Immunomodulatory Effects of Cylindrospermopsin in Human T Cells and Monocytes
Abstract
1. Introduction
2. Results
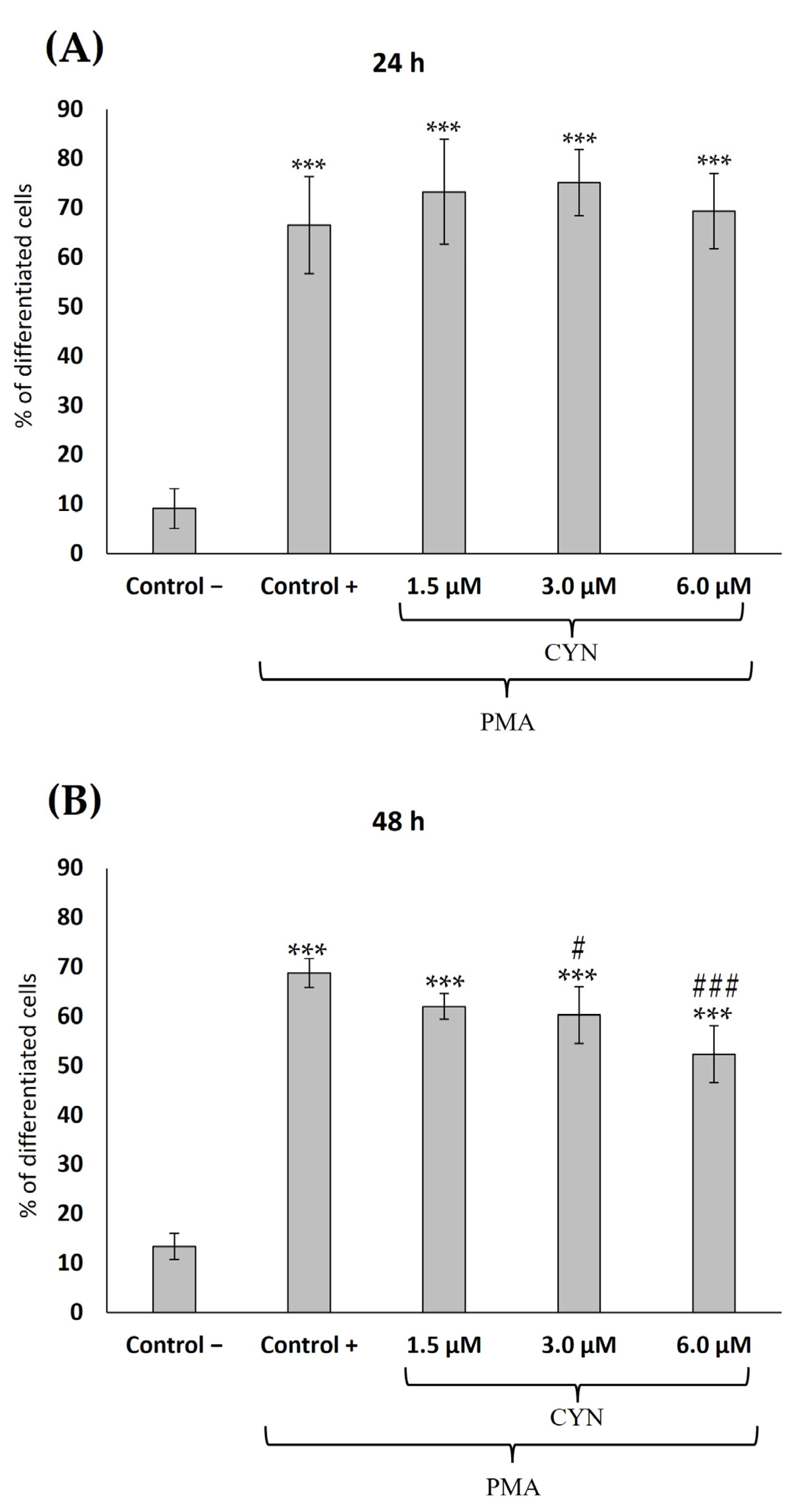
2.1. Cell Viability Determination and Influence of CYN on the Differentiation of THP-1 Monocytes into Macrophages
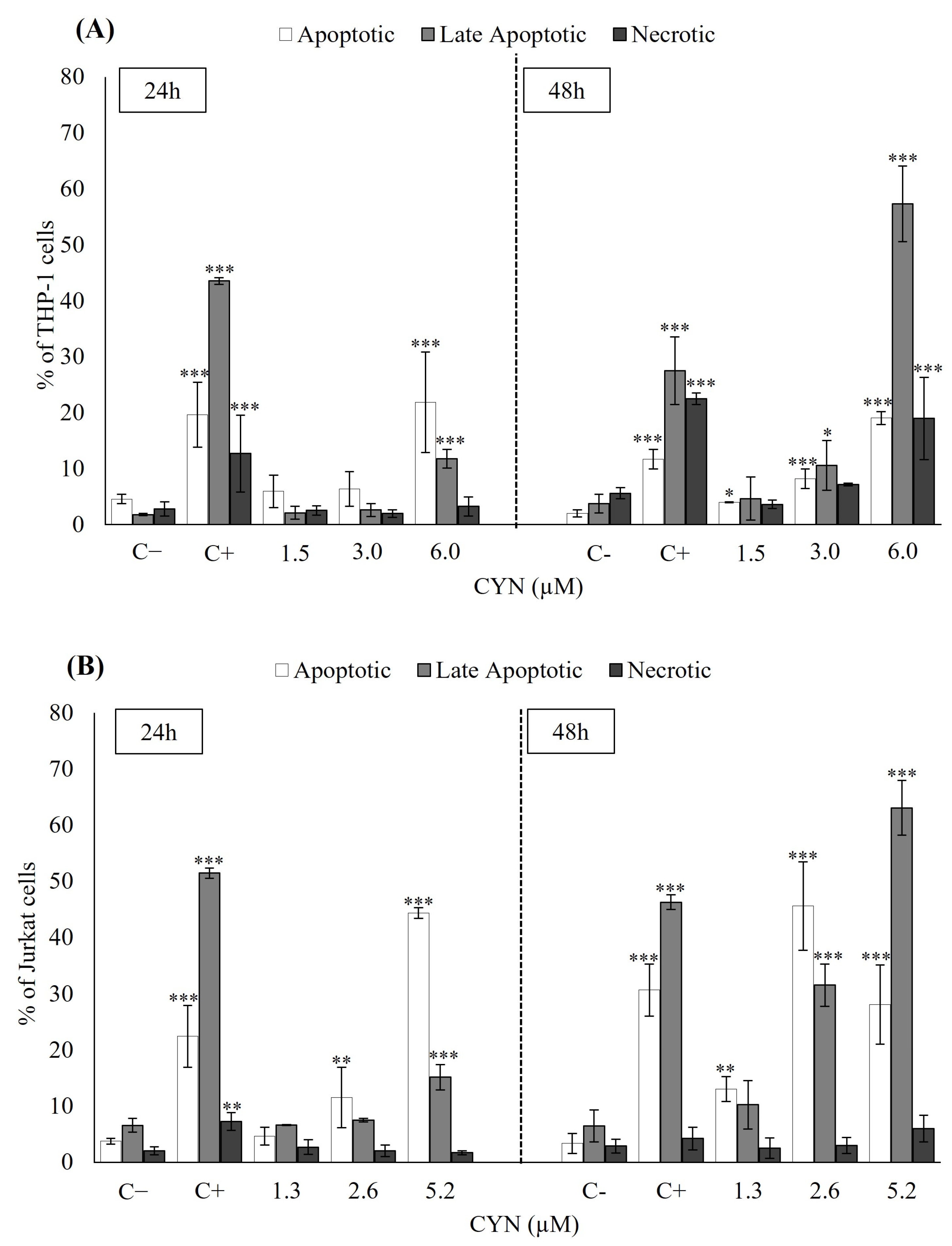
2.2. Influence of CYN on Cell Death Mechanisms (Apoptosis/Necrosis) by Flow Cytometry
2.3. Effects of CYN on the Different Cytokines’ Genetic Expression (mRNA Levels) by RT-qPCR
2.4. Effects of CYN on Cytokines Levels by ELISA
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemical and Reagents
5.2. Model System
5.3. Cytotoxicity and Differentiation
5.4. Flow Cytometry
5.5. Gene Expression
5.6. Cytokines Detection
5.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glibert, P.M. Harmful algae at the complex nexus of eutrophication and climate change. Harmful Algae 2020, 91, 101583. [Google Scholar] [CrossRef]
- Mehdizadeh Allaf, M.; Peerhossaini, H. Cyanobacteria: Model Microorganisms and Beyond. Microorganisms 2022, 10, 696. [Google Scholar] [CrossRef]
- Díez-Quijada, L.; Prieto, A.I.; Guzmán-Guillén, R.; Jos, A.; Cameán, A.M. Occurrence and toxicity of microcystin congeners other than MC-LR and MC-RR: A review. Food Chem. Toxicol. 2019, 125, 106–132. [Google Scholar] [CrossRef]
- Dulić, T.; Svirčev, Z.; Malešević, T.P.; Faassen, E.J.; Savela, H.; Hao, Q.; Meriluoto, J. Assessment of Common Cyanotoxins in Cyanobacteria of Biological Loess Crusts. Toxins 2022, 14, 215. [Google Scholar] [CrossRef]
- Weralupitiya, C.; Wanigatunge, R.P.; Gunawardana, D.; Vithanage, M.; Magana-Arachchi, D. Cyanotoxins uptake and accumulation in crops: Phytotoxicity and implications on human health. Toxicon 2022, 211, 21–35. [Google Scholar] [CrossRef]
- Hercog, K.; Štampar, M.; Štern, A.; Filipič, M.; Žegura, B. Application of advanced HepG2 3D cell model for studying genotoxic activity of cyanobacterial toxin cylindrospermopsin. Environ. Pollut. 2020, 265, 114965. [Google Scholar] [CrossRef]
- World Health Organization. Cyanobacterial Toxins: Cylindrospermopsins. Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/handle/10665/338063 (accessed on 4 April 2023).
- Puerto, M.; Prieto, A.I.; Maisanaba, S.; Gutiérrez-Praena, D.; Mellado-García, P.; Jos, A.; Cameán, A.M. Mutagenic and genotoxic potential of pure Cylindrospermopsin by a battery of in vitro test. Food Chem. Toxicol. 2018, 121, 413–422. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, G.; Chen, Y.; Jia, N.; Li, R. Four decades of progress in cylindrospermopsin research: The ins and outs of a potent cyanotoxin. J. Hazard. Mater. 2021, 406, 124653. [Google Scholar] [CrossRef]
- Casas-Rodriguez, A.; Cameán, A.M.; Jos, A. Potential Endocrine Disruption of Cyanobacterial Toxins, Microcystins and Cylindrospermopsin: A Review. Toxins 2022, 14, 882. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Xiao, G.; Chen, G.; Han, L.; Hu, T. Adverse effect of cylindrospermopsin on embryonic development in zebrafish (Danio rerio). Chemosphere 2020, 241, 125060. [Google Scholar] [CrossRef]
- De la Cruz, A.A.; Chernoff, N.; Sinclair, J.L.; Hill, D.; Diggs, D.L.; Lynch, A.T. Introduction to Cyanobacteria and Cyanotoxins. In Water Treatment for Purification from Cyanobacteria and Cyanotoxins, 1st ed.; Hiskia, A.E., Triantis, T.M., Antoniou, M.G., Kaloudis, T., Dionysiou, D.D., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 1–35. ISBN 978-1-118-92861-5. [Google Scholar]
- Puerto, M.; Jos, A.; Pichardo, S.; Gutiérrez-Praena, D.; Cameán, A.M. Acute effects of pure cylindrospermopsin on the activity and transcription of antioxidant enzymes in tilapia (Oreochromis niloticus) exposed by gavage. Ecotoxicology 2011, 20, 1852–1860. [Google Scholar] [CrossRef]
- Poniedziałek, B.; Rzymski, P.; Wiktorowicz, K. First report of cylindrospermopsin effect on human peripheral blood lymphocytes proliferation in vitro. Cent. Eur. J. Immunol. 2012, 37, 314–317. [Google Scholar] [CrossRef]
- Poniedziałek, B.; Rzymski, P.; Wiktorowicz, K. Toxicity of cylindrospermopsin in human lymphocytes: Proliferation, viability and cell cycle studies. Toxicol. Vitr. 2014, 28, 968–974. [Google Scholar] [CrossRef]
- Diez-Quijada, L.; Benítez-González, M.D.M.; Puerto, M.; Jos, A.; Cameán, A.M. Immunotoxic Effects Induced by Microcystins and Cylindrospermopsin: A Review. Toxins 2021, 13, 711. [Google Scholar] [CrossRef]
- Poniedziałek, B.; Rzymski, P.; Kokociński, M.; Karczewski, J. Toxic potencies of metabolite(s) of non-cylindrospermopsin producing Cylindrospermopsis raciborskii isolated from temperate zone in human white cells. Chemosphere 2015, 120, 608–614. [Google Scholar] [CrossRef]
- Poniedziałek, B.; Rzymski, P.; Karczewski, J. The role of the enzymatic antioxidant system in cylindrospermopsin-induced toxicity in human lymphocytes. Toxicol. Vitr. 2015, 29, 926–932. [Google Scholar] [CrossRef]
- Žegura, B.; Gajski, G.; Štraser, A.; Garaj-Vrhovac, V. Cylindrospermopsin induced DNA damage and alteration in the expression of genes involved in the response to DNA damage, apoptosis and oxidative stress. Toxicon 2011, 58, 471–479. [Google Scholar] [CrossRef]
- Poniedziałek, B.; Rzymski, P.; Karczewski, J. Cylindrospermopsin decreases the oxidative burst capacity of human neutrophils. Toxicon 2014, 87, 113–119. [Google Scholar] [CrossRef]
- Sieroslawska, A.; Rymuszka, A. Effects of cylindrospermopsin on a common carp leucocyte cell line. J. Appl Toxicol. 2015, 35, 83–89. [Google Scholar] [CrossRef]
- Sieroslawska, A.; Rymuszka, A.; Adaszek, Ł. Effects of cylindrospermopsin on the phagocytic cells of the common carp (Cyprinus carpio L.). J. Appl Toxicol. 2015, 35, 1406–1414. [Google Scholar] [CrossRef]
- Takser, L.; Benachour, N.; Husk, B.; Cabana, H.; Gris, D. Cyanotoxins at low doses induce apoptosis and inflammatory effects in murine brain cells: Potential implications for neurodegenerative diseases. Toxicol. Rep. 2016, 3, 180–189. [Google Scholar] [CrossRef]
- Moosova, Z.; Pekarova, M.; Sindlerova, L.S.; Vasicek, O.; Kubala, L.; Blaha, L.; Adamovsky, O. Immunomodulatory effects of cyanobacterial toxin cylindrospermopsin on innate immune cells. Chemosphere 2019, 226, 439–446. [Google Scholar] [CrossRef]
- Ibrahim, M.A.A. Cell Biology of the Immune System. In Goodman’s Medical Cell Biology, 4th ed.; Goodman, S.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 337–360. ISBN 9780128179277. [Google Scholar]
- Yasin, Z.N.; Mohd Idrus, F.N.; Hoe, C.H.; Yvonne-Tee, G.B. Macrophage polarization in THP-1 cell line and primary monocytes: A systematic review. Differentiation 2022, 128, 67–82. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Yunus, M.A.; Ramli, M.M.; Osman, N.H.; Mohamed, R. Stimulation of Innate and Adaptive Immune Cells with Graphene Oxide and Reduced Graphene Oxide Affect Cancer Progression. Arch. Immunol. Ther. Exp. 2021, 69, 20. [Google Scholar] [CrossRef]
- Abraham, R.T.; Weiss, A. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nat. Rev. Immunol. 2004, 4, 301–308. [Google Scholar] [CrossRef]
- Chen, J.L.; Nong, G.M. Advances in application of Jurkat cell model in research on infectious diseases. Zhongguo dang dai er ke za zhi = Chin. J. Contemp. Pediatr. 2018, 20, 236–242. [Google Scholar] [CrossRef]
- Jarczak, D.; Nierhaus, A. Cytokine Storm-Definition, Causes, and Implications. Int. J. Mol. Sci. 2022, 23, 11740. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Sieroslawska, A.; Rymuszka, A. Cylindrospermopsin induces oxidative stress and genotoxic effects in the fish CLC cell line. J. Appl. Toxicol. 2014, 35, 426–433. [Google Scholar] [CrossRef]
- USEPA. Health Effects Support Document for the Cyanobacterial Toxin Cylindrospermopsin; USEPA: Washington, DC, USA, 2015. [Google Scholar]
- Schmeits, P.C.; Volger, O.L.; Zandvliet, E.T.; van Loveren, H.; Peijnenburg, A.A.; Hendriksen, P.J. Assessment of the usefulness of the murine cytotoxic T cell line CTLL-2 for immunotoxicity screening by transcriptomics. Toxicol. Lett. 2013, 217, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Manyes, L.; Escrivá, L.; Ruiz, M.J.; Juan-García, A. Beauvericin and enniatin B effects on a human lymphoblastoid Jurkat T-cell model. Food Chem. Toxicol. 2018, 115, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Solhaug, A.; Karlsøen, L.M.; Holme, J.A.; Kristoffersen, A.B.; Eriksen, G.S. Immunomodulatory effects of individual and combined mycotoxins in the THP-1 cell line. Toxicol. Vitro 2016, 36, 120–132. [Google Scholar] [CrossRef]
- Bain, P.A. Gene Expression Profiling of Cylindrospermopsin Toxicity. Ph.D. Thesis, Griffith University, Nathan, Australia, 2007. Available online: https://research-repository.griffith.edu.au/handle/10072/367068?show=full (accessed on 15 March 2023). School of Biomolecular and Physical Sciences.
- Aceves, M.; Dueñas, A.; Gómez, C.; San Vicente, E.; Crespo, M.S.; García-Rodríguez, C. A new pharmacological effect of salicylates: Inhibition of NFAT-dependent transcription. J. Immunol. 2004, 173, 5721–5729. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhao, X.; Liu, Y.; Shi, Q.; Hua, Z.; Shen, P. Analysis of immunomodulating nitric oxide, iNOS and cytokines mRNA in mouse macrophages induced by microcystin-LR. Toxicology 2004, 197, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Lankoff, A.; Carmichael, W.W.; Grasman, K.A.; Yuan, M. The uptake kinetics and immunotoxic effects of microcystin-LR in human and chicken peripheral blood lymphocytes in vitro. Toxicology 2004, 204, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Hajighasemi, F.; Mirshafiey, A. In vitro Effects of Propranolol on T Helper Type 1 Cytokine Profile in Human Leukemic T Cells. Int. J. Hematol. Oncol. Stem Cell Res. 2016, 10, 99–105. [Google Scholar]
- Adamovsky, O.; Moosova, Z.; Pekarova, M.; Basu, A.; Babica, P.; Sindlerova, L.S.; Kubala, L.; Blaha, L. Immunomodulatory potency of microcystin, an important water-polluting cyanobacterial toxin. Environ. Sci. Technol. 2015, 49, 12457–12464. [Google Scholar] [CrossRef]
- Diez-Quijada, L.; Casas-Rodriguez, A.; Guzmán-Guillén, R.; Molina-Hernández, V.; Albaladejo, R.G.; Cameán, A.M.; Jos, A. Immunomodulatory Effects of Pure Cylindrospermopsin in Rats Orally Exposed for 28 Days. Toxins 2022, 14, 144. [Google Scholar] [CrossRef]
- Daigneault, M.; Preston, J.A.; Marriott, H.M.; Whyte, M.K.; Dockrell, D.H. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS ONE 2010, 5, e8668. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.; Rosner, H.; Rohrmann, B.; Erler, W.; Geschwend, G.; Gräfe, U.; Burkert, B.; Möller, U.; Diller, R.; Sachse, K.; et al. Effects of the mycotoxin ochratoxin A and some of its metabolites on the human cell line THP-1. Toxicology 2003, 184, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ma, C.; Gong, L.; Guo, Y.; Fu, K.; Zhang, Y.; Zhou, H.; Li, Y. Macrophage Polarization and Its Role in Liver Disease. Front. Immunol. 2021, 12, 803037. [Google Scholar] [CrossRef] [PubMed]
- Pichardo, S.; Jos, A.; Zurita, J.L.; Salguero, M.; Cameán, A.M.; Repetto, G. Acute and subacute toxic effects produced by microcystin-YR on the fish cell lines RTG-2 and PLHC-1. Toxicol. Vitr. 2007, 21, 1460–1467. [Google Scholar] [CrossRef] [PubMed]


| THP-1 | 4 h | 24 h |
|---|---|---|
| Mean ± SD (pg/mL) | ||
| TNF-α | ||
| C− | 11.86 ± 0.38 | 35.72 ± 1.20 |
| C+ (LPS 10 ng/mL) | 139.67 ± 6.29 *** | 554.39 ± 4.17 *** |
| CYN | 17.45 ± 1.20 * | 37.41 ± 2.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casas-Rodríguez, A.; Cebadero-Dominguez, Ó.; Puerto, M.; Cameán, A.M.; Jos, A. Immunomodulatory Effects of Cylindrospermopsin in Human T Cells and Monocytes. Toxins 2023, 15, 301. https://doi.org/10.3390/toxins15040301
Casas-Rodríguez A, Cebadero-Dominguez Ó, Puerto M, Cameán AM, Jos A. Immunomodulatory Effects of Cylindrospermopsin in Human T Cells and Monocytes. Toxins. 2023; 15(4):301. https://doi.org/10.3390/toxins15040301
Chicago/Turabian StyleCasas-Rodríguez, Antonio, Óscar Cebadero-Dominguez, María Puerto, Ana María Cameán, and Angeles Jos. 2023. "Immunomodulatory Effects of Cylindrospermopsin in Human T Cells and Monocytes" Toxins 15, no. 4: 301. https://doi.org/10.3390/toxins15040301
APA StyleCasas-Rodríguez, A., Cebadero-Dominguez, Ó., Puerto, M., Cameán, A. M., & Jos, A. (2023). Immunomodulatory Effects of Cylindrospermopsin in Human T Cells and Monocytes. Toxins, 15(4), 301. https://doi.org/10.3390/toxins15040301







