Diversity of Mycotoxins Produced by Fusarium Strains Infecting Weeds
Abstract
1. Introduction
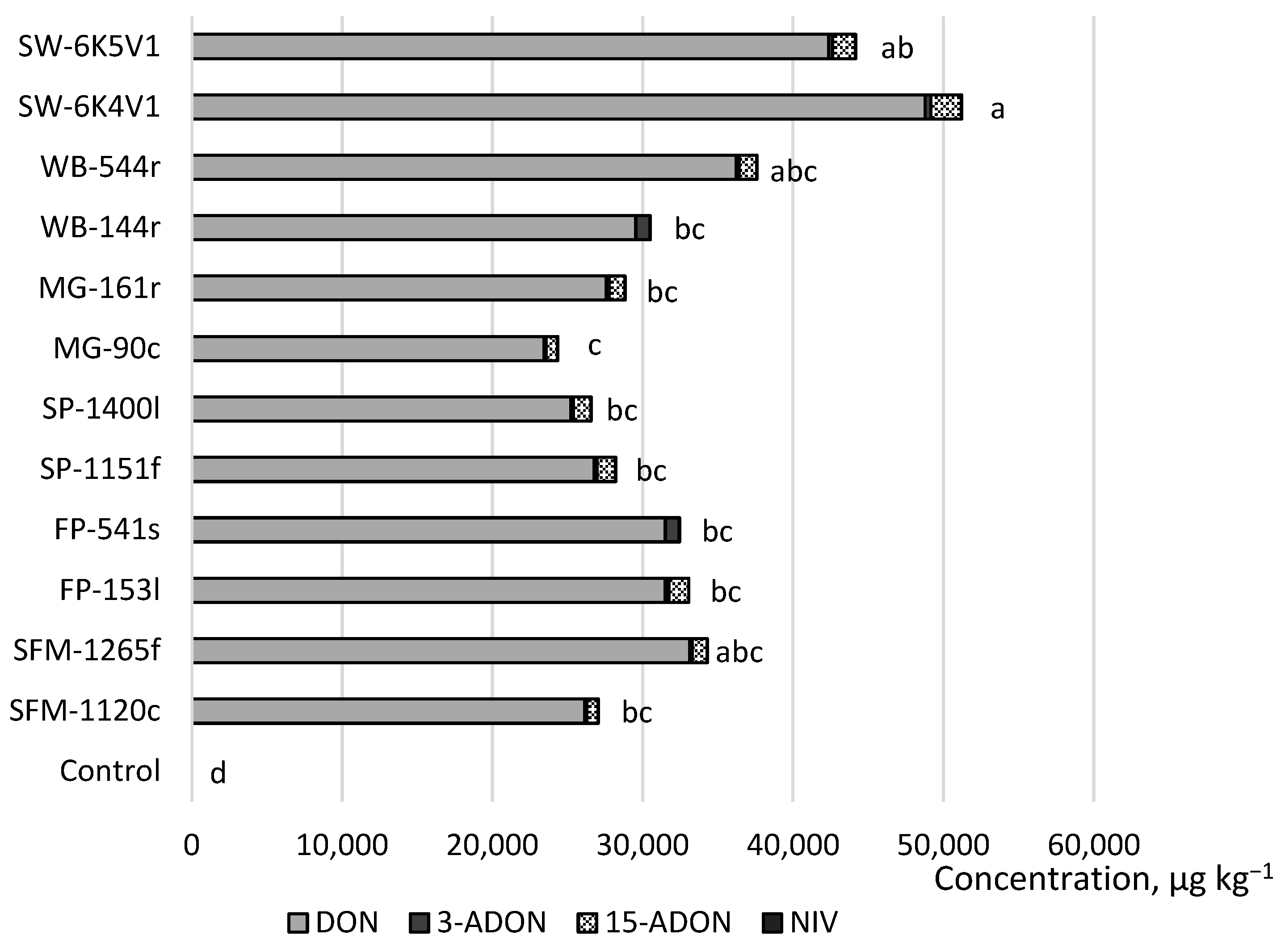
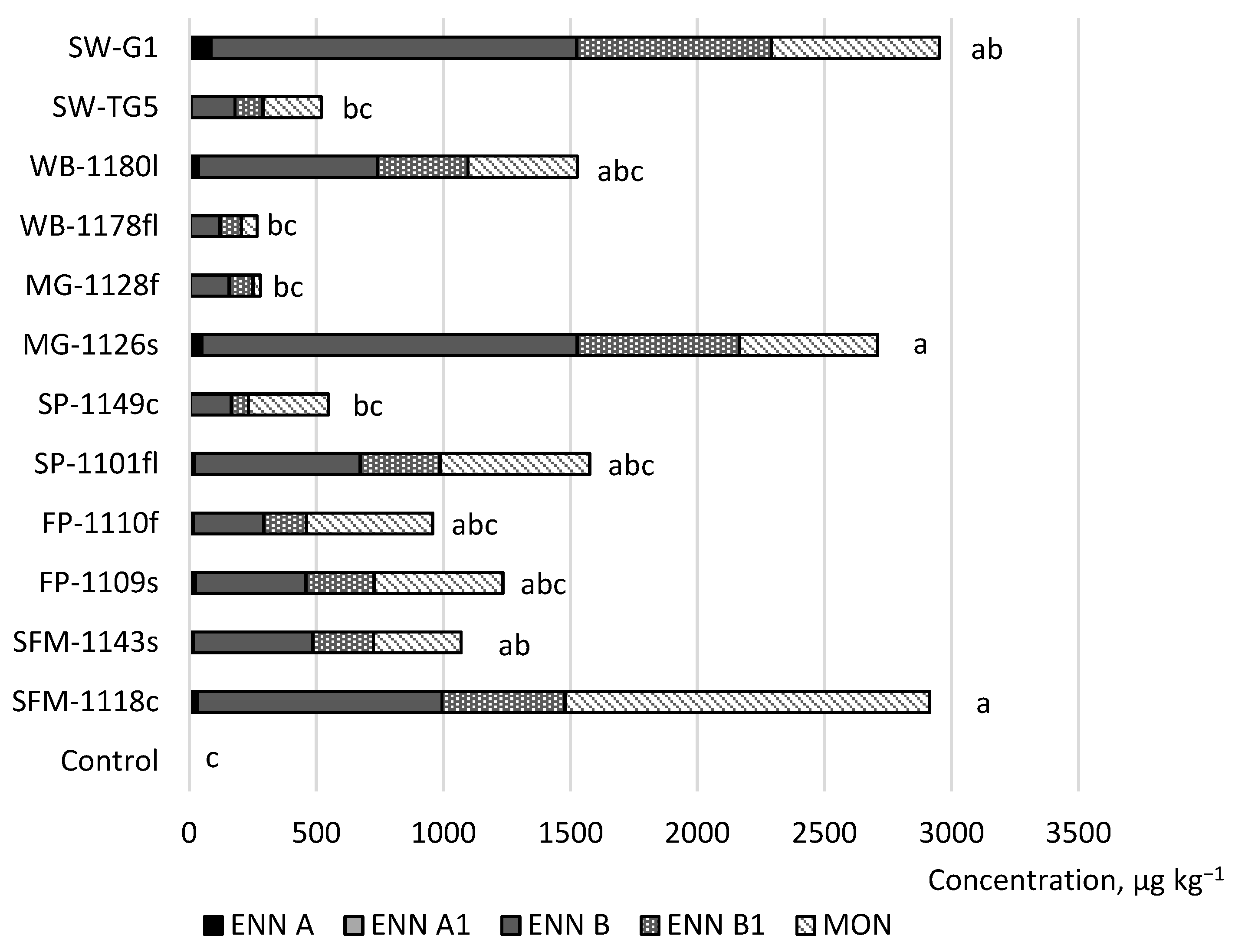
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Sample Collection
5.2. Sample Preparation for Mycotoxin Analyses
5.3. Method of Analysis
5.4. Method Validation
5.5. Meteorological Conditions
5.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kotowicz, N.K.; Frąc, M.; Lipiec, J. The importance of Fusarium fungi in wheat cultivation–pathogenicity and mycotoxins production: A review. J. Anim. Plant Sci. 2014, 21, 3326–3343. Available online: https://m.elewa.org/JAPS/2014/21.2/3BLOCKED.pdf (accessed on 26 April 2022).
- Pereira, V.L.; Fernandes, J.O.; Cunha, S.C. Mycotoxins in cereals and related foodstuffs: A review on occurrence and recent methods of analysis. Trends Food Sci. Technol. 2014, 36, 96–136. [Google Scholar] [CrossRef]
- Janaviciene, S.; Mankeviciene, A.; Suproniene, S.; Kochiieru, Y.; Keriene, I. The prevalence of deoxynivalenol and its derivatives in the spring wheat grain from different agricultural production systems in Lithuania. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Janaviciene, S.; Suproniene, S.; Kadziene, G.; Pavlenko, R.; Berzina, Z.; Bartkevics, V. Toxigenicity of F. graminearum residing on host plants alternative to wheat as influenced by environmental conditions. Toxins 2022, 14, 541. [Google Scholar] [CrossRef]
- Landschoot, S.; Audenaert, K.; Waegeman, W.; Pycke, B.; Bekaert, B.; De Baets, B.; Haesaert, G. Connection between primary Fusarium inoculum on gramineous weeds, crop residues and soil samples and the final population on wheat ears in Flanders, Belgium. Crop Prot. 2011, 30, 1297–1305. [Google Scholar] [CrossRef]
- Leplat, J.; Friberg, H.; Abid, M.; Steinberg, C. Survival of Fusarium graminearum, the causal agent of Fusarium head blight. A Review. Agron. Sustain. Dev. 2013, 33, 97–111. [Google Scholar] [CrossRef]
- Dong, F.; Xu, J.; Zhang, X.; Wang, S.; Xing, Y.; Mokoena, M.P.; Olaniran, A.O.; Shi, J. Gramineous weeds near paddy fields are alternative hosts for the Fusarium graminearum species complex that causes fusarium head blight in rice. Plant Pathol. 2020, 69, 433–441. [Google Scholar] [CrossRef]
- Heitmann, N.; Glemnitz, M.; Lentzsch, P.; Platen, R.; Müller, M.E.H. Quantifying the role of ground beetles for the dispersal of fusarium and alternaria fungi in agricultural landscapes. J. Fungi 2021, 7, 863. [Google Scholar] [CrossRef]
- Hoffmann, A.; Funk, R.; Müller, M.E.H. Blowin’ in the wind: Wind dispersal ability of phytopathogenic fusarium in a wind tunnel experiment. Atmosphere 2021, 12, 1653. [Google Scholar] [CrossRef]
- Nesic, K.; Ivanovic, S.; Nesic, V. Fusarial toxins: Secondary metabolites of Fusarium fungi. Rev. Environ. Contam. Toxicol. 2014, 228, 101–120. [Google Scholar] [CrossRef]
- Boutigny, A.L.; Ward, T.J.; Van Coller, G.J.; Flett, B.; Lamprecht, S.C.; O’Donnell, K.; Viljoen, A. Analysis of the Fusarium graminearum species complex from wheat, barley and maize in South Africa provides evidence of species-specific differences in host preference. Fungal Genet. Biol. 2011, 48, 914–920. [Google Scholar] [CrossRef]
- Kuhnem, P.R.; Ward, T.J.; Silva, C.N.; Spolti, P.; Ciliato, M.L.; Tessmann, D.J.; Del Ponte, E.M. Composition and toxigenic potential of the Fusarium graminearum species complex from maize ears, stalks and stubble in Brazil. Plant Pathol. 2016, 65, 1185–1191. [Google Scholar] [CrossRef]
- Lee, J.; Chang, I.Y.; Kim, H.; Yun, S.H.; Leslie, J.F.; Lee, Y.W. Genetic diversity and fitness of Fusarium graminearum populations from rice in Korea. Appl. Environ. Microbiol. 2009, 75, 3289–3295. [Google Scholar] [CrossRef]
- O’Donnell, K.; Ward, T.J.; Geiser, D.M.; Kistler, H.C.; Aoki, T. Genealogical concordance between the mating type locus and seven other nuclear genes supports formal recognition of nine phylogenetically distinct species within the Fusarium graminearum clade. Fungal Genet. Biol. 2004, 41, 600–623. [Google Scholar] [CrossRef]
- Gräfenhan, T.; Patrick, S.K.; Roscoe, M.; Trelka, R.; Gaba, D.; Chan, J.M.; McKendry, T.; Clear, R.M.; Tittlemier, S.A. Fusarium damage in cereal grains from Western Canada. 1. Phylogenetic analysis of moniliformin-producing Fusarium species and their natural occurrence in mycotoxin-contaminated wheat, oats, and rye. J. Agric. Food Chem. 2013, 61, 5425–5437. [Google Scholar] [CrossRef]
- Stakheev, A.A.; Khairulina, D.R.; Zavriev, S.K. Four-locus phylogeny of Fusarium avenaceum and related species and their species-specific identification based on partial phosphate permease gene sequences. Int. J. Food Microbiol. 2016, 225, 27–37. [Google Scholar] [CrossRef]
- Uhlig, S.; Jestoi, M.; Parikka, P. Fusarium avenaceum—The North European situation. Int. J. Food Microbiol. 2007, 119, 17–24. [Google Scholar] [CrossRef]
- Logrieco, A.; Rizzo, A.; Ferracane, R.; Ritieni, A. Occurrence of beauvericin and enniatins in wheat affected by Fusarium avenaceum head blight. Appl. Environ. Microbiol. 2002, 68, 82–85. [Google Scholar] [CrossRef]
- Jestoi, M.; Rokka, M.; Yli-Mattila, T.; Parikka, P.; Rizzo, A.; Peltonen, K. Presence and concentrations of the Fusarium-related mycotoxins beauvericin, enniatins and moniliformin in Finnish grain samples. Food Addit. Contam. 2004, 21, 794–802. [Google Scholar] [CrossRef]
- Kosiak, B.; Torp, M.; Skjerve, E. The prevalence and distribution of Fusarium species in Norwegian cereals: A survey. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2003, 53, 168–176. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing: Ames, IA, USA, 2006; 388p. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Risks to human and animal health related to the presence of moniliformin in food and feed. EFSA J. 2018, 16, 5082. [Google Scholar] [CrossRef]
- Uhlig, S.; Torp, M.; Jarp, J.; Parich, A.; Gutleb, A.C.; Krska, R. Moniliformin in Norwegian grain. Food Addit. Contam. 2004, 21, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Van Asselt, E.D.; Azambuja, W.; Moretti, A.; Kastelein, P.; de Rijk, T.C.; Stratakou, I.; van der Fels-Klerx, H.J. A Dutch field survey on fungal infection and mycotoxin concentrations in maize. Food Addit. Contam. 2012, 29, 1556–1565. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; van Dam, R.; Spanjer, M.; de Stoppelaar, J.; Mol, H.; de Nijs, M.; López, P. Survey of moniliformin in wheat- and corn-based products using a straightforward analytical method. Mycotoxin Res. 2017, 33, 333–341. [Google Scholar] [CrossRef] [PubMed]
- European Commission (EC). Commission Regulation, No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:364:0005:0024:EN:PDF (accessed on 10 January 2023).
- Zavtrikovienė, E.; Gorash, A.; Kadžienė, G.; Matelionienė, N.; Supronienė, S. Pathogenicity of asymptomatically residing Fusarium species in non-gramineous plants and weeds to spring wheat under greenhouse conditions. Pathogens 2022, 11, 1467. [Google Scholar] [CrossRef]
- Matelionienė, N.; Supronienė, S.; Shamshitov, A.; Zavtrikovienė, E.; Janavičienė, S.; Kadžienė, G. Weeds in cereal crop rotations may host Fusarium species that cause Fusarium head blight and grain weight losses in wheat. Agronomy 2022, 12, 2741. [Google Scholar] [CrossRef]
- Postic, J.; Cosic, J.; Vrandecic, K.; Jurkovic, D.; Saleh, A.A.; Leslie, J.F. Diversity of Fusarium Species Isolated from Weeds and Plant Debris in Croatia. J. Phytopathol. 2012, 160, 76–81. [Google Scholar] [CrossRef]
- Krnjaja, V.; Stanković, S.; Obradović, A.; Petrović, T.; Mandić, V.; Bijelić, Z.; Božić, M. Trichothecene Genotypes of Fusarium graminearum Populations Isolated from Winter Wheat Crops in Serbia. Toxins 2018, 10, 460. [Google Scholar] [CrossRef]
- Stanković, S.; Tančić, S.; Lević, J.; Krnjaja, V. Production of deoxynivalenol by Fusarium graminearum and Fusarium culmorum isolated from wheat kernels in Serbia. Cereal Res. Commun. 2008, 36, 395–396. Available online: http://r.istocar.bg.ac.rs/handle/123456789/158 (accessed on 20 March 2023).
- Obradović, A.; Stanković, S.; Krnjaja, V.; Nikolić, A.; Ignjatović-Micić, D.; Stepanović, J.; Duduk, B. Trichothecene chemotype diversity of Fusarium graminearum isolated from wheat, maize and barley in Serbia. Genetika 2017, 49, 355–364. [Google Scholar] [CrossRef]
- Gerling, M.; Petry, L.; Barkusky, D.; Büttner, C.; Müller, M. Infected grasses as inoculum for Fusarium infestation and mycotoxin accumulation in wheat with and without irrigation. Mycotoxin Res. 2023, 39, 19–31. [Google Scholar] [CrossRef]
- Dong, F.; Zhang, X.; Xu, J.H.; Shi, J.R.; Lee, Y.W.; Chen, X.Y.; Li, Y.P.; Mokoena, M.P.; Olaniran, A.O. Analysis of Fusarium graminearum species complex from freshly harvested rice in Jiangsu Province (China). Plant Dis. 2020, 104, 2138–2143. [Google Scholar] [CrossRef]
- Lofgren, L.A.; LeBlanc, N.R.; Certano, A.K.; Nachtigall, J.; LaBine, K.M.; Riddle, J.; Broz, K.; Dong, Y.H.; Bethan, B.; Kafer, C.W.; et al. Fusarium graminearum: Pathogen or endophyte of North American grasses? New Phytol. 2018, 217, 1203–1212. [Google Scholar] [CrossRef]
- Jestoi, M.N.; Paavanen-Huhtala, S.; Parikka, P.; Yli-Mattila, T. In vitro and in vivo mycotoxin production of Fusarium species isolated from Finnish grains. Arch. Phytopathol. Plant Prot. 2008, 41, 545–558. [Google Scholar] [CrossRef]
- Bertuzzi, T.; Giorni, P.; Rastelli, S.; Vaccino, P.; Lanzanova, C.; Locatelli, S. Co-occurrence of moniliformin and regulated Fusarium toxins in maize and wheat grown in Italy. Molecules 2020, 25, 2440. [Google Scholar] [CrossRef]
- Beccari, G.; Colasante, V.; Tini, F.; Senatore, M.T.; Prodi, A.; Sulyok, M.; Covarelli, L. Causal agents of Fusarium head blight of durum wheat (Triticum durum Desf.) in Central Italy and their in vitro biosynthesis of secondary metabolites. Food Microbiol. 2018, 70, 17–27. [Google Scholar] [CrossRef]
- Orlando, B.; Grignon, G.; Vitry, C.; Kashefifard, K.; Valade, R. Fusarium species and enniatin mycotoxins in wheat, durum wheat, triticale and barley harvested in France. Mycotoxin Res. 2019, 35, 369–380. [Google Scholar] [CrossRef]
- Reboud, X.; Eychenne, N.; Délos, M.; Folcher, L. Withdrawal of maize protection by herbicides and insecticides increases mycotoxins contamination near maximum thresholds. Agron. Sustain. Dev. 2016, 36, 43. [Google Scholar] [CrossRef]
- Suproniene, S.; Kadziene, G.; Irzykowski, W.; Sneideris, D.; Ivanauskas, A.; Sakalauskas, S.; Serbiak, P.; Svegzda, P.; Auskalniene, O.; Jedryczka, M. Weed species within cereal crop rotations can serve as alternative hosts for Fusarium graminearum causing Fusarium head blight of wheat. Fungal Ecol. 2019, 37, 30–37. [Google Scholar] [CrossRef]
- Bartkiene, E.; Zokaityte, E.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Klupsaite, D.; Cernauskas, D.; Ruzauskas, M.; Bartkevics, V.; Pugajeva, I.; et al. Combination of Extrusion and Fermentation with Lactobacillus plantarum and L. uvarum Strains for Improving the Safety Characteristics of Wheat Bran. Toxins 2021, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Guidance Document on the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food, European Commission, Food Safety. 2016. Available online: https://food.ec.europa.eu/system/files/2017-05/animal-feed-guidance_document_lod_en.pdf (accessed on 12 January 2021).
| Mycotoxin | F. avenaceum | F. graminearum | ||||||
|---|---|---|---|---|---|---|---|---|
| Positive (%) | Minimum (µg kg−1) | Maximum (µg kg−1) | Average (µg kg−1) | Positive (%) | Minimum (µg kg−1) | Maximum (µg kg−1) | Average (µg kg−1) | |
| DON * | 98 | <5.4 | 636 | 89 | 100 | 5120 | 84,319 | 31,849 |
| NIV * | 0 | <11.8 | <11.8 | <11.8 | 96 | <11.8 | 39 | 14 |
| 3-ADON * | 0 | <11 | <11 | <11 | 100 | 27 | 1245 | 334 |
| 15-ADON * | 0 | <42 | <42 | <42 | 84 | <42 | 3915 | 994 |
| ZEA * | 0 | <10.3 | <10.3 | <10.3 | 52 | <10.3 | 28 | 4 |
| NEO * | 0 | <4.6 | <4.6 | <4.6 | 0 | <4.6 | <4.6 | <4.6 |
| ENN A * | 98 | <5.7 | 170 | 23 | 2 | <5.7 | 1 | 0 |
| ENN A1 * | 67 | <5.3 | 17 | 2 | 0 | <5.3 | <5.3 | <5.3 |
| ENN B * | 100 | 1 | 2749 | 585 | 6 | <10.7 | 39 | 1 |
| ENN B1 * | 100 | 18 | 1541 | 299 | 10 | <9.3 | 44 | 4 |
| T-2 * | 2 | <6.1 | 2 | 0 | 4 | <6.1 | 2 | 0 |
| HT-2 * | 17 | <7.4 | 23 | 1 | 12 | <7.4 | 20 | 1 |
| MON * | 100 | 5 | 4653 | 470 | 0 | <2 | <2 | <2 |
| Treatment No. | Host Plant | F. avenaceum Strain Code | F. graminearum Strain Code |
|---|---|---|---|
| 1 | Spring wheat (Triticum aestivum) | SW-G1 | SW-6K5V1 |
| 2 | SW-TG5 | SW-6K4V1 | |
| 3 | Wild buckwheat (Fallopia convolvulus (L.) Löve) | WB-1180l | WB-544r |
| 4 | WB-1178fl | WB-144r | |
| 5 | Meadow grass (Poa annua L.) | MG-1128f | MG-161r |
| 6 | MG-1126s | MG-90c | |
| 7 | Shepherd’s purse (Capsella bursa-pastoris (L.) Medik.) | SP-1149c | SP-1400l |
| 8 | SP-1101fl | SP-1151f | |
| 9 | Field pansy (Viola arvensis Murray) | FP-1110f | FP-541s |
| 10 | FP-1109s | FP-153l | |
| 11 | Scentless false mayweed (Tripleurospermum inodorum (L.) Sch.) | SFM-1143s | SFM-1265f |
| 12 | SFM-1118c | SFM-1120c | |
| 13 | Control | Sterile distilled water | |
| Validation Parameters | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mycotoxin | Retention Time (min) | Polarity | LOD * (µg kg−1) | LOQ * (µg kg−1) | Linear Range (µg kg−1) | R2 * | Accuracy (Deviation from the Theoretical Value (%)) | Precision (RSD * (%)) | ||||
| Level of Spiked Samples (µg kg−1) | ||||||||||||
| 10 | 50 | 100 | 10 | 50 | 100 | |||||||
| NIV | 3.4 | Positive | 3.9 | 11.8 | 10–250 | 0.9992 | 3 | −5 | 2 | 11 | 6 | 2 |
| DON | 5.9 | Positive | 1.8 | 5.4 | 10–500 | 0.9993 | −2 | −6 | −6 | 6 | 3 | 4 |
| NEO | 7.9 | Positive | 1.5 | 4.6 | 10–100 | 0.9994 | 10 | −1 | −4 | 4 | 3 | 3 |
| 15-ADON | 8.4 | Positive | 14 | 42 | 10–500 | 0.9988 | x | 4 | 2 | x | 8 | 2 |
| 3-ADON | 8.6 | Positive | 3.6 | 11 | 10–500 | 0.9991 | −24 | −11 | −9 | 14 | 14 | 11 |
| HT-2 | 11.2 | Positive | 2.4 | 7.4 | 10–500 | 0.9998 | 8 | −5 | 8 | 7 | 2 | 2 |
| T-2 | 11.9 | Positive | 2.0 | 6.1 | 10–100 | 0.9989 | −14 | −6 | −7 | 7 | 5 | 4 |
| ZEA | 12.5 | Negative | 3.4 | 10.3 | 10–500 | 0.9992 | 19 | −4 | 1 | 9 | 7 | 6 |
| ENN B | 14.1 | Positive | 3.5 | 10.7 | 10–100 | 0.9972 | −29 | 0 | −7 | 14 | 4 | 4 |
| ENN B1 | 14.1 | Positive | 3.0 | 9.3 | 10–500 | 0.9998 | −15 | 4 | −7 | 11 | 7 | 5 |
| ENN A | 14.3 | Positive | 1.9 | 5.7 | 10–500 | 0.9998 | −15 | 1 | −11 | 7 | 3 | 5 |
| ENN A1 | 14.4 | Positive | 1.7 | 5.3 | 10–500 | 0.9998 | −5 | −1 | −14 | 6 | 7 | 8 |
| Level of spiked samples (µg kg−1) | ||||||||||||
| 100 | 800 | 100 | 800 | |||||||||
| MON | 11 | Negative | 0.6 | 2 | 50–1000 | 0.9974 | 7 | 4 | 23 | 9 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janaviciene, S.; Venslovas, E.; Kadziene, G.; Matelioniene, N.; Berzina, Z.; Bartkevics, V.; Suproniene, S. Diversity of Mycotoxins Produced by Fusarium Strains Infecting Weeds. Toxins 2023, 15, 420. https://doi.org/10.3390/toxins15070420
Janaviciene S, Venslovas E, Kadziene G, Matelioniene N, Berzina Z, Bartkevics V, Suproniene S. Diversity of Mycotoxins Produced by Fusarium Strains Infecting Weeds. Toxins. 2023; 15(7):420. https://doi.org/10.3390/toxins15070420
Chicago/Turabian StyleJanaviciene, Sigita, Eimantas Venslovas, Grazina Kadziene, Neringa Matelioniene, Zane Berzina, Vadims Bartkevics, and Skaidre Suproniene. 2023. "Diversity of Mycotoxins Produced by Fusarium Strains Infecting Weeds" Toxins 15, no. 7: 420. https://doi.org/10.3390/toxins15070420
APA StyleJanaviciene, S., Venslovas, E., Kadziene, G., Matelioniene, N., Berzina, Z., Bartkevics, V., & Suproniene, S. (2023). Diversity of Mycotoxins Produced by Fusarium Strains Infecting Weeds. Toxins, 15(7), 420. https://doi.org/10.3390/toxins15070420









