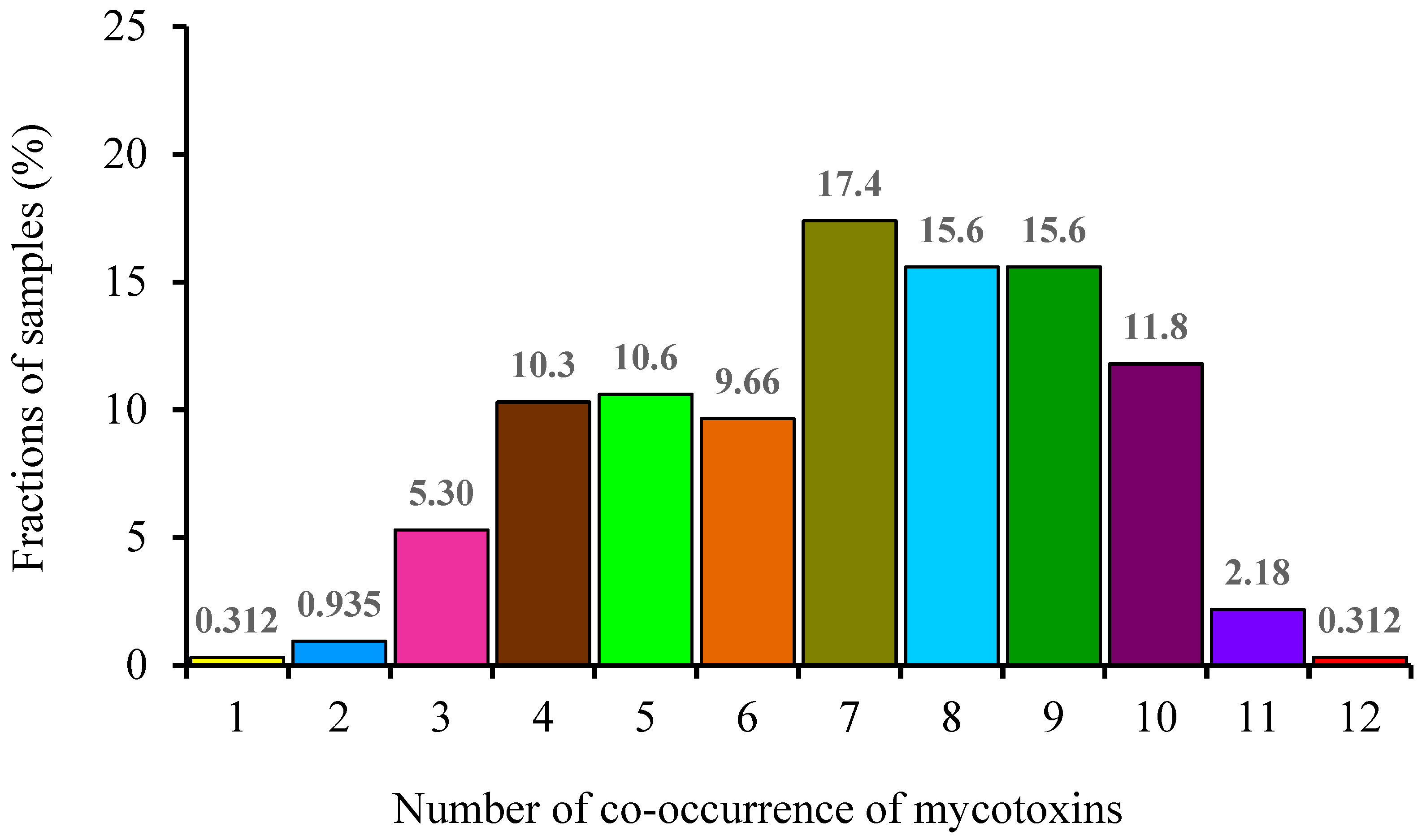2.1. Method Validation
The developed method was validated for linearity, accuracy (recoveries), precision (RSDs), the limit of detection (LOD), and the limit of quantification (LOQ) under guidance from SANCO/12495/2011 [
15]. Recoveries of analyzed mycotoxins in the wheat grain samples were evaluated by spiking the samples with standards at three different concentrations (
n = 6). In the national standard of People’s Republic of China GB 2761-2017 [
16], the maximum limits for DON and ZEN in wheat grains are 1000 μg/kg and 60 μg/kg, respectively. Recoveries for DON were determined at 100, 1000, and 2000 μg/kg fortification levels, whereas those for ZEN were determined at 20, 50, and 100 μg/kg fortification levels. Due to the lack of maximum limits for emerging mycotoxins, low, medium and high fortification levels (in the range of 1 to 100 μg/kg) were applied for the recovery tests for emerging mycotoxins analyzed in this study (
Table 1). As shown in
Table 1, the average recoveries were in the range of 87.9–112%, while the relative standard deviations (RSD%) were all <11.9%. The LODs and LOQs were defined based on the signal-to-noise ratio (S/N) of 3:1 and 10:1, respectively, with the lowest matrix standard concentration under guidance from SANCO/12495/2011 [
15]. As shown in
Table 2, the LODs ranged from 0.03 to 3.0 µg/kg, and the LOQs ranged from 0.1 to 10.0 µg/kg in wheat grain samples. The results of method validation indicated the feasibility of the developed method. The method validation results verified the feasibility of the developed method for the determination of regulated and emerging mycotoxins in wheat grains.
Figure S1 shows the LC–MS/MS chromatogram of mycotoxins DON, TeA, BEA, ENA, ENA1, ENB, ENB1, AME, AOH, TeA, TEN, and ALT in the wheat grain matrix.
2.3. Geographical Distribution of Mycotoxins in Wheat Grains
The investigation outcomes of mycotoxins in wheat grains harvested across eight provinces in China are summarized in
Table 5.
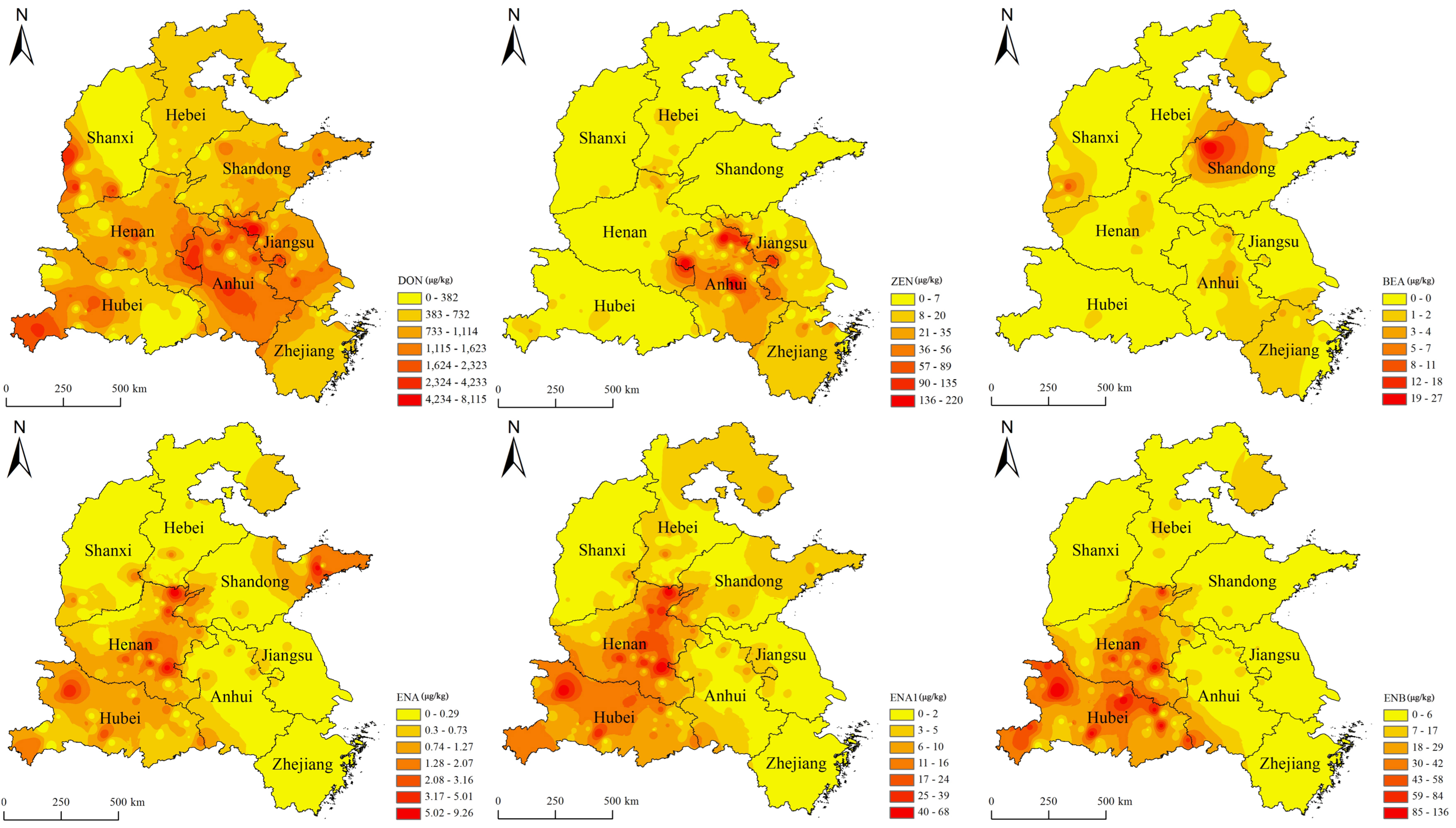
Figure 1 depicts the spatial distribution of mycotoxins in wheat grain samples collected across China in 2021.
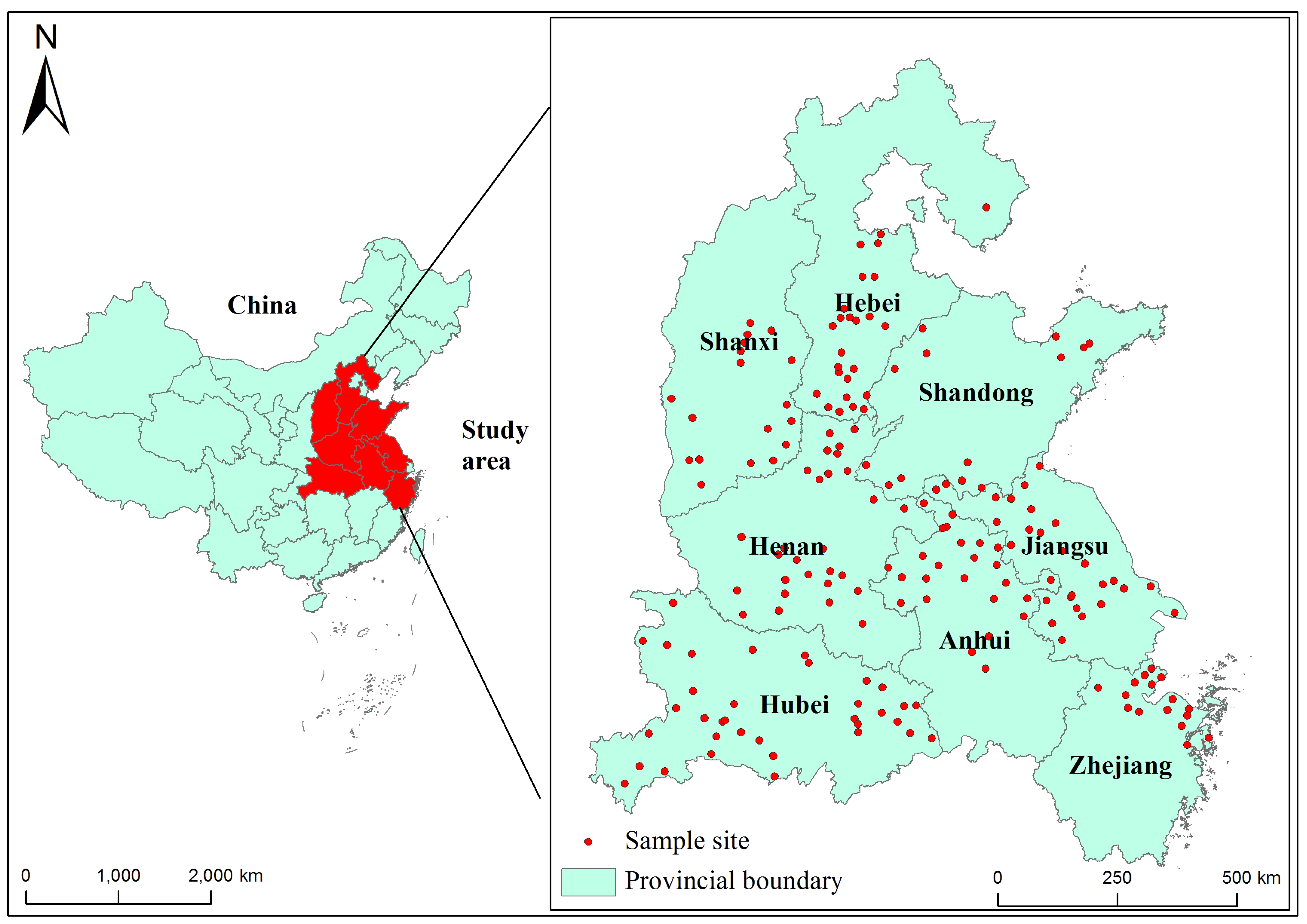
In this study, the wheat grain samples were collected from eight major wheat-producing areas in China, including four provinces in the north of the Huaihe River (e.g., Zhejaing, Jiangsu, Anhui, and Hubei provinces) and four provinces in the south of Huaihe River (e.g., Henan, Shandong, Shanxi, and Hebei). Huaihe River acts as the climatic boundary between the southern and northern regions of China, with the northern region of the river being a warm temperate zone and the southern region of the river being a subtropical zone [
33]. The level of contamination of wheat samples with mycotoxins has been observed to vary across different geographies in China. Regarding the detection rates of mycotoxins in wheat grains, the Fusarium toxins (i.e., DON, ZEN, BEA, ENA, ENA1, ENB, ENB1) were detected in the order from high to low as follows: Hubei (50.8%) > Henan (50.7%) > Hebei (47.5%) > Anhui > (46.3%) > Jiangsu (40.0%) > Zhejiang (34.8%) > Shandong (32.2%) > Shanxi (21.3%), while the Alternaria toxins (AME, AOH, TEN, TeA, and ALT) were detected in the order from high to low were as follows: Hubei (80.0%) > Hebei (76.3%) > Henan (75.3%) > Jiangsu (70.2%) > Shandong (67.8%) > Anhui (64.9%) > Zhejiang (58.5%) > Shanxi (53.6%).
According to the spatial analysis, Anhui showed the highest average of DON (1649 µg/kg) and ZEN (59.7 µg/kg), Shandong demonstrated the highest average of BEA (13.9 µg/kg) and TeA (537µg/kg), Henan showed the highest average of ENNs (23.6 µg/kg), Henan demonstrated the highest average of AME (7.18 µg/kg) and ALT (3.73 µg/kg), Hubei showed the highest average of TEN (80.1 µg/kg), and Shanxi demonstrated the highest average of AOH (25.9 µg/kg). A study reported that the top four regions for DON contamination in wheat grains are located mainly in southern China (e.g., Anhui, Zhejiang, and Jiangsu), which is consistent with our findings [
34]. The present study results provide new insights into the geographic profile of mycotoxins in wheat grains, especially for emerging toxins, suggesting that special attention must be paid to the contamination of wheat grain with certain toxins in different provinces in China and an effective management strategy must be implemented for controlling mycotoxin production.
2.4. Co-Occurrence of Mycotoxins in Wheat Grains
The co-occurrence of mycotoxins in wheat grains was statistically analyzed in this study.
Figure 2 shows the frequency of co-occurrence of mycotoxins in wheat grain samples. The co-occurrence of more than one toxin was detected in 99.7% (320/321) samples. The co-occurrence of seven toxins accounted for the largest proportion (17.4%), followed by that of eight and nine toxins (15.6%), ten toxins (11.8%), five toxins (10.6%), four toxins (10.3%), six toxins (9.66%), three toxins (5.30%), eleven toxins (2.18%), two toxins (0.935%), one toxin (0.312%), and twelve toxins (0.312%).
Table 6 summarizes the combinations of co-occurrence of regulated and emerging mycotoxins detected in the wheat grain samples. The top three combinations showing co-occurrence of a large number of mycotoxins were as follows: co-occurrence of ten toxins (DON + ZEN + ENA + ENA1 + ENB + ENB1 + AME + AOH + TeA + TEN), co-occurrence of nine toxins (DON + ENA + ENA1 + ENB + ENB1 + AME + AOH + TeA + TEN), and co-occurrence of eight toxins (DON + ZEN + ENB + ENB1 + AME + AOH + TeA + TEN). Blesa, Moltó, Akhdari, Mañes, and Zinedine (2014) reported that 51% of the wheat grains from Morocco were contaminated with two to six mycotoxins. The most frequent co-occurrence was noted with the combination ENA + ENA1 + ENB + ENB1 [
35]. Juan et al., (2013) reported that 81% of cereal samples were contaminated with more than one mycotoxin in Italy [
26].
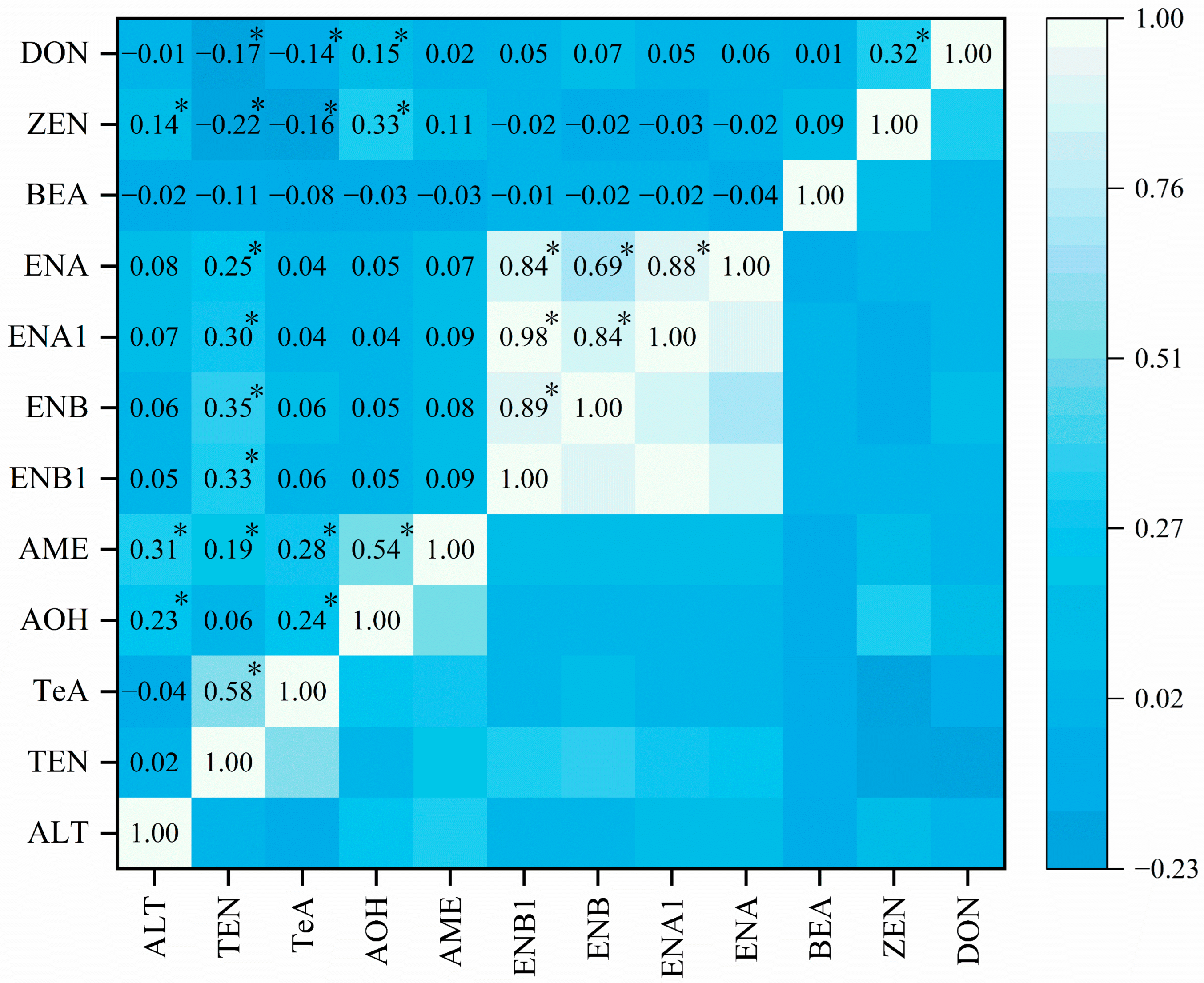
Correlation analysis was performed to determine the correlation among the mycotoxins detected in wheat grain samples (
Figure 3). DON and ZEN (
r = 0.32,
p < 0.05), AME and AOH (
r = 0.54,
p < 0.05), TeA and TEN (
r = 0.58,
p < 0.05), among ENNs (
r = 0.69–0.98,
p < 0.05) demonstrated significant linear regressions of correlation, which are in line with the results of previous studies [
8,
36]. The co-occurrence of mycotoxins is a serious concern to human health owing to the possible synergistic and additive effects induced by multi-mycotoxins [
37,
38]. Exploring the co-occurrence of mycotoxins in wheat grains can provide comprehensive information for human health risk assessment.
2.5. Risk Assessment
A deterministic approach was applied to estimate the dietary risk of natural mycotoxins detected in wheat grains to Chinese consumers aged 4–70 years. The mean concentrations of DON, ZEN, BEN, ENNs, AME, AOH, TeA, TEN, and ALT in the wheat grain samples were 881.7, 10.6, 0.377, 5.85, 3.33, 5.98, 331.9, 36.8, and 0.286 μg/kg, respectively. Regarding the loss of mycotoxins during the process of wheat to wheat-based products, the processing factor of 0.28 was applied for dietary exposure estimation in this study.
Table 7 summarizes the dietary exposure to twelve mycotoxins detected in wheat grain samples for different age subgroups in China. The dietary intake of DON through wheat consumption was in the range of 0.592–0.992 µg/kg b.w./day, not exceeding the PMTDI of 1 µg/kg b.w./day set by JECFA [
39], with the highest exposure found in children aged 4–7 years. The HQs of DON for different age groups among the Chinese population were less than 1, indicating a tolerable exposure level for Chinese consumers. Although DON is reported to be stable during processing such as heating, extrusion, cooking, brewing, or baking, physical removal techniques such as sorting, cleaning, and grinding are extremely effective in removing DON from food commodities [
40]. As reported by the FAO/WHO JECFA [
39], the dietary exposure to DON from wheat consumption was 5.3 µg/kg b.w./day for GEMS/Food Cluster B (Lebanon, United Arab Emirates, Cyprus, Greece, Israel, Italy, Portugal, Spain, and Turkey) and 9.13 µg/kg b.w./day for GEMS/Food Cluster M (the United States and Canada), which are higher than the PMTDI of 1 µg/kg b.w./day [
39]. This dietary exposure to DON from wheat consumption ranged from 2.11 to 3.54 µg/kg b.w./day, exceeding the PMTDI value (1 µg/kg b.w./day), when processing factors were not considered, which is consistent with the FAO/WHO JECFA report. As shown in
Table 7, the dietary intake of ZEN was 0.007–0.012 µg/kg bw/day, which is lower than the PMTDI of 0.5 µg/kg b.w./day [
41], with the corresponding HQs being far lower than 1, implying a tolerable health risk for Chinese consumers. In 2011, the EFSA reported that dietary exposure to ZEN through consumption of grains and grain-based food was acceptable for all age groups [
21]. However, it was reported that people of Rajasthan (a state in India) were exposed to three and six tenths to four-fold of TDI of ZEN through wheat consumption [
25].
BEA and ENNs are frequently detected in various foodstuffs, and cereal grains and cereal-based food products are the main contributors to their dietary exposure. BEA and ENNs exert cytotoxic effects, thereby affecting the cellular ionic homeostasis [
42]. In this study, dietary exposures to BEA and ENNs were evaluated in terms of the TCC value (0.025 µg/kg b.w./day for BEA and 1.5 µg/kg b.w./day for the sum of ENNs) as proposed by the EFSA Panel on Contaminants in the Food Chain (CONTAM) [
43] to obtain a rough estimation of the possible health risks to humans. Herein, the estimated levels of dietary exposure to BEA and ENNs were 0.0003–0.0004 μg/kg b.w./day and 0.004–0.007 μg/kg b.w./day, respectively, indicating acceptable health risks for the Chinese population. Similar results were reported in a study conducted in the Romanian population [
44]. The dietary exposure to BEA ranged from 0 μg/kg b.w./day (lower-bound scenario, LB) to 0.0053 μg/kg b.w./day (upper-bound scenario, UB), while the dietary exposure to ENNs ranged from 0.0312 μg/kg b.w./day (LB) to 0.0805 μg/kg b.w./day (UB) through the consumption of wheat samples. Although the results indicated that the chronic dietary exposure may not have adverse effects on human health, attention should be paid to the synergistic or additive effects of multi-mycotoxin co-occurrence in wheat grains. Further assessment should be conducted once data on toxicity-guided fractionation of the BEA and ENNs are available in the future.
Owing to the limited toxicological data available for Alternaria toxins, such as AME, AOH, TeA, and TEN, the TTC approach (0.0025 µg/kg b.w./day for AME and AOH; 1.5 µg/kg b.w./day for TeA and TEN) was adopted to assess the effects of these mycotoxins on human health [
45]. Regarding AME and AOH, the results of the dietary exposure estimates ranged from 0.003 to 0.007 µg/kg b.w./day, exceeding the TTC value of 0.0025 µg/kg b.w./day, with the corresponding HQs for AME and AOH being >1. Overall, the risk characterization results emphasized that special attention must be paid to AME and AOH contamination in wheat and additional toxicity data and occurrence data must be acquired for a detailed and accurate assessment of health risks. For TeA, the dietary exposure was estimated to be 0.223–0.373 µg/kg b.w./day (
Table 7), which is less than the TTC value of 1.5 µg/kg b.w./day, consistent with the reports published by EFSA suggesting that TeA is unlikely to be of human health concern [
21]. Compared with AME, AOH, and TeA, TEN has been relatively understudied in food commodities [
28]. In this study, the dietary exposure to TEN was in the range of 0.025 to 0.041 µg/kg b.w./day, which was lower than the TCC value of 1.5 µg/kg b.w./day, and the corresponding HQs were <1, indicating tolerable health risk for the Chinese population. The CONTAM Panel of EFSA also reported that TEN is unlikely to have adverse effects on human health [
21]. The dietary exposure assessment was not performed for ALT due to the lack of health reference values.
Notably, some uncertainties are involved in the dietary exposure assessment for mycotoxins among Chinese consumers. The dietary risk assessment conducted in this study was only a preliminary analysis due to limited data on the toxicological properties of the emerging BEA, ENNs, and Alternaria toxins. The present study did not evaluate the total dietary exposure but only determined the intake of mycotoxins from wheat grains. Generally, consumers are usually exposed to various chemical hazards with multi-exposure pathways in daily life, and therefore, the possible synergistic or additive effects of multi-mycotoxins or other toxic chemicals (e.g., pesticides or heavy metal) should not be ignored.