Domperidone Inhibits Clostridium botulinum C2 Toxin and Bordetella pertussis Toxin
Abstract
1. Introduction
2. Results
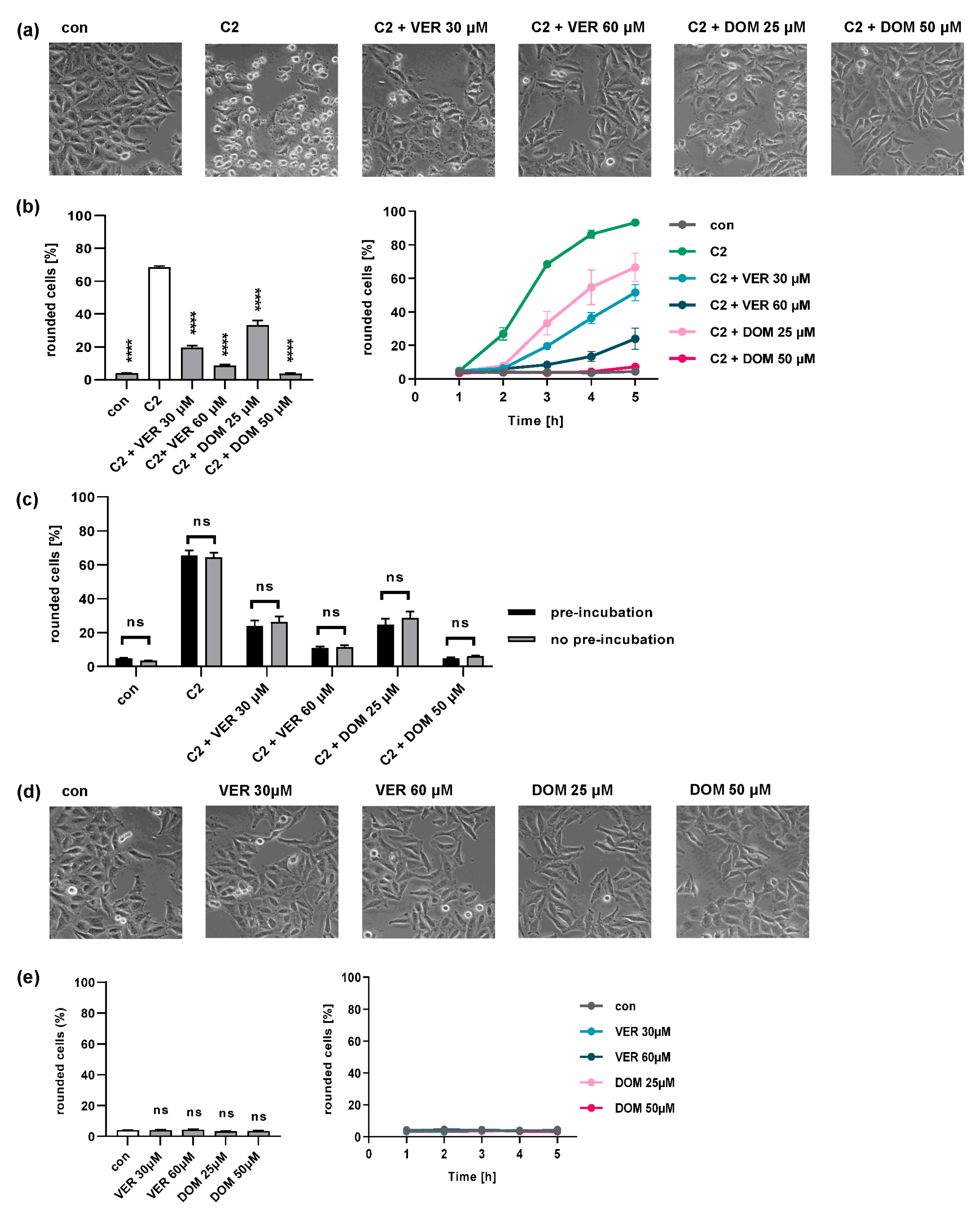
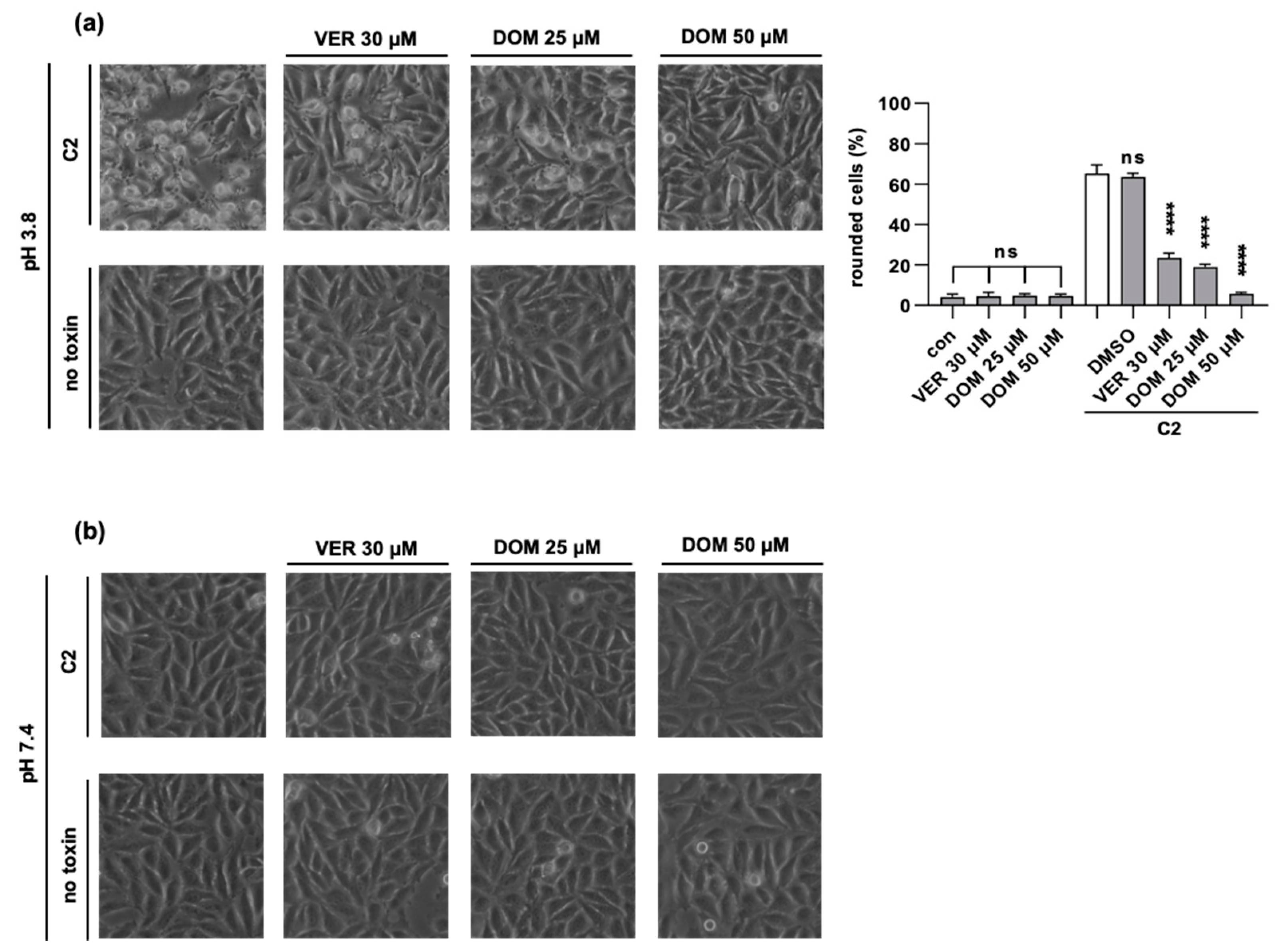
2.1. Domperidone Protects Cells from Intoxication with C2 Toxin
2.2. Domperidone Reduces the Amount of ADP-Ribosylated G-Actin in Cells without Affecting the C2I Enzyme Activity In Vitro or the Binding of C2 Toxin to Cells
2.3. Domperidone Inhibits the pH-Driven Membrane Translocation of C2I into the Cytosol
2.4. Domperidone Reduces the Amount of ADP-Ribosylated Gαi in PT-Treated Cells without Interfering with the Enzyme Activity In Vitro or the Cell Binding of PT to Cells
2.5. In the Presence of Domperidone, Less Free PTS1 Is Detectable in Cells
2.6. Inhibitors Reduce the Chaperone-Mediated Increase in PTS1 Enzyme Activity
2.7. Domperidone Reduces the PT-Mediated Effects on cAMP Signaling
3. Discussion
4. Materials and Methods
4.1. Protein Expression and Purification
4.2. Cell Culture and Intoxication Experiments
4.3. Sequential ADP-Ribosylation of Gαi or G-Actin in Lysates from Toxin-Treated Cells
4.4. In Vitro Enzyme Activity of C2I and PTS1
4.5. Binding of C2 Toxin and PT to Cells
4.6. C2 Toxin Translocation Assay
4.7. Fluorescence Microscopy
4.8. iGIST Bioassay
4.9. Reproducibility of Experiments and Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ohishi, I. Lethal and Vascular Permeability Activities of Botulinum C2 Toxin Induced by Separate Injections of the Two Toxin Components. Infect. Immun. 1983, 40, 336–339. [Google Scholar] [CrossRef]
- Ohishi, I. Response of Mouse Intestinal Loop to Botulinum C2 Toxin: Enterotoxic Activity Induced by Cooperation of Nonlinked Protein Components. Infect. Immun. 1983, 40, 691–695. [Google Scholar] [CrossRef]
- Barth, H.; Blocker, D.; Behlke, J.; Bergsma-Schutter, W.; Brisson, A.; Benz, R.; Aktories, K. Cellular Uptake of Clostridium Botulinum C2 Toxin Requires Oligomerization and Acidification. J. Biol. Chem. 2000, 275, 18704–18711. [Google Scholar] [CrossRef]
- Eckhardt, M.; Barth, H.; Blöcker, D.; Aktories, K. Binding of Clostridium Botulinum C2 Toxin to Asparagine-Linked Complex and Hybrid Carbohydrates. J. Biol. Chem. 2000, 275, 2328–2334. [Google Scholar] [CrossRef]
- Barth, H.; Hofmann, F.; Olenik, C.; Just, I.; Aktories, K. The N-Terminal Part of the Enzyme Component (C2I) of the Binary Clostridium Botulinum C2 Toxin Interacts with the Binding Component C2II and Functions as a Carrier System for a Rho ADP-Ribosylating C3-Like Fusion Toxin. Infect. Immun. 1998, 66, 1364–1369. [Google Scholar] [CrossRef]
- Blöcker, D.; Barth, H.; Maier, E.; Benz, R.; Barbieri, J.T.; Aktories, K. The C Terminus of Component C2II of Clostridium Botulinum C2 Toxin Is Essential for Receptor Binding. Infect. Immun. 2000, 68, 4566–4573. [Google Scholar] [CrossRef]
- Kaiser, E.; Haug, G.; Hliscs, M.; Aktories, K.; Barth, H. Formation of a Biologically Active Toxin Complex of the Binary Clostridium Botulinum C2 Toxin without Cell Membrane Interaction. Biochemistry 2006, 45, 13361–13368. [Google Scholar] [CrossRef]
- Schleberger, C.; Hochmann, H.; Barth, H.; Aktories, K.; Schulz, G.E. Structure and Action of the Binary C2 Toxin from Clostridium Botulinum. J. Mol. Biol. 2006, 364, 705–715. [Google Scholar] [CrossRef]
- Haug, G.; Wilde, C.; Leemhuis, J.; Meyer, D.K.; Aktories, K.; Barth, H. Cellular Uptake of Clostridium Botulinum C2 Toxin: Membrane Translocation of a Fusion Toxin Requires Unfolding of Its Dihydrofolate Reductase Domain. Biochemistry 2003, 42, 15284–15291. [Google Scholar] [CrossRef]
- Aktories, K.; Bärmann, M.; Ohishi, I.; Tsuyama, S.; Jakobs, K.H.; Habermann, E. Botulinum C2 Toxin ADP-Ribosylates Actin. Nature 1986, 322, 390–392. [Google Scholar] [CrossRef]
- Wegner, A.; Aktories, K. ADP-Ribosylated Actin Caps the Barbed Ends of Actin Filaments. J. Biol. Chem. 1988, 263, 13739–13742. [Google Scholar] [CrossRef]
- Kurazono, H.; Hosokawa, M.; Matsuda, H.; Sakaguchi, G. Fluid Accumulation in the Ligated Intestinal Loop and Histopathological Changes of the Intestinal Mucosa Caused by Clostridium Botulinum C2 Toxin in the Pheasant and Chicken. Res. Vet. Sci. 1987, 42, 349–353. [Google Scholar] [CrossRef]
- Tamura, M.; Nogimori, K.; Murai, S.; Yajima, M.; Ito, K.; Katada, T.; Ui, M.; Ishii, S. Subunit Structure of Islet-Activating Protein, Pertussis Toxin, in Conformity with the A-B Model. Biochemistry 1982, 21, 5516–5522. [Google Scholar] [CrossRef]
- Stein, P.E.; Boodhoo, A.; Armstrong, G.D.; Cockle, S.A.; Klein, M.H.; Read, R.J. The Crystal Structure of Pertussis Toxin. Structure 1994, 2, 45–57. [Google Scholar] [CrossRef]
- Locht, C.; Coutte, L.; Mielcarek, N. The Ins and Outs of Pertussis Toxin. FEBS J. 2011, 278, 4668–4682. [Google Scholar] [CrossRef]
- Witvliet, M.H.; Burns, D.L.; Brennan, M.J.; Poolman, J.T.; Manclark, C.R. Binding of Pertussis Toxin to Eucaryotic Cells and Glycoproteins. Infect. Immun. 1989, 57, 3324–3330. [Google Scholar] [CrossRef]
- Plaut, R.D.; Carbonetti, N.H. Retrograde Transport of Pertussis Toxin in the Mammalian Cell. Cell. Microbiol. 2008, 10, 1130–1139. [Google Scholar] [CrossRef]
- Teter, K. Intracellular Trafficking and Translocation of Pertussis Toxin. Toxins 2019, 11, 437. [Google Scholar] [CrossRef]
- el Bayâ, A.; Linnemann, R.; von Olleschik-Elbheim, L.; Robenek, H.; Schmidt, M.A. Endocytosis and Retrograde Transport of Pertussis Toxin to the Golgi Complex as a Prerequisite for Cellular Intoxication. Eur. J. Cell Biol. 1997, 73, 40–48. [Google Scholar]
- Hazes, B.; Boodhoo, A.; Cockle, S.A.; Read, R.J. Crystal Structure of the Pertussis Toxin-ATP Complex: A Molecular Sensor. J. Mol. Biol. 1996, 258, 661–671. [Google Scholar] [CrossRef]
- Plaut, R.D.; Scanlon, K.M.; Taylor, M.; Teter, K.; Carbonetti, N.H. Intracellular Disassembly and Activity of Pertussis Toxin Require Interaction with ATP. Pathog. Dis. 2016, 74, ftw065. [Google Scholar] [CrossRef]
- Pande, A.H.; Moe, D.; Jamnadas, M.; Tatulian, S.A.; Teter, K. The Pertussis Toxin S1 Subunit Is a Thermally Unstable Protein Susceptible to Degradation by the 20S Proteasome. Biochemistry 2006, 45, 13734–13740. [Google Scholar] [CrossRef]
- Banerjee, T.; Cilenti, L.; Taylor, M.; Showman, A.; Tatulian, S.A.; Teter, K. Thermal Unfolding of the Pertussis Toxin S1 Subunit Facilitates Toxin Translocation to the Cytosol by the Mechanism of Endoplasmic Reticulum-Associated Degradation. Infect. Immun. 2016, 84, 3388–3398. [Google Scholar] [CrossRef]
- Worthington, Z.E.V.; Carbonetti, N.H. Evading the Proteasome: Absence of Lysine Residues Contributes to Pertussis Toxin Activity by Evasion of Proteasome Degradation. Infect. Immun. 2007, 75, 2946–2953. [Google Scholar] [CrossRef]
- Bokoch, G.M.; Katada, T.; Northup, J.K.; Hewlett, E.L.; Gilman, A.G. Identification of the Predominant Substrate for ADP-Ribosylation by Islet Activating Protein. J. Biol. Chem. 1983, 258, 2072–2075. [Google Scholar] [CrossRef]
- Katada, T. The Inhibitory G Protein G(i) Identified as Pertussis Toxin-Catalyzed ADP-Ribosylation. Biol. Pharm. Bull. 2012, 35, 2103–2111. [Google Scholar] [CrossRef]
- Paramonov, V.M.; Sahlgren, C.; Rivero-Müller, A.; Pulliainen, A.T. IGIST-A Kinetic Bioassay for Pertussis Toxin Based on Its Effect on Inhibitory GPCR Signaling. ACS Sens. 2020, 5, 3438–3448. [Google Scholar] [CrossRef]
- Carbonetti, N.H. Contribution of Pertussis Toxin to the Pathogenesis of Pertussis Disease. Pathog. Dis. 2015, 73, ftv073. [Google Scholar] [CrossRef]
- Pittman, M. The Concept of Pertussis as a Toxin-Mediated Disease. Pediatr. Infect. Dis. 1984, 3, 467–486. [Google Scholar] [CrossRef]
- Scanlon, K.; Skerry, C.; Carbonetti, N. Association of Pertussis Toxin with Severe Pertussis Disease. Toxins 2019, 11, 373. [Google Scholar] [CrossRef]
- Domenech de Cellès, M.; Magpantay, F.M.G.; King, A.A.; Rohani, P. The Pertussis Enigma: Reconciling Epidemiology, Immunology and Evolution. Proc. Biol. Sci. 2016, 283, 20152309. [Google Scholar] [CrossRef]
- Mattoo, S.; Cherry, J.D. Molecular Pathogenesis, Epidemiology, and Clinical Manifestations of Respiratory Infections Due to Bordetella Pertussis and Other Bordetella Subspecies. Clin. Microbiol. Rev. 2005, 18, 326–382. [Google Scholar] [CrossRef]
- Surridge, J.; Segedin, E.R.; Grant, C.C. Pertussis Requiring Intensive Care. Arch. Dis. Child. 2007, 92, 970–975. [Google Scholar] [CrossRef]
- Haug, G.; Leemhuis, J.; Tiemann, D.; Meyer, D.K.; Aktories, K.; Barth, H. The Host Cell Chaperone Hsp90 Is Essential for Translocation of the Binary Clostridium Botulinum C2 Toxin into the Cytosol. J. Biol. Chem. 2003, 278, 32266–32274. [Google Scholar] [CrossRef]
- Kaiser, E.; Pust, S.; Kroll, C.; Barth, H. Cyclophilin A Facilitates Translocation of the Clostridium Botulinum C2 Toxin across Membranes of Acidified Endosomes into the Cytosol of Mammalian Cells. Cell. Microbiol. 2009, 11, 780–795. [Google Scholar] [CrossRef]
- Ernst, K.; Langer, S.; Kaiser, E.; Osseforth, C.; Michaelis, J.; Popoff, M.R.; Schwan, C.; Aktories, K.; Kahlert, V.; Malesevic, M.; et al. Cyclophilin-Facilitated Membrane Translocation as Pharmacological Target to Prevent Intoxication of Mammalian Cells by Binary Clostridial Actin ADP-Ribosylated Toxins. J. Mol. Biol. 2015, 427, 1224–1238. [Google Scholar] [CrossRef]
- Kaiser, E.; Böhm, N.; Ernst, K.; Langer, S.; Schwan, C.; Aktories, K.; Popoff, M.; Fischer, G.; Barth, H. FK506-Binding Protein 51 Interacts with Clostridium Botulinum C2 Toxin and FK506 Inhibits Membrane Translocation of the Toxin in Mammalian Cells. Cell. Microbiol. 2012, 14, 1193–1205. [Google Scholar] [CrossRef]
- Ernst, K.; Schmid, J.; Beck, M.; Hägele, M.; Hohwieler, M.; Hauff, P.; Ückert, A.K.; Anastasia, A.; Fauler, M.; Jank, T.; et al. Hsp70 Facilitates Trans-Membrane Transport of Bacterial ADP-Ribosylating Toxins into the Cytosol of Mammalian Cells. Sci. Rep. 2017, 7, 2724. [Google Scholar] [CrossRef]
- Ernst, K.; Sailer, J.; Braune, M.; Barth, H. Intoxication of Mammalian Cells with Binary Clostridial Enterotoxins Is Inhibited by the Combination of Pharmacological Chaperone Inhibitors. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 941–954. [Google Scholar] [CrossRef]
- Ernst, K.; Kling, C.; Landenberger, M.; Barth, H. Combined Pharmacological Inhibition of Cyclophilins, FK506-Binding Proteins, Hsp90, and Hsp70 Protects Cells From Clostridium Botulinum C2 Toxin. Front. Pharmacol. 2018, 9, 1287. [Google Scholar] [CrossRef]
- Ernst, K.; Eberhardt, N.; Mittler, A.-K.; Sonnabend, M.; Anastasia, A.; Freisinger, S.; Schiene-Fischer, C.; Malešević, M.; Barth, H. Pharmacological Cyclophilin Inhibitors Prevent Intoxication of Mammalian Cells with Bordetella pertussis Toxin. Toxins 2018, 10, 181. [Google Scholar] [CrossRef]
- Ernst, K.; Mittler, A.-K.; Winkelmann, V.; Kling, C.; Eberhardt, N.; Anastasia, A.; Sonnabend, M.; Lochbaum, R.; Wirsching, J.; Sakari, M.; et al. Pharmacological Targeting of Host Chaperones Protects from Pertussis Toxin In Vitro and In Vivo. Sci. Rep. 2021, 11, 5429. [Google Scholar] [CrossRef]
- Kellner, A.; Taylor, M.; Banerjee, T.; Britt, C.B.T.; Teter, K. A Binding Motif for Hsp90 in the A Chains of ADP-Ribosylating Toxins That Move from the Endoplasmic Reticulum to the Cytosol. Cell. Microbiol. 2019, 21, e13074. [Google Scholar] [CrossRef]
- Kellner, A.; Cherubin, P.; Harper, J.K.; Teter, K. Proline Isomerization as a Key Determinant for Hsp90-Toxin Interactions. Front. Cell. Infect. Microbiol. 2021, 11, 771653. [Google Scholar] [CrossRef]
- Ernst, K. Novel Strategies to Inhibit Pertussis Toxin. Toxins 2022, 14, 187. [Google Scholar] [CrossRef]
- Ernst, K. Requirement of Peptidyl-Prolyl Cis/Trans Isomerases and Chaperones for Cellular Uptake of Bacterial AB-Type Toxins. Front. Cell. Infect. Microbiol. 2022, 12, 938015. [Google Scholar] [CrossRef]
- Sakari, M.; Laisi, A.; Pulliainen, A.T. Exotoxin-Targeted Drug Modalities as Antibiotic Alternatives. ACS Infect. Dis. 2022, 8, 433–456. [Google Scholar] [CrossRef]
- Williamson, D.S.; Borgognoni, J.; Clay, A.; Daniels, Z.; Dokurno, P.; Drysdale, M.J.; Foloppe, N.; Francis, G.L.; Graham, C.J.; Howes, R.; et al. Novel Adenosine-Derived Inhibitors of 70 KDa Heat Shock Protein, Discovered through Structure-Based Design. J. Med. Chem. 2009, 52, 1510–1513. [Google Scholar] [CrossRef]
- Concilli, M.; Petruzzelli, R.; Parisi, S.; Catalano, F.; Sirci, F.; Napolitano, F.; Renda, M.; Galietta, L.J.V.; Di Bernardo, D.; Polishchuk, R.S. Pharmacoproteomics Pinpoints HSP70 Interaction for Correction of the Most Frequent Wilson Disease-Causing Mutant of ATP7B. Proc. Natl. Acad. Sci. USA 2020, 117, 32453–32463. [Google Scholar] [CrossRef]
- Sandvig, K.; Olsnes, S. Diphtheria Toxin Entry into Cells Is Facilitated by Low PH. J. Cell Biol. 1980, 87, 828–832. [Google Scholar] [CrossRef]
- Falnes, P.O.; Sandvig, K. Penetration of Protein Toxins into Cells. Curr. Opin. Cell Biol. 2000, 12, 407–413. [Google Scholar] [CrossRef]
- Barth, H.; Pfeifer, G.; Hofmann, F.; Maier, E.; Benz, R.; Aktories, K. Low PH-Induced Formation of Ion Channels by Clostridium Difficile Toxin B in Target Cells. J. Biol. Chem. 2001, 276, 10670–10676. [Google Scholar] [CrossRef]
- Gray, M.C.; Guerrant, R.L.; Hewlett, E.L. The CHO Cell Clustering Response to Pertussis Toxin: History of Its Discovery and Recent Developments in Its Use. Toxins 2021, 13, 815. [Google Scholar] [CrossRef]
- Pande, A.H.; Scaglione, P.; Taylor, M.; Nemec, K.N.; Tuthill, S.; Moe, D.; Holmes, R.K.; Tatulian, S.A.; Teter, K. Conformational Instability of the Cholera Toxin A1 Polypeptide. J. Mol. Biol. 2007, 374, 1114–1128. [Google Scholar] [CrossRef]
- Burress, H.; Taylor, M.; Banerjee, T.; Tatulian, S.A.; Teter, K. Co- and Post-Translocation Roles for HSP90 in Cholera Intoxication. J. Biol. Chem. 2014, 289, 33644–33654. [Google Scholar] [CrossRef]
- Stiles, B.G.; Wigelsworth, D.J.; Popoff, M.R.; Barth, H. Clostridial Binary Toxins: Iota and C2 Family Portraits. Front. Cell. Infect. Microbiol. 2011, 1, 11. [Google Scholar] [CrossRef]
- Ernst, K.; Liebscher, M.; Mathea, S.; Granzhan, A.; Schmid, J.; Popoff, M.R.; Ihmels, H.; Barth, H.; Schiene-Fischer, C. A Novel Hsp70 Inhibitor Prevents Cell Intoxication with the Actin ADP-Ribosylating Clostridium Perfringens Iota Toxin. Sci. Rep. 2016, 6, 20301. [Google Scholar] [CrossRef]
- Haug, G.; Aktories, K.; Barth, H. The Host Cell Chaperone Hsp90 Is Necessary for Cytotoxic Action of the Binary Iota-like Toxins. Infect. Immun. 2004, 72, 3066–3068. [Google Scholar] [CrossRef]
- Kaiser, E.; Kroll, C.; Ernst, K.; Schwan, C.; Popoff, M.; Fischer, G.; Buchner, J.; Aktories, K.; Barth, H. Membrane Translocation of Binary Actin-ADP-Ribosylating Toxins from Clostridium Difficile and Clostridium Perfringens Is Facilitated by Cyclophilin A and Hsp90. Infect. Immun. 2011, 79, 3913–3921. [Google Scholar] [CrossRef]
- Schuster, M.; Schnell, L.; Feigl, P.; Birkhofer, C.; Mohr, K.; Roeder, M.; Carle, S.; Langer, S.; Tippel, F.; Buchner, J.; et al. The Hsp90 Machinery Facilitates the Transport of Diphtheria Toxin into Human Cells. Sci. Rep. 2017, 7, 613. [Google Scholar] [CrossRef]
- Ratts, R.; Zeng, H.; Berg, E.A.; Blue, C.; McComb, M.E.; Costello, C.E.; vanderSpek, J.C.; Murphy, J.R. The Cytosolic Entry of Diphtheria Toxin Catalytic Domain Requires a Host Cell Cytosolic Translocation Factor Complex. J. Cell Biol. 2003, 160, 1139–1150. [Google Scholar] [CrossRef]
- Dmochewitz, L.; Lillich, M.; Kaiser, E.; Jennings, L.D.; Lang, A.E.; Buchner, J.; Fischer, G.; Aktories, K.; Collier, R.J.; Barth, H. Role of CypA and Hsp90 in Membrane Translocation Mediated by Anthrax Protective Antigen. Cell. Microbiol. 2011, 13, 359–373. [Google Scholar] [CrossRef]
- Rosenzweig, R.; Nillegoda, N.B.; Mayer, M.P.; Bukau, B. The Hsp70 Chaperone Network. Nat. Rev. Mol. Cell Biol. 2019, 20, 665–680. [Google Scholar] [CrossRef]
- Clerico, E.M.; Tilitsky, J.M.; Meng, W.; Gierasch, L.M. How Hsp70 Molecular Machines Interact with Their Substrates to Mediate Diverse Physiological Functions. J. Mol. Biol. 2015, 427, 1575–1588. [Google Scholar] [CrossRef]
- Jensen, R.E.; Johnson, A.E. Protein Translocation: Is Hsp70 Pulling My Chain? Curr. Biol. 1999, 9, R779–R782. [Google Scholar] [CrossRef]
- Sousa, R.; Lafer, E.M. The Physics of Entropic Pulling: A Novel Model for the Hsp70 Motor Mechanism. Int. J. Mol. Sci. 2019, 20, 2334. [Google Scholar] [CrossRef]
- Biebl, M.M.; Buchner, J. Structure, Function, and Regulation of the Hsp90 Machinery. Cold Spring Harb. Perspect. Biol. 2019, 11, a034017. [Google Scholar] [CrossRef]
- Howe, M.K.; Bodoor, K.; Carlson, D.A.; Hughes, P.F.; Alwarawrah, Y.; Loiselle, D.R.; Jaeger, A.M.; Darr, D.B.; Jordan, J.L.; Hunter, L.M.; et al. Identification of an Allosteric Small-Molecule Inhibitor Selective for the Inducible Form of Heat Shock Protein 70. Chem. Biol. 2014, 21, 1648–1659. [Google Scholar] [CrossRef]
- Reddymasu, S.C.; Soykan, I.; McCallum, R.W. Domperidone: Review of Pharmacology and Clinical Applications in Gastroenterology. Am. J. Gastroenterol. 2007, 102, 2036–2045. [Google Scholar] [CrossRef]
- Ou, L.B.; Moriello, C.; Douros, A.; Filion, K.B. Domperidone and the Risks of Sudden Cardiac Death and Ventricular Arrhythmia: A Systematic Review and Meta-Analysis of Observational Studies. Br. J. Clin. Pharmacol. 2021, 87, 3649–3658. [Google Scholar] [CrossRef]
- Scanlon, K.; Skerry, C.; Carbonetti, N. Role of Major Toxin Virulence Factors in Pertussis Infection and Disease Pathogenesis. Adv. Exp. Med. Biol. 2019, 1183, 35–51. [Google Scholar] [CrossRef]
- Liu, J.; Farmer, J.D.; Lane, W.S.; Friedman, J.; Weissman, I.; Schreiber, S.L. Calcineurin Is a Common Target of Cyclophilin-Cyclosporin A and FKBP-FK506 Complexes. Cell 1991, 66, 807–815. [Google Scholar] [CrossRef]
- Ashok, Y.; Miettinen, M.; Oliveira, D.K.H.D.; Tamirat, M.Z.; Näreoja, K.; Tiwari, A.; Hottiger, M.O.; Johnson, M.S.; Lehtiö, L.; Pulliainen, A.T. Discovery of Compounds Inhibiting the ADP-Ribosyltransferase Activity of Pertussis Toxin. ACS Infect. Dis. 2020, 6, 588–602. [Google Scholar] [CrossRef]
- Barth, H.; Preiss, J.C.; Hofmann, F.; Aktories, K. Characterization of the Catalytic Site of the ADP-Ribosyltransferase Clostridium Botulinum C2 Toxin by Site-Directed Mutagenesis. J. Biol. Chem. 1998, 273, 29506–29511. [Google Scholar] [CrossRef]
- Freeman, B.C.; Michels, A.; Song, J.; Kampinga, H.H.; Morimoto, R.I. Analysis of Molecular Chaperone Activities Using In Vitro and In Vivo Approaches. Methods Mol. Biol. 2000, 99, 393–419. [Google Scholar] [CrossRef]
- Richter, K.; Soroka, J.; Skalniak, L.; Leskovar, A.; Hessling, M.; Reinstein, J.; Buchner, J. Conserved Conformational Changes in the ATPase Cycle of Human Hsp90. J. Biol. Chem. 2008, 283, 17757–17765. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, J.; Braune-Yan, M.; Lietz, S.; Wahba, M.; Pulliainen, A.T.; Barth, H.; Ernst, K. Domperidone Inhibits Clostridium botulinum C2 Toxin and Bordetella pertussis Toxin. Toxins 2023, 15, 412. https://doi.org/10.3390/toxins15070412
Jia J, Braune-Yan M, Lietz S, Wahba M, Pulliainen AT, Barth H, Ernst K. Domperidone Inhibits Clostridium botulinum C2 Toxin and Bordetella pertussis Toxin. Toxins. 2023; 15(7):412. https://doi.org/10.3390/toxins15070412
Chicago/Turabian StyleJia, Jinfang, Maria Braune-Yan, Stefanie Lietz, Mary Wahba, Arto T. Pulliainen, Holger Barth, and Katharina Ernst. 2023. "Domperidone Inhibits Clostridium botulinum C2 Toxin and Bordetella pertussis Toxin" Toxins 15, no. 7: 412. https://doi.org/10.3390/toxins15070412
APA StyleJia, J., Braune-Yan, M., Lietz, S., Wahba, M., Pulliainen, A. T., Barth, H., & Ernst, K. (2023). Domperidone Inhibits Clostridium botulinum C2 Toxin and Bordetella pertussis Toxin. Toxins, 15(7), 412. https://doi.org/10.3390/toxins15070412





