; Pohland, A.E.; Dickens, J.W.; Park, D.L. Sampling, sample preparation, and sampling plans for foodstuffs for mycotoxin analysis. Pure Appl. Chem. 1986, 58, 305–314. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 401/2006 of 23 February 2006 Laying Down the Methods of Sampling and Analysis for the Official Control of the Levels of Mycotoxins in Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R0401&from=EN (accessed on 6 June 2023).
- Food and Drug Administration. The Investigations Operations Manual (IOM) Chapter 4—Sampling. Available online: https://www.fda.gov/media/75243/download (accessed on 3 July 2023).
- Crosby, N.T. Review of current and future analytical methods for the determination of mycotoxins? Food Addit. Contam. 1984, 1, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Krska, R.; Schubert-Ullrich, P.; Molinelli, A.; Sulyok, M.; MacDonald, S.; Crews, C. Mycotoxin analysis: An update. Food Addit. Contam. Part A 2008, 25, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Brera, C.; Crews, C.; Iha, M.H.; Krska, R.; Lattanzio, V.; Macdonald, S.; Malone, R.; Maragos, C.; Solfrizzo, M.; et al. Developments in mycotoxin analysis: An update for 2015–2016. World Mycotoxin J. 2016, 9, 5–30. [Google Scholar] [CrossRef]
- Zhang, K.; Banerjee, K. A Review: Sample Preparation and Chromatographic Technologies for Detection of Aflatoxins in Foods. Toxins 2020, 12, 539. [Google Scholar] [CrossRef]
- ISO Guide 35:2017; Reference Materials—Guidance for Characterization and Assessment of Homogeneity and Stability. International Organisation for Standardisation (ISO): Geneva, Switzerland, 2017.
- Thompson, M.; Wood, R. The international harmonized protocol for the proficiency testing of (chemical) analytical laboratories (Technical Report). Pure Appl. Chem. 1993, 65, 2123–2144. [Google Scholar] [CrossRef]
- Thompson, M.; Ellison, S.L.R.; Wood, R. The international harmonized protocol for the proficiency testing of (chemical) analytical laboratories (Technical Report). Pure Appl. Chem. 2006, 78, 145–196. [Google Scholar] [CrossRef]
- Albert, R.; Horwitz, W. A Heuristic Derivation of the Horwitz Curve. Anal. Chem. 1997, 69, 789–790. [Google Scholar] [CrossRef]
- Fearn, T.; Thompson, M. A new test for ‘sufficient homogeneity’. Analyst 2001, 126, 1414–1417. [Google Scholar] [CrossRef]
- AOAC International. Official Method 965.22, Sorting Corn Grits. Available online: http://www.aoacofficialmethod.org/index.php?main_page=product_info&cPath=1&products_id=1846 (accessed on 3 July 2023).
- Allen, T. 4—Particle Size Analysis by Sieving; Terence Allen, T., Sampling, P., Determination, P.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 208–250. ISBN 9780444515643. [Google Scholar] [CrossRef]
- De Boer, G.B.J.; de Weerd, C.; Thoenes, D.; Goossens, H.W.J. Laser Diffraction Spectrometry: Fraunhofer Diffraction Versus Mie Scattering. Part. Part. Syst. Charact. 1987, 4, 14–19. [Google Scholar] [CrossRef]
- Lock, J.A.; Gouesbet, G. Generalized Lorenz-Mie theory and applications. J. Quant. Spectrosc. Radiat. Transf. 2009, 110, 800–807. [Google Scholar] [CrossRef]
- Wriedt, T. Mie Theory: A Review. In The Mie Theory; Hergert, W., Wriedt, T., Eds.; Springer Series in Optical Sciences; Springer: Berlin/Heidelberg, Germany, 2012; Volume 169. [Google Scholar] [CrossRef]
- Black, D.L.; McQuay, M.Q.; Bonin, M.P. Laser-based techniques for particle-size measurement: A review of sizing methods and their industrial applications. Prog. Energy Combust. Sci. 1996, 22, 267–306. [Google Scholar] [CrossRef]
- Pitard, F.F. Pierre Gy’s Sampling Theory and Sampling Practice; CRC Press, Inc.: Boca Raton, FL, USA, 1989; Volume 2. [Google Scholar]
- Gerlach, R.W.; Nocerino, J.M. EPA/600/R-03/027 Guidance for Obtaining Representative Laboratory Analytical Subsamples from Particulate Laboratory Samples; United States Environmental Protection Agency: Washington, DC, USA, 2003.
- AOAC International. Official Methods 977.16, Sampling for Aflatoxins—Preparation for Sample Procedure. Available online: http://www.aoacofficialmethod.org/index.php?main_page=product_info&products_id=2065 (accessed on 3 July 2023).
- Ferraris, C.; Bullard, J.; Hackley, V. Particle Size Distribution by LASER Diffraction Spectrometry: Application to Cementitious Powders. Aiche J. 2006. Available online: https://www.nist.gov/publications/particle-size-distribution-laser-diffraction-spectrometry-application-cementitious (accessed on 21 October 2022).
- Zhang, K.; Tan, S.; Xu, D. Determination of Mycotoxins in Dried Fruits Using LC-MS/MS—A Sample Homogeneity, Troubleshooting and Confirmation of Identity Study. Foods 2022, 11, 894. [Google Scholar] [CrossRef]
- Tan, S.; Zhang, K.; Martinez, C.L. Laser Diffraction Particle Size Analysis of Mycotoxin Samples. Poster Presentation. The FDA Annual Student Scientific Research Day. 2021. Available online: https://www.fda.gov/science-research/fda-stem-outreach-education-and-engagement/laser-diffraction-particle-size-analysis-mycotoxin-samples (accessed on 3 July 2023).
- De Cleyn, E.; Holm, R.; Mooter, G.V.D. Size Analysis of Small Particles in Wet Dispersions by Laser Diffractometry: A Guidance to Quality Data. J. Pharm. Sci. 2019, 108, 1905–1914. [Google Scholar] [CrossRef]
- Malvern Panalytical. Mastersizer-3000-User-Manual-English-MAN0474-2-1. 2013. Available online: https://www.malvernpanalytical.com/en/learn/knowledge-center/user-manuals/man0474en (accessed on 6 June 2023).
- Lin, L.I.-K. A Concordance Correlation Coefficient to Evaluate Reproducibility. Biometrics 1989, 45, 255. [Google Scholar] [CrossRef]
- Lin, L.I. A note on the concordance correlation coefficient. Biometrics 2000, 56, 324–325. [Google Scholar]
- Hlavacek, R.G.; Freeman, W.G. Low-heat, 200 mesh milling provides smooth, uniform product. Food Process. Mark. 1968, 29, 44–45. [Google Scholar]
- Mohd Rozalli, N.H.; Chin, N.L.; Yusof, Y.A. Grinding characteristics of Asian originated peanuts (Arachishypogaea L.) and specific energy consumption during ultra-high speed grinding for natural peanut butter production. J. Food Eng. 2015, 152, 1–7. [Google Scholar] [CrossRef]
- Codex Stan 152-1985. Codex Standard for Wheat Flour. Available online: www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B152-1985%252FCXS_152e.pdf (accessed on 3 July 2023).
- Agimelen, O.S.; Mulholland, A.J.; Sefcik, J. Modelling of artefacts in estimations of particle size of needle-like particles from laser diffraction measurements. Chem. Eng. Sci. 2017, 158, 445–452. [Google Scholar] [CrossRef]
- Nathier-Dufour, N.; Bougeard, L.; Devaus, M.-F.; Bertrand, D.; de Monredon, F.L.D. Comparison of sieving and laser diffraction for the particle size measurements of raw materials used in foodstuff. Powder Technol. 1993, 76, 191–200. [Google Scholar] [CrossRef]
- Beals, M.J.; Fugal, J.P.; Shaw, R.A.; Lu, J.; Spuler, S.M.; Stith, J.L. Holographic measurements of inhomogeneous cloud mixing at the centimeter scale. Science 2015, 350, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.; Nimmo-Smith, A. The application of holography to the analysis of size and settling velocity of suspended cohesive sediments. Limnol. Oceanogr. Methods 2010, 8, 1–15. [Google Scholar] [CrossRef]
- Kumar, S.S.; He, Z.; Hogan, C.J.; Fredericks, S.A.; Hong, J. Evaluation of laser diffraction-based particle size measurements using digital inline holography. Meas. Sci. Technol. 2020, 31, 125201. [Google Scholar] [CrossRef]
- Zhang, K.; Wong, J.W.; Krynitsky, A.J.; Trucksess, M.W. Determining mycotoxins in baby foods and animal feeds using stable isotope dilution and liquid chromatography tandem mass spectrometry. J. Agric. Food Chem. 2014, 62, 8935–8943. [Google Scholar] [CrossRef]
- Whitaker, T.B.; Springer, J.; Defize, P.R.; de Koe, W.J.; Coker, R. Evaluation of sampling plans used in the United States, United Kingdom, and The Netherlands to test raw shelled peanuts for aflatoxin. J. AOAC Int. 1995, 78, 1010–1018. [Google Scholar] [CrossRef]
- Champeil, A.; Fourbet, J.-F.; Doré, T. Effects of Grain Sampling Procedures on Fusarium Mycotoxin Assays in Wheat Grains. J. Agric. Food Chem. 2004, 52, 6049–6054. [Google Scholar] [CrossRef]
- Casado, M.R.; Parsons, D.; Weightman, R.; Magan, N.; Origgi, S. Geostatistical analysis of the spatial distribution of mycotoxin concentration in bulk cereals. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2009, 26, 867–873. [Google Scholar] [CrossRef]
- Castells, M.; Marín, S.; Sanchis, V.; Ramos, A.J. Distribution of fumonisins and aflatoxins in corn fractions during industrial cornflake processing. Int. J. Food Microbiol. 2008, 123, 81–87. [Google Scholar] [CrossRef]
- Zhang, K.; Schaab, M.R.; Southwood, G.; Tor, E.R.; Aston, L.S.; Song, W.; Eitzer, B.; Majumdar, S.; Lapainis, T.; Mai, H.; et al. A Collaborative Study: Determination of Mycotoxins in Corn, Peanut Butter, and Wheat Flour Using Stable Isotope Dilution Assay (SIDA) and Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS). J. Agric. Food Chem. 2017, 65, 7138–7152. [Google Scholar] [CrossRef]
- Zhang, K. FDA Foods Program Compendium of Analytical Laboratory Methods: Chemical Analytical Manual (CAM). Method C-003.01. Determination of Mycotoxins in Corn, Peanut Butter, and Wheat Flour Using Stable Isotope Dilution Assay (SIDA) and Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). Available online: https://www.fda.gov/media/114240/download (accessed on 3 July 2023).
- Zhang, K. Evaluation of Automated Sample Preparation for Mycotoxin Analysis in Foods. J. AOAC Int. 2020, 103, 1052–1059. [Google Scholar] [CrossRef]
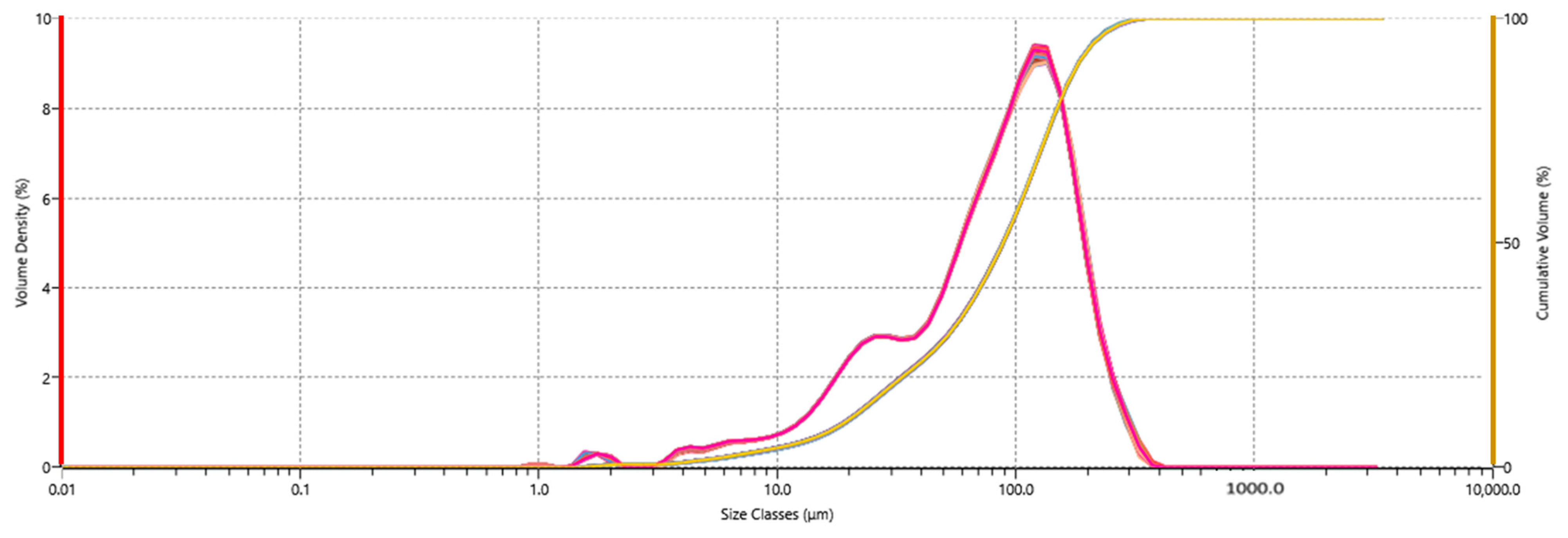
| Obscuration | Refractive Index | Absorption Index | Dv10 (µm) | Dv50 (µm) | Dv90 (µm) | Lin’s CCC | Residual | Weighted Residual |
|---|---|---|---|---|---|---|---|---|
| 10 | 1.4 | 0.01 | 18 (5) | 79 (3) | 192 (2) | 0.9980 | 0.30% | 0.36% |
| 10 | 1.6 | 0 | 20 (5) | 80 (3) | 193 (2) | 0.9981 | 0.31% | 0.31% |
| 10 | 1.6 | 0.001 | 21 (5) | 80 (3) | 193 (2) | 0.9981 | 0.29% | 0.29% |
| 10 | 1.6 | 0.01 | 19 (5) | 80 (3) | 193 (2) | 1.0000 | 0.33% | 0.32% |
| 10 | 1.6 | 0.1 | 19 (5) | 79 (3) | 193 (2) | 0.9980 | 0.27% | 0.30% |
| 10 | 1.6 | 1 | 18 (5) | 79 (3) | 192 (2) | 0.9979 | 0.26% | 0.26% |
| 10 | 1.8 | 0.01 | 19 (5) | 79 (3) | 193 (2) | 0.9999 | 0.30% | 0.28% |
| 20 | 1.4 | 0.01 | 18 (1) | 85 (1) | 182 (1) | 0.9943 | 0.32% | 0.38% |
| 20 | 1.6 | 0.01 | 21 (1) | 87 (1) | 183 (1) | 0.9943 | 0.33% | 0.34% |
| 20 | 1.8 | 0.01 | 20 (0.5) | 86 (0.5) | 183 (1) | 0.9943 | 0.34% | 0.33% |
| Sample #- Test Portion # | Corn | Compound Feed | Peanut Butter | Wheat Flour | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dv10 (µm) | Dv50 (µm) | Dv90 (µm) | Dv10 (µm) | Dv50 (µm) | Dv90 (µm) | Dv10 (µm) | Dv50 (µm) | Dv90 (µm) | Dv10 (µm) | Dv50 (µm) | Dv90 (µm) | |
| 1–1 | 14 (2) | 161 (4) | 577 (2) | 27 (3) | 244 (2) | 665 (2) | 4 (1) | 17 (1) | 85 (12) | 19 (5) | 80 (3) | 193 (2) |
| 1–2 | 14 (2) | 161 (3) | 564 (2) | 27 (3) | 244 (3) | 670 (4) | 4 (1) | 17 (1) | 79 (8) | 17 (2) | 73 (2) | 186 (2) |
| 2–1 | 14 (3) | 165 (5) | 582 (3) | 25 (5) | 219 (7) | 614 (5) | 4 (1) | 17 (1) | 76 (6) | 17 (7) | 89 (6) | 209 (2) |
| 2–2 | 13 (1) | 147 (2) | 557 (2) | 24 (5) | 202 (4) | 552 (6) | 4 (1) | 17 (1) | 76 (9) | 15 (2) | 79 (2) | 199 (1) |
| 3–1 | 14 (3) | 160 (6) | 561 (3) | 25 (2) | 208 (2) | 566 (4) | 4 (1) | 17 (1) | 73 (6) | 17 (6) | 85 (6) | 203 (3) |
| 3–2 | 13 (1) | 150 (3) | 561 (3) | 24 (5) | 209 (5) | 588 (12) | 3 (1) | 17 (1) | 75 (5) | 15 (3) | 75 (3) | 190 (2) |
| 4–1 | 14 (2) | 163 (4) | 577 (2) | 28 (5) | 239 (4) | 627 (5) | 4 (1) | 17 (1) | 73 (8) | 16 (6) | 79 (6) | 198 (3) |
| 4–2 | 13 (3) | 145 (4) | 554 (2) | 29 (4) | 242 (2) | 625 (4) | 4 (1) | 16 (1) | 72 (6) | 15 (2) | 74 (3) | 187 (2) |
| 5–1 | 14 (3) | 172 (8) | 575 (4) | 24 (2) | 234 (2) | 619 (2) | 4 (1) | 17 (1) | 73 (5) | 17 (6) | 86 (6) | 205 (3) |
| 5–2 | 13 (1) | 153 (3) | 549 (2) | 25 (2) | 239 (1) | 630 (3) | 4 (1) | 17 (1) | 74 (14) | 15 (2) | 75 (2) | 189 (1) |
| 6–1 | 14 (2) | 164 (4) | 591 (4) | 27 (3) | 232 (3) | 671 (4) | 4 (1) | 17 (1) | 73 (4) | 17 (7) | 86 (7) | 205 (3) |
| 6–2 | 14 (2) | 154 (4) | 589 (4) | 24 (4) | 215 (3) | 617 (10) | 4 (1) | 17 (2) | 80 (23) | 15 (2) | 74 (3) | 191 (2) |
| 7–1 | 14 (3) | 159 (6) | 568 (2) | 24 (3) | 218 (3) | 658 (7) | 4 (1) | 17 (1) | 72 (9) | 27 (7) | 81 (5) | 183 (1) |
| 7–2 | 13 (1) | 145 (3) | 563 (2) | 24 (1) | 216 (2) | 629 (3) | 4 (1) | 17 (1) | 72 (2) | 21 (7) | 75 (1) | 185 (1) |
| 8–1 | 14 (3) | 160 (6) | 613 (2) | 27 (4) | 233 (3) | 634 (8) | 4 (1) | 16 (1) | 71 (7) | 17 (4) | 85 (5) | 201 (3) |
| 8–2 | 14 (1) | 154 (2) | 594 (1) | 27 (1) | 235 (2) | 622 (3) | 4 (1) | 16 (1) | 69 (5) | 15 (2) | 75 (2) | 186 (1) |
| 9–1 | 14 (2) | 162 (5) | 531 (2) | 27 (2) | 227 (1) | 577 (2) | 4 (1) | 16 (1) | 70 (5) | 17 (6) | 85 (7) | 204 (3) |
| 9–2 | 14 (2) | 149 (4) | 522 (3) | 27 (4) | 236 (3) | 609 (7) | 4 (1) | 16 (1) | 70 (2) | 15 (2) | 74 (3) | 188 (2) |
| 10–1 | 14 (3) | 179 (3) | 596 (2) | 27 (4) | 224 (3) | 587 (3) | 4 (1) | 17 (1) | 72 (5) | 16 (1) | 83 (5) | 203 (6) |
| 10–2 | 14 (1) | 171 (3) | 599 (2) | 26 (3) | 219 (1) | 565 (2) | 4 (1) | 16 (1) | 71 (6) | 15 (1) | 74 (2) | 188 (3) |
| Range (µm) | 13-14 | 145-179 | 522-613 | 24-27 | 202-244 | 552-671 | 3-4 | 16-17 | 69-85 | 15-27 | 73-89 | 183-209 |
| Grand mean (RSD %) | 14 (3) | 159 (6) | 571 (4) | 26 (6) | 267 (6) | 616 (6) | 4 (6) | 17 (3) | 74 (5) | 17 (17) | 79 (7) | 195 (4) |
| QC (CRM 3310) | 39 (1) | 74 (1) | 111 (1) | 41 (1) | 75 (1) | 110 (1) | 39 (1) | 73 (1) | 111 (3) | 41 (1) | 76 (1) | 117 (3) |
| Subsample | Test Portion Analyzed (3) | Sum (ng/g) | Average | Variance | SD | RSD (%) |
|---|---|---|---|---|---|---|
| 1 | 3 | 1026.99 | 342.33 | 200.89 | 14.17 | 4.14 |
| 2 | 3 | 967.31 | 322.47 | 19.36 | 4.40 | 1.36 |
| 3 | 3 | 923.40 | 307.80 | 923.23 | 30.38 | 9.87 |
| 4 | 3 | 1028.10 | 342.70 | 36.27 | 6.02 | 1.76 |
| 5 | 3 | 1020.81 | 340.27 | 271.36 | 16.47 | 4.84 |
| 6 | 3 | 1032.09 | 344.03 | 96.20 | 9.81 | 2.85 |
| 7 | 3 | 1017.39 | 339.13 | 707.56 | 26.60 | 7.84 |
| 8 | 3 | 1049.19 | 349.73 | 42.89 | 6.55 | 1.87 |
| 9 | 3 | 1004.91 | 334.97 | 175.57 | 13.25 | 3.96 |
| 10 | 3 | 980.91 | 326.97 | 10.57 | 3.25 | 0.99 |
| ANOVA | ||||||
| Source of Variation | SS | df | MS | F | p-value | F crit |
| Between Groups | 4254.02 | 9 | 472.67 | 1.90 | 0.11 | 2.39 |
| Within Groups | 4967.85 | 20 | 248.39 | |||
| Total | 9221.872 | 29 |
| Subsample | Test Portion Analyzed (3) | Sum (ng/g) | Average (ng/g) | Variance | SD | RSD (%) |
|---|---|---|---|---|---|---|
| 1 | 3 | 2461.30 | 820.43 | 826.80 | 28.75 | 3.50 |
| 2 | 3 | 2382.00 | 794.00 | 1806.79 | 42.51 | 5.35 |
| 3 | 3 | 2501.80 | 833.93 | 498.49 | 22.33 | 2.68 |
| 4 | 3 | 2491.80 | 830.60 | 3175.69 | 56.35 | 6.78 |
| 5 | 3 | 2349.80 | 783.27 | 1432.90 | 37.85 | 4.83 |
| 6 | 3 | 2513.90 | 837.97 | 1361.65 | 36.90 | 4.40 |
| 7 | 3 | 2630.50 | 876.83 | 2264.90 | 47.59 | 5.43 |
| 8 | 3 | 2518.90 | 839.63 | 660.04 | 25.69 | 3.06 |
| 9 | 3 | 2481.80 | 827.27 | 2085.32 | 45.67 | 5.52 |
| 10 | 3 | 2503.10 | 834.37 | 5407.14 | 73.53 | 8.81 |
| ANOVA | ||||||
| Source of Variation | SS | df | MS | F | p-value | F crit |
| Between Groups | 17,749.31 | 9 | 1972.15 | 1.01 | 0.46 | 2.39 |
| Within Groups | 39,039.49 | 20 | 1951.97 | |||
| Total | 56,788.80 | 29 |
| Subsample | Test Portion Analyzed (3) | Sum (ng/g) | Average (ng/g) | Variance | SD | RSD (%) |
|---|---|---|---|---|---|---|
| 1 | 3 | 789.10 | 263.03 | 1655.42 | 40.69 | 15.47 |
| 2 | 3 | 717.70 | 239.23 | 599.86 | 24.49 | 10.24 |
| 3 | 3 | 721.90 | 240.63 | 108.70 | 10.43 | 4.33 |
| 4 | 3 | 770.00 | 256.67 | 1102.94 | 33.21 | 12.94 |
| 5 | 3 | 658.40 | 219.47 | 121.96 | 11.04 | 5.03 |
| 6 | 3 | 794.30 | 264.77 | 283.42 | 16.84 | 6.36 |
| 7 | 3 | 838.30 | 279.43 | 20.84 | 4.57 | 1.63 |
| 8 | 3 | 781.80 | 260.60 | 604.11 | 24.58 | 9.43 |
| 9 | 3 | 804.10 | 268.03 | 59.62 | 7.72 | 2.88 |
| 10 | 3 | 689.50 | 229.83 | 172.76 | 13.14 | 5.72 |
| ANOVA | ||||||
| Source of Variation | SS | df | MS | F | p-value | F crit |
| Between Groups | 9695.38 | 9 | 1077.26 | 2.28 | 0.06 | 2.39 |
| Within Groups | 9459.32 | 20 | 472.97 | |||
| Total | 19,154.70 | 29 |
| Subsample | Test Portion Analyzed (3) | Sum (ng/g) | Average (ng/g) | Variance | SD | RSD (%) |
|---|---|---|---|---|---|---|
| 1 | 3 | 185.71 | 61.90 | 89.87 | 9.48 | 15.31 |
| 2 | 3 | 176.85 | 58.95 | 56.47 | 7.51 | 12.75 |
| 3 | 3 | 181.03 | 60.34 | 3.74 | 1.93 | 3.20 |
| 4 | 3 | 194.75 | 64.92 | 124.56 | 11.16 | 17.19 |
| 5 | 3 | 151.60 | 50.53 | 4.72 | 2.17 | 4.30 |
| 6 | 3 | 194.37 | 64.79 | 46.49 | 6.82 | 10.52 |
| 7 | 3 | 200.90 | 66.97 | 4.24 | 2.06 | 3.07 |
| 8 | 3 | 187.33 | 62.44 | 15.98 | 4.00 | 6.40 |
| 9 | 3 | 214.29 | 71.43 | 28.88 | 5.37 | 7.52 |
| 10 | 3 | 170.79 | 56.93 | 0.43 | 0.66 | 1.16 |
| ANOVA | ||||||
| Source of Variation | SS | df | MS | F | p-value | F crit |
| Between Groups | 897.79 | 9 | 99.75 | 2.66 | 0.03 | 2.39 |
| Within Groups | 750.73 | 20 | 37.54 | |||
| Total | 1648.52 | 29 |
| Subsample | Test Portion Analyzed (3) | Sum (ng/g) | Average (ng/g) | Variance | SD | RSD (%) |
|---|---|---|---|---|---|---|
| 1 | 3 | 76.12 | 25.37 | 10.23 | 3.20 | 12.61 |
| 2 | 3 | 61.48 | 20.49 | 0.89 | 0.94 | 4.61 |
| 3 | 3 | 69.74 | 23.25 | 0.89 | 0.94 | 4.05 |
| 4 | 3 | 72.27 | 24.09 | 11.26 | 3.36 | 13.93 |
| 5 | 3 | 55.11 | 18.37 | 0.85 | 0.92 | 5.01 |
| 6 | 3 | 64.86 | 21.62 | 9.79 | 3.13 | 14.47 |
| 7 | 3 | 67.88 | 22.63 | 2.84 | 1.69 | 7.45 |
| 8 | 3 | 71.81 | 23.94 | 1.34 | 1.16 | 4.84 |
| 9 | 3 | 68.95 | 22.98 | 9.84 | 3.14 | 13.65 |
| 10 | 3 | 63.23 | 21.08 | 11.92 | 3.45 | 16.38 |
| ANOVA | ||||||
| Source of Variation | SS | df | MS | F | p-value | F crit |
| Between Groups | 112.20 | 9 | 12.47 | 2.08 | 0.08 | 2.39 |
| Within Groups | 119.68 | 20 | 5.98 | |||
| Total | 231.88 | 29 |
| Subsample | Test Portion Analyzed (n = 3) | Sum (ng/g) | Average (ng/g) | Variance | SD | RSD (%) |
|---|---|---|---|---|---|---|
| 1 | 3 | 168.57 | 56.19 | 25.78 | 5.08 | 9.04 |
| 2 | 3 | 195.38 | 65.13 | 9.66 | 3.11 | 4.77 |
| 3 | 3 | 175.26 | 58.42 | 2.57 | 1.60 | 2.75 |
| 4 | 3 | 157.28 | 52.43 | 74.36 | 8.62 | 16.45 |
| 5 | 3 | 158.96 | 52.99 | 8.87 | 2.98 | 5.62 |
| 6 | 3 | 185.42 | 61.81 | 45.18 | 6.72 | 10.88 |
| 7 | 3 | 178.35 | 59.45 | 6.07 | 2.46 | 4.14 |
| 8 | 3 | 160.04 | 53.35 | 88.88 | 9.43 | 17.67 |
| 9 | 3 | 178.08 | 59.36 | 13.55 | 3.68 | 6.20 |
| 10 | 3 | 170.70 | 56.90 | 83.01 | 9.11 | 16.01 |
| ANOVA | ||||||
| Source of Variation | SS | df | MS | F | p-value | F crit |
| Between Groups | 450.46 | 9 | 50.05 | 1.40 | 0.25 | 2.39 |
| Within Groups | 715.85 | 20 | 35.79 | |||
| Total | 1166.31 | 29 |
| Subsample | Test Portion Analyzed (n = 3) | Sum (ng/g) | Average (ng/g) | Variance | SD | RSD (%) |
|---|---|---|---|---|---|---|
| 1 | 3 | 2.74 | 0.91 | 0.004 | 0.06 | 6.88 |
| 2 | 3 | 3.04 | 1.01 | 0.002 | 0.05 | 4.88 |
| 3 | 3 | 3.02 | 1.01 | 0.022 | 0.15 | 14.76 |
| 4 | 3 | 2.58 | 0.86 | 0.018 | 0.14 | 15.78 |
| 5 | 3 | 2.51 | 0.84 | 0.036 | 0.19 | 22.78 |
| 6 | 3 | 2.96 | 0.99 | 0.009 | 0.10 | 9.70 |
| 7 | 3 | 2.65 | 0.88 | 0.027 | 0.17 | 18.74 |
| 8 | 3 | 2.80 | 0.93 | 0.002 | 0.04 | 4.26 |
| 9 | 3 | 2.45 | 0.82 | 0.006 | 0.08 | 9.73 |
| 10 | 3 | 2.70 | 0.90 | 0.004 | 0.06 | 7.11 |
| ANOVA | ||||||
| Source of Variation | SS | df | MS | F | p-value | F crit |
| Between Groups | 0.13 | 9 | 0.01 | 1.10 | 0.41 | 2.39 |
| Within Groups | 0.26 | 20 | 0.01 | |||
| Total | 0.39 | 29 |
| Subsample | Test Portion Analyzed (n = 3) | Sum (ng/g) | Average (ng/g) | Variance | SD | RSD (%) |
|---|---|---|---|---|---|---|
| 1 | 3 | 339.90 | 113.30 | 75.73 | 8.70 | 7.68 |
| 2 | 3 | 375.09 | 125.03 | 16.06 | 4.01 | 3.21 |
| 3 | 3 | 358.99 | 119.63 | 8.36 | 2.89 | 2.42 |
| 4 | 3 | 365.49 | 121.83 | 21.49 | 4.64 | 3.81 |
| 5 | 3 | 377.10 | 125.70 | 0.76 | 0.87 | 0.69 |
| 6 | 3 | 368.61 | 122.87 | 30.49 | 5.52 | 4.49 |
| 7 | 3 | 368.91 | 122.97 | 2.24 | 1.50 | 1.22 |
| 8 | 3 | 355.50 | 118.50 | 0.13 | 0.36 | 0.30 |
| 9 | 3 | 368.70 | 122.90 | 24.52 | 4.95 | 4.03 |
| 10 | 3 | 360.39 | 120.13 | 55.61 | 7.46 | 6.21 |
| ANOVA | ||||||
| Source of Variation | SS | df | MS | F | p-value | F crit |
| Between Groups | 352.05 | 9 | 39.12 | 1.66 | 0.16 | 2.39 |
| Within Groups | 470.82 | 20 | 23.54 | |||
| Total | 822.87 | 29 |
| Subsample # | Test Portion Set # | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| 1 | 55.47 | 57.45 | 72.79 |
| 2 | 56.39 | 67.41 | 53.05 |
| 3 | 60.81 | 58.22 | 62.00 |
| 4 | 58.21 | 58.74 | 77.80 |
| 5 | 49.83 | 52.97 | 48.80 |
| 6 | 59.96 | 61.82 | 72.59 |
| 7 | 68.80 | 64.74 | 67.36 |
| 8 | 63.15 | 66.04 | 58.14 |
| 9 | 72.78 | 65.51 | 76.00 |
| 10 | 56.53 | 57.69 | 56.57 |
| Set 1 and 2 | Set 1 and 3 | Set 2 and 3 | |
| Analytical variance | 0.000011 | 0.000045 | 0.000057 |
| Between-sample variance | 0.003352 | 0.006687 | 0.000141 |
| Allowable between-sample variance | 0.000020 | 0.000020 | 0.000020 |
| Sampling variance | 0.000022 | 0.000033 | 0.000007 |
| Critical value | 0.000048 | 0.000082 | 0.000094 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Tran, I.; Tan, S. Characterization of Particle-Size-Based Homogeneity and Mycotoxin Distribution Using Laser Diffraction Particle Size Analysis. Toxins 2023, 15, 450. https://doi.org/10.3390/toxins15070450
Zhang K, Tran I, Tan S. Characterization of Particle-Size-Based Homogeneity and Mycotoxin Distribution Using Laser Diffraction Particle Size Analysis. Toxins. 2023; 15(7):450. https://doi.org/10.3390/toxins15070450
Chicago/Turabian StyleZhang, Kai, Ivy Tran, and Steven Tan. 2023. "Characterization of Particle-Size-Based Homogeneity and Mycotoxin Distribution Using Laser Diffraction Particle Size Analysis" Toxins 15, no. 7: 450. https://doi.org/10.3390/toxins15070450
APA StyleZhang, K., Tran, I., & Tan, S. (2023). Characterization of Particle-Size-Based Homogeneity and Mycotoxin Distribution Using Laser Diffraction Particle Size Analysis. Toxins, 15(7), 450. https://doi.org/10.3390/toxins15070450




