The Venom Composition of the Snake Tribe Philodryadini: ‘Omic’ Techniques Reveal Intergeneric Variability among South American Racers
Abstract
1. Introduction
2. Results
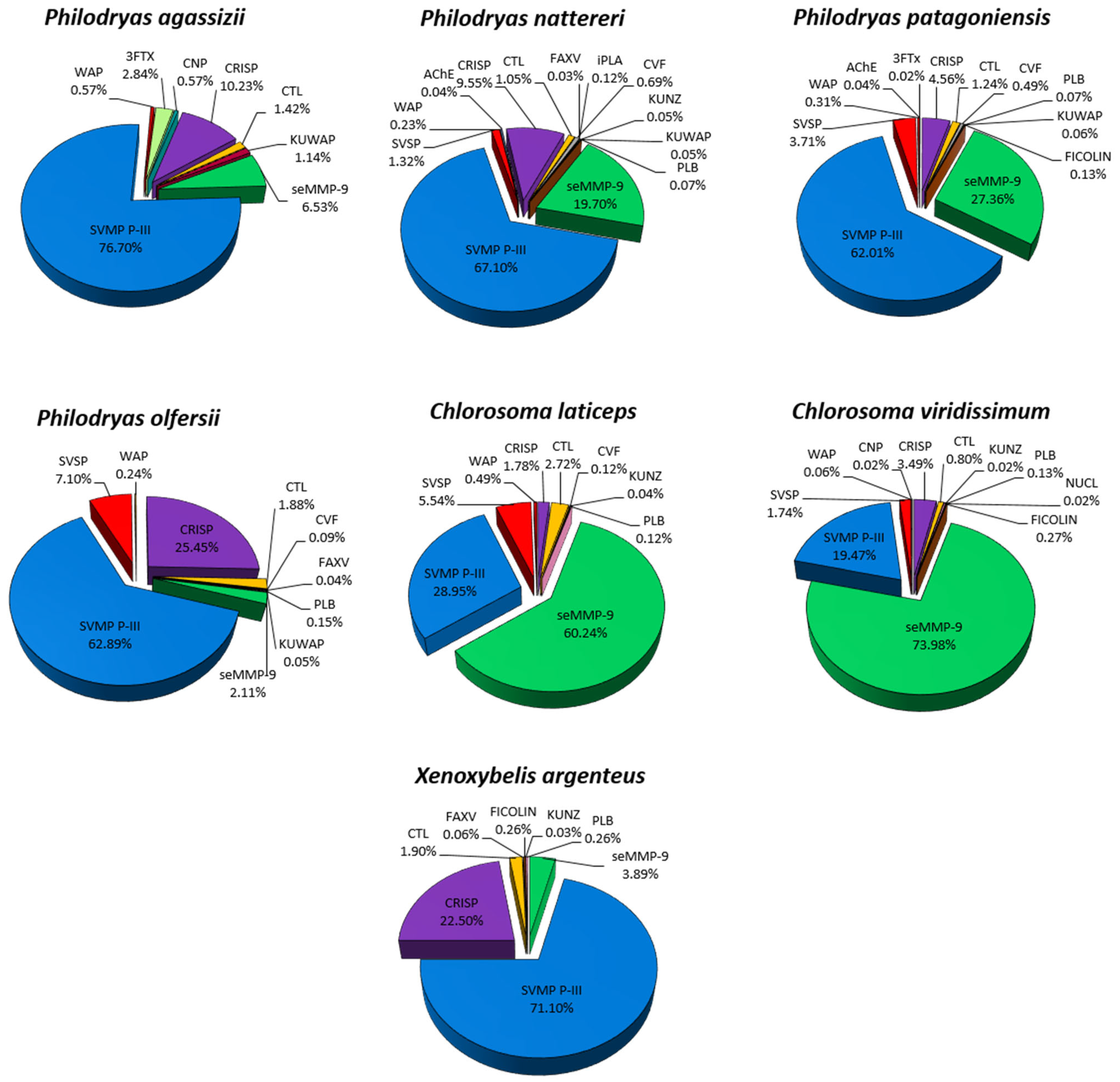
2.1. Philodryadini Duvernoy’s Venom Gland Transcriptome and Venom Composition
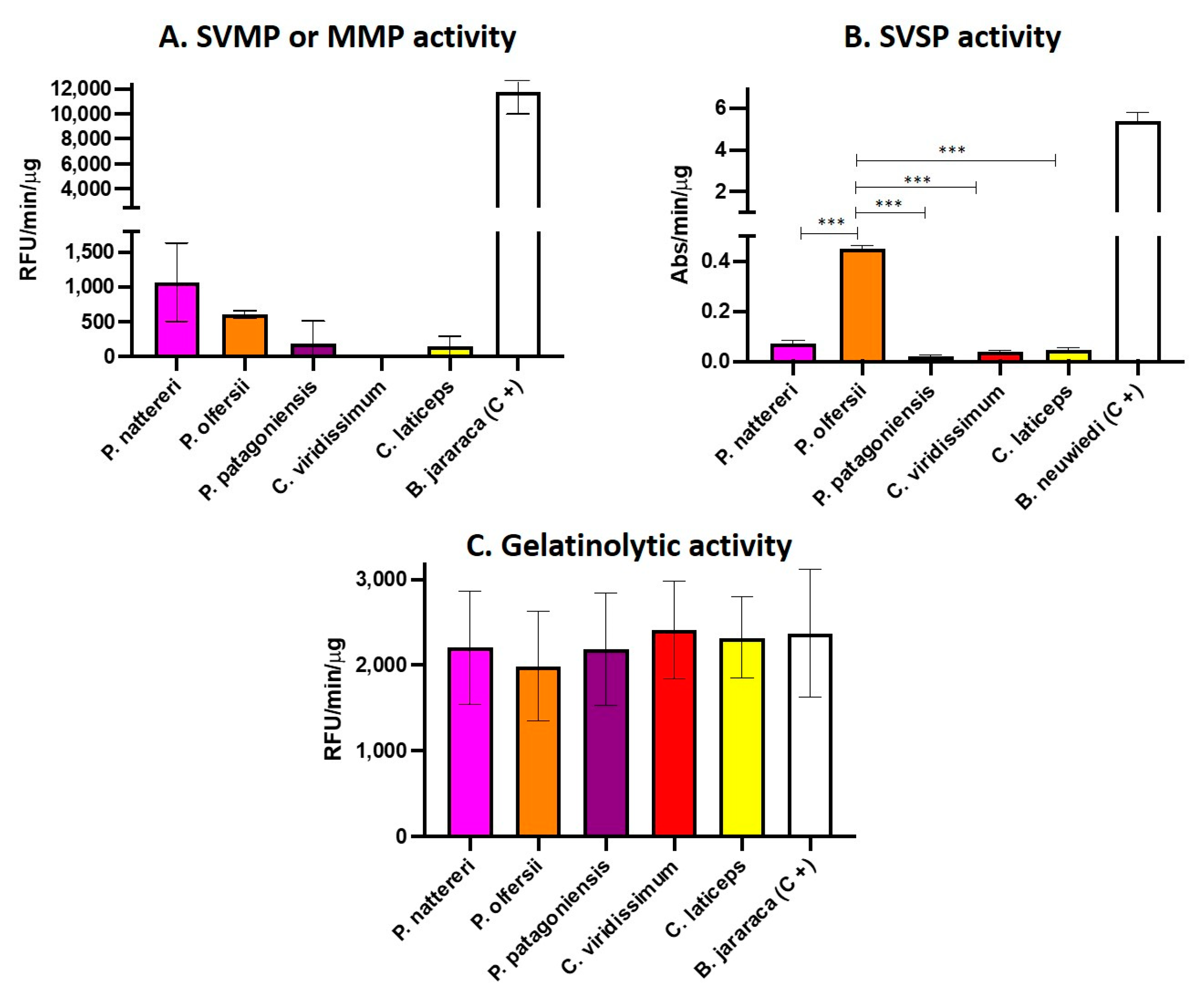
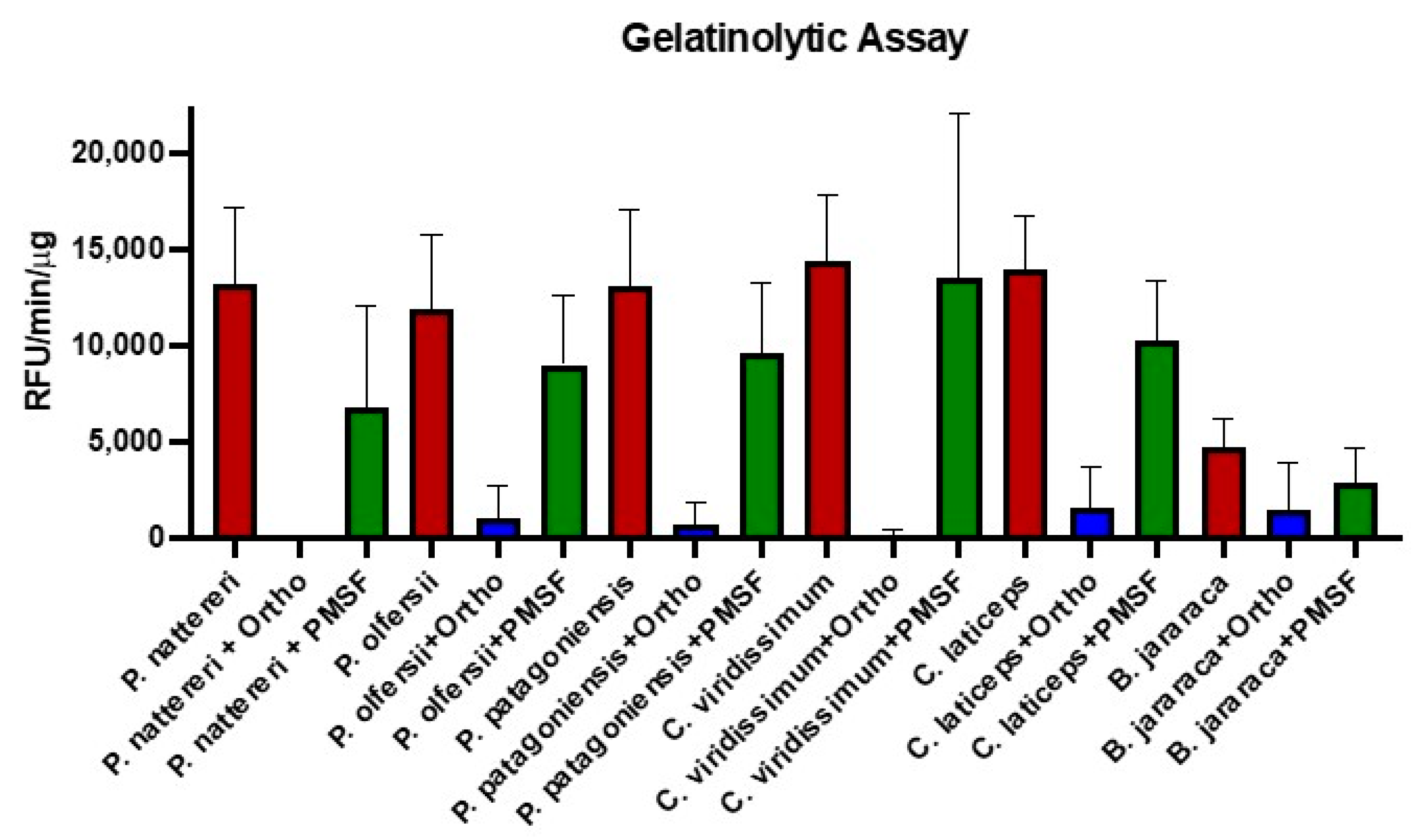
2.2. Functional Analysis of Philodryadini Venoms
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Snakes and Venoms
5.2. Electrophoresis in Polyacrylamide Gel in the Presence of SDS (SDS-PAGE)
5.3. Transcriptomic Analysis
5.3.1. Transcriptome Assembly
5.3.2. Toxins Annotation
5.4. Analyses of Venoms by Mass Spectrometry (LC-MS/MS)
5.5. Functional Analysis of Venoms
5.5.1. Enzymatic Assays on Synthetic Substrates
5.5.2. Gelatinolytic Assay on SDS-PAGE Gel
5.5.3. Gelatinolytic Assay against 1,10-Phenanthroline and PMSF Inhibitors
5.6. Identification of Gel Interest Bands by Mass Spectrometry (Q-TOF-LC-MS)
5.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zaher, H.; Grazziotin, F.G.; Cadle, J.E.; Murphy, R.W.; Moura-Leite, J.C.; Bonatto, S.L. Molecular phylogeny of advanced snakes (Serpentes, Caenophidia) with an emphasis on South American Xenodontines: A revised classification and descriptions of new taxa. Pap. Avulsos Zool. 2009, 49, 115–153. [Google Scholar] [CrossRef]
- Grazziotin, F.; Zaher, H.; Murphy, R.; Scrocchi, G.; Benavides, M.; Zhang, Y.; Bonatto, S. Molecular phylogeny of the New World Dipsadidae (Serpentes: Colubroidea): A reappraisal. Cladistics 2012, 28, 437–459. [Google Scholar] [CrossRef] [PubMed]
- Minton, S.A. Venomous bites by nonvenomous snakes: An annotated bibliography of colubrid envenomation. J. Wilderness Med. 1990, 1, 119–127. [Google Scholar] [CrossRef]
- Arredondo, J.C.; Grazziotin, F.G.; Scrocchi, G.J.; Rodrigues, M.T.U.; Bonatto, S.L.; Zaher, H.E.D. Molecular phylogeny of the tribe Philodryadini Cope, 1886 (Dipsadidae: Xenodontinae): Rediscovering the diversity of the South American Racers. Pap. Avulsos Zool. 2020, 60, e20206053. [Google Scholar] [CrossRef]
- Junqueira-de-Azevedo, I.L.; Campos, P.F.; Ching, A.T.; Mackessy, S.P. Colubrid Venom Composition: An -Omics Perspective. Toxins 2016, 8, 230. [Google Scholar] [CrossRef]
- Modahl, C.M.; Saviola, A.J.; Mackessy, S.P. Venoms of Colubrids. In Venom Genomics and Proteomics. Toxinology; Gopalakrishnakone, P., Calvete, J., Eds.; Springer Science+Business Media Dordrecht: Berlin, Germany, 2016; pp. 51–79. [Google Scholar] [CrossRef]
- Weinstein, S.A.; White, J.; Keyler, D.E.; Warrell, D.A. Non-front-fanged colubroid snakes: A current evidence-based analysis of medical significance. Toxicon 2013, 69, 103–113. [Google Scholar] [CrossRef]
- Ribeiro, L.A.; Puorto, G.; Jorge, M.T. Bites by the colubrid snake Philodryas olfersii: A clinical and epidemiological study of 43 cases. Toxicon 1999, 37, 943–948. [Google Scholar] [CrossRef]
- França, F.G.R.; Mesquita, D.O.; Nogueira, C.C.; Araújo, A.F.B. Phylogeny and ecology determine morphological structure in a snake assemblage in the central Brazilian Cerrado. Copeia 2008, 1, 23–38. [Google Scholar] [CrossRef]
- Hartmann, P.; Marques, O. Diet and habitat use of two sympatric species of Philodryas (Colubridae), in south Brazil. Amphib-Reptil 2005, 26, 25–31. [Google Scholar] [CrossRef]
- Thomas, R.A. A Revision of the South American Colubrid Snake Genus Philodryas Wagler, 1830. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 1976. [Google Scholar]
- Di-Bernardo, M. História Natural de uma Comunidade de Serpentes da Borda Oriental do Planalto das Araucárias Rio Grande do Sul Brasil. Ph.D. Thesis, Universidade Estadual Paulista, Rio Claro, São Paulo, Brazil, 1998. [Google Scholar]
- Hartmann, P.A. Hábito alimentar e utilização do ambiente em duas espécies simpátricas de Philodryas (Serpentes Colubridae) no sul do Brasil. Master’s Dissertation, Universidade Estadual Paulista, Rio Claro, São Paulo, Brazil, 2001. [Google Scholar]
- Sawaya, R.J.; Marques, O.A.V.; Martins, M. Composition and natural history of a Cerrado snake assemblage at Itirapina, São Paulo State, southeastern Brazil. Biota Neotrop. 2008, 8, 127–149. [Google Scholar] [CrossRef]
- Mesquita, P.C.M.D.; Borges-Nojosa, D.M.; Passos, D.C.; Bezerra, C.H. Ecology of Philodryas nattereri in the Brazilian semi-arid region. Herpetol. J. 2011, 21, 193–198. [Google Scholar]
- Guedes, T.B.; Nogueira, C.; Marques, O.A.V. Diversity, natural history, and geographic distribution of snakes in the Caatinga, Northeastern Brazil. Zootaxa 2014, 3863, 1–93. [Google Scholar] [CrossRef]
- Machado-Filho, P.R. Evolução do Hábito Alimentar e utilização do Substrato Pelo Gênero Philodryas Wagler, 1830. Master’s Dissertation, State University of São Paulo Julio de Mesquita Filho, Institute of Biosciences, Institute of Biosciences, São Paulo, Brazil, 2015. [Google Scholar]
- Guedes, T.B.; Sazima, I.; Marques, O.A.V. Does swallowing a toad require any specialisation? Feeding behaviour of the dipsadid snake Philodryas nattereri on the bufonid toad Rhinella jimi. Herpetol. Notes 2018, 11, 825–828. [Google Scholar]
- Quintela, F.M.; Loebmann, D. Diet, sexual dimorphism and reproduction of sympatric racers Philodryas aestiva and Philodryas patagoniensis from the coastal Brazilian Pampa. An Acad. Bras. Cien. 2019, 91, e20180296. [Google Scholar] [CrossRef]
- Zaher, H.; Scroocch, G.; Masiero, R. Rediscovery and redescription of the type of Philodryas laticeps Werner, 1900 and the taxonomic status of P. oligolepis Gomes, 1921 (Serpentes, Colubridae). Zootaxa 2008, 1940, 25–40. [Google Scholar] [CrossRef]
- Barros, M.A.S.; Pinto, L.C.; Pfau, R.O.; Kislowski, F.; Freire, M.D. Philodryas olfersii (Serpentes, Dipsadidae) feeding on bats in southern Brazil. Braz. J. Biol. 2015, 13, 231–236. [Google Scholar]
- Chávez-Arribasplata, J.C.; Almora, C.E.; Pellón, J.J.; Venegas, P.J. Bat consumption by Philodryas viridissima (Serpentes: Colubridae) in the Amazon Basin of southeastern Peru. Phyllomedusa 2016, 15, 195–197. [Google Scholar] [CrossRef]
- Guedes, T. Philodryas nattereri (Paraguay Green Racer). Diet. Herpetol. Rev. 2017, 50, 679–680. [Google Scholar]
- Marques, O.A.V.; Sawaya, R.J.; Stender-Oliveira, F.; Franca, F.G.R. Ecology of the colubrid snake Pseudablabes agassizii in South-Eastern South America. Herpetol. J. 2006, 16, 37–45. [Google Scholar]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef]
- Casewell, N.R.; Jackson, T.N.W.; Laustsen, A.H.; Sunagar, K. Causes and Consequences of Snake Venom Variation. Trends Pharmacol. Sci. 2020, 41, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Holding, M.L.; Strickland, J.L.; Rautsaw, R.M.; Hofmann, E.P.; Mason, A.J.; Hogan, M.P.; Nystrom, G.S.; Ellsworth, S.A.; Colston, T.J.; Borja, M.; et al. Phylogenetically diverse diets favor more complex venoms in North American pitvipers. Proc. Natl. Acad. Sci. USA 2021, 118, e2015579118. [Google Scholar] [CrossRef] [PubMed]
- Tasoulis, T.; Isbister, G.K. A current perspective on snake venom composition and constituent protein families. Arch. Toxicol. 2023, 97, 133–153. [Google Scholar] [CrossRef]
- Calvete, J.J.; Sanz, L.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M. Venoms, venomics, antivenomics. FEBS Lett. 2009, 583, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.S.; Georgieva, D.; Genov, N.; Murakami, M.T.; Sinha, M.; Kumar, R.P.; Kaur, P.; Kumar, S.; Dey, S.; Sharma, S.; et al. Enzymatic toxins from snake venom: Structural characterization and mechanism of catalysis. FEBS J. 2011, 278, 4544–4576. [Google Scholar] [CrossRef]
- Li, L.; Huang, J.; Lin, Y. Snake Venoms in Cancer Therapy: Past, Present and Future. Toxins 2018, 10, 346. [Google Scholar] [CrossRef]
- Zelanis, A. Abordagens sistêmicas em toxinologia: Perspectivas e implicações de metodologias ômicas no estudo de toxinas de venenos de serpentes. Estud. Biol. 2012, 34, 143–147. [Google Scholar] [CrossRef]
- van Thiel, J.; Alonso, L.L.; Slagboom, J.; Dunstan, N.; Wouters, R.M.; Modahl, C.M.; Vonk, F.J.; Jackson, T.N.W.; Kool, J. Highly Evolvable: Investigating Interspecific and Intraspecific Venom Variation in Taipans (Oxyuranus spp.) and Brown Snakes (Pseudonaja spp.). Toxins 2023, 15, 74. [Google Scholar] [CrossRef]
- Amazonas, D.R.; Portes-Junior, J.A.; Nishiyama-Jr, M.Y.; Nicolau, C.A.; Chalkidis, H.M.; Mourão, R.H.V.; Grazziotin, F.G.; Rokyta, D.R.; Gibbs, H.L.; Valente, R.H.; et al. Molecular mechanisms underlying intraspecific variation in snake venom. J. Proteom. 2018, 181, 60–72. [Google Scholar] [CrossRef]
- Freitas-de-Sousa, L.A.; Nachtigall, P.G.; Portes-Junior, J.A.; Holding, M.L.; Nystrom, G.S.; Ellsworth, S.A.; Guimarães, N.C.; Tioyama, E.; Ortiz, F.; Silva, B.R.; et al. Size Matters: An Evaluation of the Molecular Basis of Ontogenetic Modifications in the Composition of Bothrops jararacussu snake venom. Toxins 2020, 12, 791. [Google Scholar] [CrossRef]
- Glenn, J.L.; Straight, R. Mojave rattlesnake (Crotalus scutulatus scutulatus) venom: Variation in toxicity with geographic origin. Toxicon 1978, 16, 81–84. [Google Scholar] [CrossRef]
- Alape-Girón, A.; Sanz, L.; Escolano, J.; Flores-Díaz, M.; Madrigal, M.; Sasa, M.; Calvete, J.J. Snake venomics of the lancehead pitviper Bothrops asper: Geographic, individual, and ontogenetic variations. J. Proteome Res. 2008, 7, 3556–3571. [Google Scholar] [CrossRef]
- Calvete, J.J.; Sanz, L.; Pérez, A.; Borges, A.; Vargas, A.M.; Lomonte, B.; Angulo, Y.; Gutiérrez, J.M.; Chalkidis, H.M.; Mourão, R.H.; et al. Snake population venomics and antivenomics of Bothrops atrox: Paedomorphism along its transamazonian dispersal and implications of geographic venom variability on snakebite management. J. Proteom. 2011, 74, 510–527. [Google Scholar] [CrossRef]
- Moretto Del-Rei, T.H.; Sousa, L.F.; Rocha, M.M.T.; Freitas-de-Sousa, L.A.; Travaglia-Cardoso, S.R.; Grego, K.; Sant’Anna, S.S.; Chalkidis, H.M.; Moura-da-Silva, A.M. Functional variability of Bothrops atrox venoms from three distinct areas across the Brazilian Amazon and consequences for human envenomings. Toxicon 2019, 164, 61–70. [Google Scholar] [CrossRef]
- Sousa, L.F.; Portes-Junior, J.A.; Nicolau, C.A.; Bernardoni, J.L.; Nishiyama, M.Y.; Amazonas, D.R.; Freitas-de-Sousa, L.A.; Mourão, R.H.; Chalkidis, H.M.; Valente, R.H.; et al. Functional proteomic analyses of Bothrops atrox venom reveals phenotypes associated with habitat variation in the Amazon. J. Proteom. 2017, 159, 32–46. [Google Scholar] [CrossRef]
- Menezes, M.C.; Furtado, M.F.; Travaglia-Cardoso, S.R.; Camargo, A.C.; Serrano, S.M. Sex-based individual variation of snake venom proteome among eighteen Bothrops jararaca siblings. Toxicon 2006, 47, 304–312. [Google Scholar] [CrossRef]
- Daltry, J.C.; Wüster, W.; Thorpe, R.S. Diet and snake venom evolution. Nature 1996, 379, 537–540. [Google Scholar] [CrossRef]
- Andrade, D.V.; Abe, A.S. Relationship of venom ontogeny and diet in Bothrops. Herpetologica 1999, 55, 200–204. [Google Scholar]
- Pawlak, J.; Mackessy, S.P.; Fry, B.G.; Bhatia, M.; Mourier, G.; Fruchart-Gaillard, C.; Servent, D.; Ménez, R.; Stura, E.; Ménez, A.; et al. Denmotoxin, a three-finger toxin from the colubrid snake Boiga dendrophila (Mangrove Catsnake) with birdspecific activity. J. Biol. Chem. 2006, 281, 29030–29041. [Google Scholar] [CrossRef]
- Saldarriaga, M.M.; Otero, R.; Núñez, V.; Toro, M.F.; Díaz, A.; Gutiérrez, J.M. Ontogenetic variability of Bothrops atrox and Bothrops asper snake venoms from Colombia. Toxicon 2003, 42, 405–411. [Google Scholar] [CrossRef]
- Guércio, R.A.; Shevchenko, A.; López-Lozano, J.L.; Paba, J.; Sousa, M.V.; Ricart, C.A. Ontogenetic variations in the venom proteome of the Amazonian snake Bothrops atrox. Proteome Sci. 2006, 4, 11. [Google Scholar] [CrossRef]
- Zelanis, A.; Tashima, A.K.; Rocha, M.M.; Furtado, M.F.; Camargo, A.C.; Ho, P.L.; Serrano, S.M. Analysis of the ontogenetic variation in the venom proteome/peptidome of Bothrops jararaca reveals different strategies to deal with prey. J. Proteome Res. 2010, 9, 2278–2291. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Sitnikova, T. Evolution by the birth-and-death process in multigene families of the vertebrate immune system. Proc. Natl. Acad. Sci. USA 1997, 94, 7799–7806. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Wagstaff, S.C.; Harrison, R.A.; Renjifo, C.; Wüster, W. Domain loss facilitates accelerated evolution and neofunctionalization of duplicate snake venom metalloproteinase toxin genes. Mol. Biol. Evol. 2011, 28, 2637–2649. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, A.D.; Swain, M.T.; Hegarty, M.J.; Logan, D.W.; Mulley, J.F. Restriction and recruitment-gene duplication and the origin and evolution of snake venom toxins. Genome Biol. Evol. 2014, 6, 2088–2095. [Google Scholar] [CrossRef]
- Vonk, F.J.; Casewell, N.R.; Henkel, C.V.; Heimberg, A.M.; Jansen, H.J.; McCleary, R.J.R.; Kerkkamp, H.M.E.; Vos, R.A.; Guerreiro, I.; Calvete, J.J.; et al. The king cobra genome reveals dynamic gene evolution and adaptation in the snake venom system. Proc. Natl. Acad. Sci. USA 2013, 110, 20651–20656. [Google Scholar] [CrossRef]
- Prado-Franceschi, J.; Hyslop, S.; Cogo, J.C.; Andrade, A.L.; Assakura, M.T.; Reichl, A.P.; Cruz-Höfling, M.A.; Rodrigues-Simioni, L. Characterization of a myotoxin from the Duvernoy’s gland secretion of the xenodontine colubrid Philodryas olfersii (green snake): Effects on striated muscle and the neuromuscular junction. Toxicon 1998, 36, 1407–1421. [Google Scholar] [CrossRef]
- Assakura, M.T.; Reichl, A.P.; Mandelbaum, F.R. Isolation and characterization of five fibrin(ogen)olytic enzymes from the venom of Philodryas olfersii (green snake). Toxicon 1994, 32, 819–831. [Google Scholar] [CrossRef]
- Ching, A.T.; Rocha, M.M.; Paes Leme, A.F.; Pimenta, D.C.; de Fátima, D.; Furtado, M.; Serrano, S.M.; Ho, P.L.; Junqueira-de-Azevedo, I.L. Some aspects of the venom proteome of the Colubridae snake Philodryas olfersii revealed from a Duvernoy’s (venom) gland transcriptome. FEBS Lett. 2006, 580, 4417–4422. [Google Scholar] [CrossRef]
- Peichoto, M.E.; Teibler, P.; Mackessy, S.P.; Leiva, L.; Acosta, O.; Gonçalves, L.R.; Tanaka-Azevedo, A.M.; Santoro, M.L. Purification and characterization of patagonfibrase, a metalloproteinase showing alpha-fibrinogenolytic and hemorrhagic activities, from Philodryas patagoniensis snake venom. Biochim. Biophys. Acta 2007, 1770, 810–819. [Google Scholar] [CrossRef]
- Peichoto, M.E.; Paes Leme, A.F.; Pauletti, B.A.; Batista, I.C.; Mackessy, S.P.; Acosta, O.; Santoro, M.L. Autolysis at the disintegrin domain of patagonfibrase, a metalloproteinase from Philodryas patagoniensis (Patagonia Green Racer; Dipsadidae) venom. Biochim. Biophys. Acta 2010, 1804, 1937–1942. [Google Scholar] [CrossRef]
- Peichoto, M.E.; Zychar, B.C.; Tavares, F.L.; de Camargo Gonçalves, L.R.; Acosta, O.; Santoro, M.L. Inflammatory effects of patagonfibrase, a metalloproteinase from Philodryas patagoniensis (Patagonia Green Racer; Dipsadidae) venom. Exp. Biol. Med. 2011, 236, 1166–1172. [Google Scholar] [CrossRef]
- Peichoto, M.E.; Mackessy, S.P.; Teibler, P.; Tavares, F.L.; Burckhardt, P.L.; Breno, M.C.; Acosta, O.; Santoro, M.L. Purification and characterization of a cysteine-rich secretory protein from Philodryas patagoniensis snake venom. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 150, 79–84. [Google Scholar] [CrossRef]
- Badari, J.C.; Díaz-Roa, A.; Teixeira Rocha, M.M.; Mendonça, R.Z.; da Silva Junior, P.I. Patagonin-CRISP: Antimicrobial Activity and Source of Antimicrobial Molecules in Duvernoy’s Gland Secretion (Philodryas patagoniensis snake). Front. Pharmacol. 2020, 11, 586705. [Google Scholar] [CrossRef]
- Kini, R.M.; Doley, R. Structure, function and evolution of three-finger toxins: Mini proteins with multiple targets. Toxicon 2010, 56, 855–867. [Google Scholar] [CrossRef]
- Fox, J.W.; Serrano, S.M. Insights into and speculations about snake venom metalloproteinase (SVMP) synthesis, folding and disulfide bond formation and their contribution to venom complexity. FEBS J. 2008, 275, 3016–3030. [Google Scholar] [CrossRef]
- Markland, F.S.; Swenson, S. Snake venom metalloproteinases. Toxicon 2013, 62, 3–18. [Google Scholar] [CrossRef]
- Morita, T. Structures and functions of snake venom CLPs (C-type lectin-like proteins) with anticoagulant-, procoagulant-, and platelet-modulating activities. Toxicon 2005, 45, 1099–1114. [Google Scholar] [CrossRef]
- Peichoto, M.E.; Tavares, F.L.; Santoro, M.L.; Mackessy, S.P. Venom proteomes of South and North American opisthoglyphous (Colubridae and Dipsadidae) snake species: A preliminary approach to understanding their biological roles. Comp. Biochem. Physiol. Part D Genom. Proteom. 2012, 7, 361–369. [Google Scholar] [CrossRef]
- Cidade, D.A.; Simão, T.A.; Dávila, A.M.; Wagner, G.; Junqueira-de-Azevedo, I.L.M.; Ho, P.L.; Bon, C.; Zingali, R.B.; Albano, R.M. Bothrops jararaca venom gland transcriptome: Analysis of the gene expression pattern. Toxicon 2006, 48, 437–461. [Google Scholar] [CrossRef]
- Zelanis, A.; Tashima, A.K.; Pinto, A.F.; Leme, A.F.; Stuginski, D.R.; Furtado, M.F.; Sherman, N.E.; Ho, P.L.; Fox, J.W.; Serrano, S.M. Bothrops jararaca venom proteome rearrangement upon neonate to adult transition. Proteomics 2011, 11, 4218–4228. [Google Scholar] [CrossRef] [PubMed]
- Moura-da-Silva, A.M.; Theakston, R.D.; Crampton, J.M. Evolution of disintegrin cysteine-rich and mammalian matrix-degrading metalloproteinases: Gene duplication and divergence of a common ancestor rather than convergent evolution. J. Mol. Evol. 1996, 43, 263–269. [Google Scholar] [CrossRef] [PubMed]
- McGivern, J.J.; Wray, K.P.; Margres, M.J.; Couch, M.E.; Mackessy, S.P.; Rokyta, D.R. RNA-seq and high-definition mass spectrometry reveal the complex and divergent venoms of two rear-fanged colubrid snakes. BMC Genom. 2014, 15, 1601. [Google Scholar] [CrossRef] [PubMed]
- Pla, D.; Sanz, L.; Whiteley, G.; Wagstaff, S.C.; Harrison, R.A.; Casewell, N.R.; Calvete, J.J. What killed Karl Patterson Schmidt? Combined venom gland transcriptomic, genomic and metagenomic analysis of the South African green tree snake (the boomslang), Dispholidus typus. Biochim. Biophys. Acta 2017, 1861, 814–823. [Google Scholar] [CrossRef]
- Pla, D.; Petras, D.; Saviola, A.J.; Modahl, C.M.; Sanz, L.; Pérez, A.; Juárez, E.; Frietze, S.; Dorrestein, P.C.; Mackessy, S.P.; et al. Transcriptomics-guided bottom-up and top-down venomics of neonate and adult specimens of the arboreal rear-fanged brown treesnake, Boiga irregularis, from Guam. J. Proteom. 2018, 174, 71–84. [Google Scholar] [CrossRef]
- Mackessy, S.P. Biochemistry and pharmacology of Colubrid snake venoms. J. Toxicol. Toxin. Rev. 2002, 21, 43–83. [Google Scholar] [CrossRef]
- Moura-da-Silva, A.M.; Butera, D.; Tanjoni, I. Importance of snake venom metalloproteinases in cell biology: Effects on platelets, inflammatory and endothelial cells. Curr. Pharm. Des. 2007, 13, 2893–2905. [Google Scholar] [CrossRef]
- Bernardoni, J.L.; Sousa, L.F.; Wermelinger, L.S.; Lopes, A.S.; Prezoto, B.C.; Serrano, S.M.T.; Zingali, R.B.; Moura-da-Silva, A.M. Functional variability of snake venom metalloproteinases: Adaptive advantages in targeting different prey and implications for human envenomation. PLoS ONE 2014, 9, e109651. [Google Scholar] [CrossRef]
- Shannon, J.D.; Baramova, E.N.; Bjarnason, J.B.; Fox, J.W. Amino acid sequence of a Crotalus atrox venom metalloproteinase which cleaves type IV collagen and gelatin. J. Biol. Chem. 1989, 264, 11575–11583. [Google Scholar] [CrossRef]
- Gutierrez, J.M.; Rucavado, A.; Escalante, T.; Díaz, C. Hemorrhage induced by snake venom metalloproteinases: Biochemical and biophysical mechanisms involved in microvessel damage. Toxicon 2005, 45, 997–1011. [Google Scholar] [CrossRef]
- Baldo, C.; Jamora, C.; Yamanouye, N.; Zorn, T.M.; Moura-da-Silva, A.M. Mechanisms of vascular damage by hemorrhagic snake venom metalloproteinases: Tissue distribution and in situ hydrolysis. PLoS Negl. Trop. Dis. 2010, 4, e727. [Google Scholar] [CrossRef]
- Siigur, E.; Tõnismägi, K.; Trummal, K.; Samel, M.; Vija, H.; Subbi, J.; Siigur, J. Factor X activator from vipera lebetina snake venom, molecular characterization and substrate specificity. Biochim. Biophys. Acta 2001, 1568, 90–98. [Google Scholar] [CrossRef]
- Modesto, J.C.; Junqueira-de-Azevedo, I.L.; Neves-Ferreira, A.G.; Fritzen, M.; Oliva, M.L.; Ho, P.L.; Perales, J.; Chudzinski-Tavassi, A.M. Insularinase A, a prothrombin activator from Bothrops insularis venom, is a metalloprotease derived from a gene encoding protease and disintegrin domains. Biol. Chem. 2005, 386, 589–600. [Google Scholar] [CrossRef]
- Kamiguti, A.S.; Slupsky, J.R.; Zuzel, M.; Hay, C.R. Properties of fibrinogen cleaved by jararhagin, a metalloproteinase from the venom of Bothrops jararaca. Thromb. Haemost. 1994, 72, 244–249. [Google Scholar] [CrossRef]
- Escalante, T.; Shannon, J.; Moura-da-Silva, A.M.; Gutiérrez, J.M.; Fox, J.W. Novel insights into capillary vessel basement membrane damage by snake venom hemorrhagic metalloproteinases: A biochemical and immunohistochemical study. Arch. Biochem. Biophys. 2006, 455, 144–153. [Google Scholar] [CrossRef]
- Escalante, T.; Rucavado, A.; Fox, J.W.; Gutierrez, J.M. Key events in microvascular damage induced by snake venom hemorrhagic metalloproteinases. J. Proteom. 2011, 74, 1781–1794. [Google Scholar] [CrossRef]
- Kamiguti, A.S.; Cardoso, J.L.; Theakston, R.D.; Sano-Martins, I.S.; Hutton, R.A.; Rugman, F.P.; Warrell, D.A.; Hay, C.R. Coagulopathy and haemorrhage in human victims of Bothrops jararaca envenoming in brazil. Toxicon 1991, 29, 961–972. [Google Scholar] [CrossRef]
- Vu, T.H.; Werb, Z. Matrix metalloproteinases: Effectors of development and normal physiology. Genes Dev. 2000, 14, 2123–2133. [Google Scholar] [CrossRef]
- Mccawley, L.J.; Matrisian, L.M. Matrix metalloproteinases: They’re not just for matrix anymore! Curr. Opin. Cell Biol. 2001, 13, 534–540. [Google Scholar] [CrossRef]
- Parks, W.C.; Wilson, C.L.; López-Boado, Y.S. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol. 2004, 4, 617–629. [Google Scholar] [CrossRef]
- Page-Mccaw, A.; Ewald, A.J.; Werb, Z. Matrix metaloproteinases and the regulation of tissue remodeling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Nagase, H. Progress in matrix metalloproteinase research. Mol. Asp. Med. 2008, 29, 290–308. [Google Scholar] [CrossRef] [PubMed]
- Komori, K.; Konishi, M.; Maruta, Y.; Toriba, M.; Sakai, A.; Matsuda, A.; Hori, T.; Nakatani, M.; Minamino, N.; Akizawa, T. Characterization of a novel metalloproteinase in duvernoy’s gland of Rhabdophis tigrinus tigrinus. J. Toxicol. Sci. 2006, 31, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Ching, A.T.; Leme, A.F.P.; Zelanis, A.; Rocha, M.M.T.; Furtado, M.F.D.; Andrade-Silva, D.; Trugilho, M.R.O.; Rocha, S.L.G.; Perales, J.; Ho, P.L.; et al. Venomics Profiling of Thamnodynastes strigatus Unveils Matrix Metalloproteinases and Other Novel Proteins Recruited to the Toxin Arsenal of Rear-Fanged Snakes. J. Proteome Res. 2012, 11, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Bayona-Serrano, J.D.; Viala, V.L.; Rautsaw, R.M.; Schramer, T.D.; Barros-Carvalho, G.A.; Nishiyama-Junior, M.Y.; Freitas-de-Sousa, L.A.; Moura-da-Silva, A.M.; Parkinson, C.L.; Grazziotin, F.G.; et al. Replacement and Parallel Simplification of Nonhomologous Proteinases Maintain Venom Phenotypes in Rear-Fanged Snakes. Mol. Biol. Evol. 2020, 37, 3563–3575. [Google Scholar] [CrossRef]
- True, J.R.; Carroll, S.B. Gene co-option in physiological and morphological evolution. Annu. Rev. Cell Dev. Biol. 2002, 18, 53–80. [Google Scholar] [CrossRef]
- Almeida, D.D.; Viala, V.L.; Nachtigall, P.G.; Broe, M.; Gibbs, H.L.; Serrano, S.M.T.; Moura-da-Silva, A.M.; Ho, P.L.; Nishiyama-Jr, M.Y.; Junqueira-de-Azevedo, I.L.M. Tracking the recruitment and evolution of snake toxins using the evolutionary context provided by the Bothrops jararaca genome. Proc. Nat. Acad. Sci. USA 2020, 118, e2015159118. [Google Scholar] [CrossRef]
- Lodovicho, M.E.; Costa, T.R.; Bernardes, C.P.; Menaldo, D.L.; Zoccal, K.F.; Carone, S.E.; Rosa, J.C.; Pucca, M.B.; Cerni, F.A.; Arantes, E.C.; et al. Investigating possible biological targets of Bj-CRP, the first cysteine-rich secretory protein (CRISP) isolated from Bothrops jararaca snake venom. Toxicol. Lett. 2017, 265, 156–169. [Google Scholar] [CrossRef]
- Fry, B.G.; Scheib, H.; van der Weerd, L.; Young, B.; McNaughtan, J.; Ramjan, S.F.; Vidal, N.; Poelmann, R.E.; Norman, J.A. Evolution of an arsenal: Structural and functional diversification of the venom system in the advanced snakes (Caenophidia). Mol. Cell Proteom. 2008, 7, 215–246. [Google Scholar] [CrossRef]
- Lu, Q.; Navdaev, A.; Clemetson, J.M.; Clemetson, K.J. Snake venom C-type lectins interacting with platelet receptors. Structure-function relationships and effects on haemostasis. Toxicon 2005, 45, 1089–1098. [Google Scholar] [CrossRef]
- Braud, S.; Bon, C.; Wisner, A. Snake venom proteins acting on hemostasis. Biochimie 2000, 82, 851–859. [Google Scholar] [CrossRef]
- Pawlak, J.; Mackessy, S.P.; Sixberry, N.M.; Stura, E.A.; Le Du, M.H.; Ménez, R.; Foo, C.S.; Ménez, A.; Nirthanan, S.; Kini, R.M. Irditoxin, a novel covalently linked heterodimeric three-finger toxin with high taxon-specific neurotoxicity. FASEB J. 2009, 23, 534–545. [Google Scholar] [CrossRef]
- Heyborne, W.H.; Mackessy, S.P. Identification and characterization of a taxon-specific three-finger toxin from the venom of the Green Vinesnake (Oxybelis fulgidus; family Colubridae). Biochimie 2013, 95, 1923–1932. [Google Scholar] [CrossRef]
- Scartozzoni, R.R. Reproductive Strategies and Feeding Ecology of the Aquatic Snakes of the Tribe Hydropsini (Dipsadidae, Xenodontinae). Ph.D Thesis, Biotechnology, Universidade de São Paulo, São Paulo, Brazil, 27 January 2010. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Bernheimer, A.W.; Linder, R.; Weinstein, S.A.; Kim, K.S. Isolation and characterization of a phospholipase B from venom of collett’s snake, Pseudechis colletti. Toxicon 1987, 25, 547–554. [Google Scholar] [CrossRef]
- Nair, D.G.; Fry, B.G.; Alewood, P.; Kumar, P.P.; Kini, R.M. Antimicrobial Activity of Omwaprin, a New Member of the Waprin Family of Snake Venom Proteins. Biochem. J. 2007, 402, 93–104. [Google Scholar] [CrossRef]
- St Pierre, L.; Earl, S.T.; Filippovich, I.; Sorokina, N.; Masci, P.P.; de Jersey, J.; Lavin, M.F. Common Evolution of Waprin and Kunitz-Like Toxin Families in Australian Venomous Snakes. Cell. Mol. Life Sci. 2008, 65, 4039–4054. [Google Scholar] [CrossRef]
- Campos, P.F.; Andrade-Silva, D.; Zelanis, A.; Leme, A.F.P.; Rocha, M.M.T.; Menezes, M.C.; Serrano, S.M.T.; Junqueira-de-Azevedo, I.L.M. Trends in the Evolution of Snake Toxins Underscored by an Integrative Omics Approach to Profile the Venom of the Colubrid Phalotris mertensi. Genome Biol. Evol. 2016, 8, 2266–2287. [Google Scholar] [CrossRef]
- Hagiwara, K.; Kikuchi, T.; Endo, Y.; Huqun; Usui, K.; Takahashi, M.; Shibata, N.; Kusakabe, T.; Xin, H.; Hoshi, S.; et al. Mouse SWAM1 and SWAM2 are antibacterial proteins composed of a single whey acidic protein motif. J. Immunol. 2003, 170, 1973–1979. [Google Scholar] [CrossRef]
- Fessler, J.H.; Kramerova, I.; Kramerov, A.; Chen, Y.; Fessler, L.I. Papilin, a novel component of basement membranes, in relation to ADAMTS metalloproteases and ECM development. Int. J. Biochem. Cell Biol. 2004, 36, 1079–1084. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, E.P.; Rautsaw, R.M.; Strickland, J.L.; Holding, M.L.; Hogan, M.P.; Mason, A.J.; Rokyta, D.R.; Parkinson, C.L. Comparative venom-gland transcriptomics and venom proteomics of four Sidewinder Rattlesnake (Crotalus cerastes) lineages reveal little differential expression despite individual variation. Sci. Rep. 2018, 8, 15534. [Google Scholar] [CrossRef]
- Krueger, F. A Wrapper Tool around Cutadapt and FastQC to Consistently Apply Quality and Adapter Trimming to FastQ Files. Available online: https://www.bioinformatics.babraham.ac.uk/projects/trim_galore (accessed on 7 December 2020).
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Chang, Z.; Li, G.; Liu, J.; Zhang, Y.; Ashby, C.; Liu, D.; Cramer, C.L.; Huang, X. Bridger: A new framework for de novo transcriptome assembly using RNA-seq data. Genome Biol. 2015, 16, 30. [Google Scholar] [CrossRef]
- Rokyta, D.R.; Lemmon, A.R.; Magres, M.J.; Aronow, K. The venom-gland transcriptome of the eastern diamondback rattlesnake (Crotalus adamanteus). BMC Genom. 2012, 13, 312. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Bankar, K.G.; Todur, V.N.; Shukla, R.N.; Vasudevan, M. Ameliorated de novo transcriptome assembly using Illumina paired end sequence data with Trinity Assembler. Genom. Data 2015, 5, 352–359. [Google Scholar] [CrossRef]
- Knittel, P.S.; Long, P.F.; Brammall, L.; Marques, A.C.; Almeida, M.T.; Padilla, G.; Moura-da-Silva, A.M. Characterising the enzymatic profile of crude tentacle extracts from the South Atlantic jellyfish Olindias sambaquiensis (Cnidaria: Hydrozoa). Toxicon 2016, 119, 1–7. [Google Scholar] [CrossRef]
- Westermeier, R.; Naven, T.; Höpker, H.R. Proteomics in Practice Proteomics in Practice: A Guide to Successful Experimental Design, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2008; p. 230. [Google Scholar] [CrossRef]
- Nogueira, C.C.; Argôlo, A.J.S.; Arzamendia, V.; Azevedo, J.A.; Barbo, F.E.; Bérnils, R.S.; Bolochio, B.E.; Borges-Martins, M.; Brasil-Godinho, M.; Braz, H.; et al. Atlas of Brazilian snakes: Verified point-locality maps to mitigate the Wallacean shortfall in a megadiverse snake fauna. South Am. J. Herpetol. 2019, 14, 1–274. [Google Scholar] [CrossRef]


| Philodryadini Database | |||||
|---|---|---|---|---|---|
| Species | Band | −10lgP | Coverage | Peptides | Identification |
| C. laticeps | 1 | 404.70 | 49 | 56 | seMMP9-SB0512_CLATSeMMP007 |
| C. viridissimum | 2 | 438.68 | 45 | 70 | seMMP9-SB0559_CVIRSeMMP011 |
| C. viridissimum | 3 | 496.05 | 55 | 94 | seMMP9-SB0559_CVIRSeMMP018 |
| P. patagoniensis | 4 | 442.28 | 51 | 57 | seMMP9-SB0235_PPATSeMMP001 |
| P. nattereri | 5 | 302.95 | 30 | 22 | seMMP9-SB0307_PNATSeMMP001 |
| P. olfersii | 6 | 289.52 | 28 | 18 | seMMP9-SB0559_CVIRSeMMP011 |
| P. olfersii | 7 | 293.78 | 43 | 13 | SVSP-SB0001_POLFSVSP002 |
| Species | Number Biota Project | Sex | Reproductive Status | Sample Origin | SVL (mm) | Use |
|---|---|---|---|---|---|---|
| Philodryas olfersii | SB0001 | F | adult | Pirenópolis/GO | 728 | T |
| SB0026 | M | adult | Porto Alegre/RS | 565 | P | |
| SB0130 | M | adult | Rio Grande do Sul | 774 | P | |
| SB0131 | F | adult | Rio Grande do Sul | 782 | P | |
| SB0132 | Nd | juvenile | Aquidauana/MS | 352 | T/P | |
| SB0370 | M | adult | Alegrete/RS | 750 | P | |
| Philodryas patagoniensis | SB0136 | M | adult | Rio Grande do Sul | 882 | P |
| SB0235 | M | adult | Rosário do Sul/RS | 525 | T/P | |
| SB0255 | F | juvenile | Bom Jardim da Serra/SC | 426 | P | |
| Philodryas nattereri | SB0307 | F | juvenile | Nd | 468 | T |
| SB0707 | F | adult | Jaíba/MG | 1095 | P | |
| SB1027 | F | adult | Araguari/MG | 1155 | P | |
| SB1028 | F | adult | Araguari/MG | 1190 | P | |
| Philodryas agassizii | SB0183 | M | adult | Painel/SC | 265 | T/P |
| Philodryas mattogrossensis | SB1881 | F | adult | Nova Ponte/MG | 930 | T |
| Chlorosoma lacticeps | SB0512 | M | adult | Sooretama/ES | 844 | T/P |
| Chlorosoma viridissimum | SB0559 | M | adult | Sena Madureira/AC | 808 | T/P |
| Xenoxybelis argenteus | SB1590 | F | adult | Mâncio Lima/AC | 584 | T |
| SB1934 | F | adult | Cruzeiro do Sul/AC | 797 | P |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tioyama, E.C.; Bayona-Serrano, J.D.; Portes-Junior, J.A.; Nachtigall, P.G.; de Souza, V.C.; Beraldo-Neto, E.; Grazziotin, F.G.; Junqueira-de-Azevedo, I.L.M.; Moura-da-Silva, A.M.; Freitas-de-Sousa, L.A. The Venom Composition of the Snake Tribe Philodryadini: ‘Omic’ Techniques Reveal Intergeneric Variability among South American Racers. Toxins 2023, 15, 415. https://doi.org/10.3390/toxins15070415
Tioyama EC, Bayona-Serrano JD, Portes-Junior JA, Nachtigall PG, de Souza VC, Beraldo-Neto E, Grazziotin FG, Junqueira-de-Azevedo ILM, Moura-da-Silva AM, Freitas-de-Sousa LA. The Venom Composition of the Snake Tribe Philodryadini: ‘Omic’ Techniques Reveal Intergeneric Variability among South American Racers. Toxins. 2023; 15(7):415. https://doi.org/10.3390/toxins15070415
Chicago/Turabian StyleTioyama, Emilly Campos, Juan David Bayona-Serrano, José A. Portes-Junior, Pedro Gabriel Nachtigall, Vinicius Carius de Souza, Emidio Beraldo-Neto, Felipe Gobbi Grazziotin, Inácio L. M. Junqueira-de-Azevedo, Ana Maria Moura-da-Silva, and Luciana Aparecida Freitas-de-Sousa. 2023. "The Venom Composition of the Snake Tribe Philodryadini: ‘Omic’ Techniques Reveal Intergeneric Variability among South American Racers" Toxins 15, no. 7: 415. https://doi.org/10.3390/toxins15070415
APA StyleTioyama, E. C., Bayona-Serrano, J. D., Portes-Junior, J. A., Nachtigall, P. G., de Souza, V. C., Beraldo-Neto, E., Grazziotin, F. G., Junqueira-de-Azevedo, I. L. M., Moura-da-Silva, A. M., & Freitas-de-Sousa, L. A. (2023). The Venom Composition of the Snake Tribe Philodryadini: ‘Omic’ Techniques Reveal Intergeneric Variability among South American Racers. Toxins, 15(7), 415. https://doi.org/10.3390/toxins15070415






