Exploring the Profile of Cell Populations and Soluble Immunological Mediators in Bothrops atrox Envenomations
Abstract
1. Introduction
2. Results
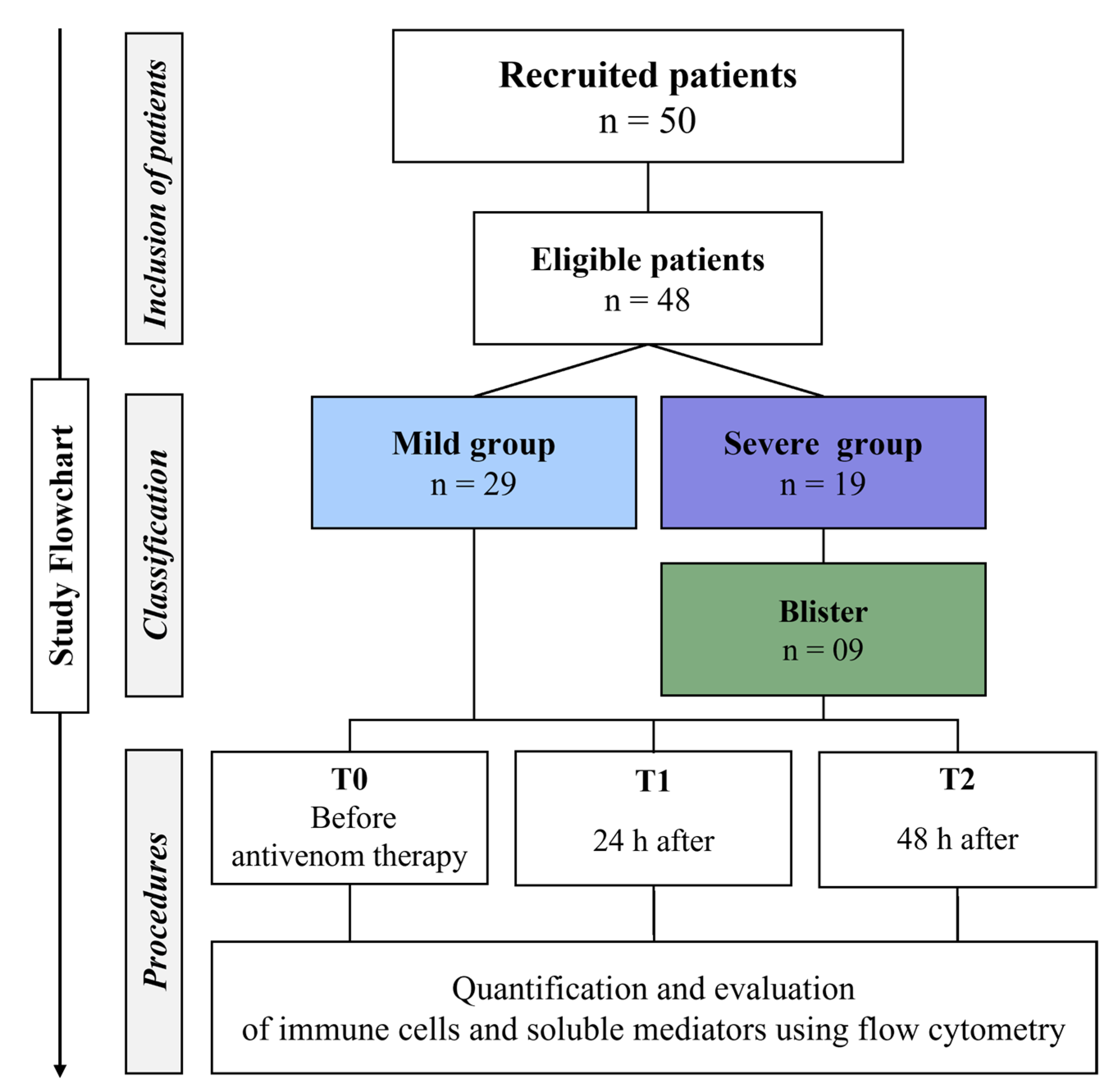
2.1. Characterization of the Study Population and Laboratory Data
2.2. Blood Count and Immunophenotyping Data in Bothrops atrox Snakebite Patients, Stratified into Mild and Severe Groups, before and after the Administration of Antivenom
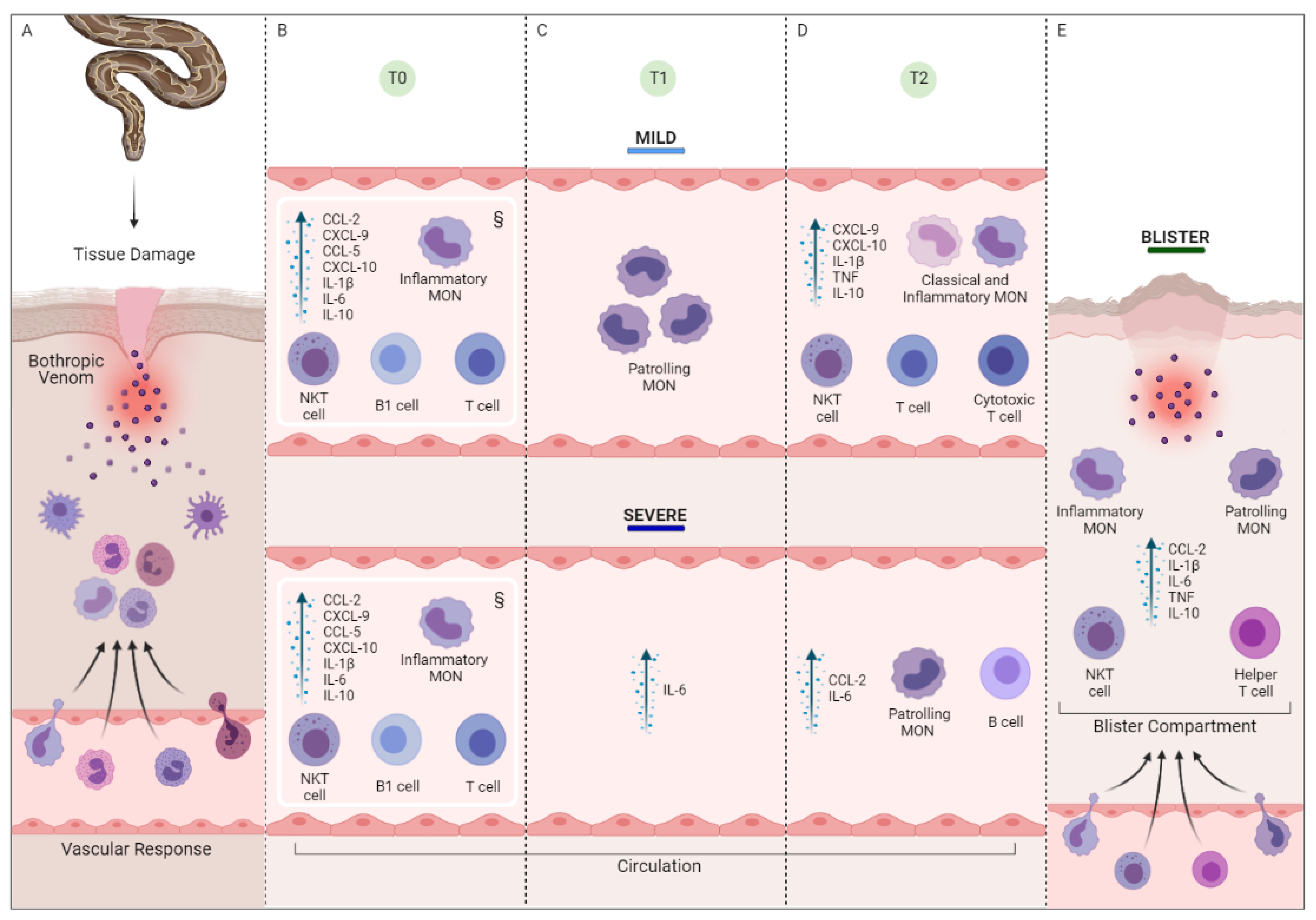
2.3. Snakebite Patients Are Marked by an Inflammatory Profile That Is Driven by a Substantial Increase in Immunological Mediators
2.4. Follow-Up of the Clinical Course of Mild and Severe Bothrops atrox Snakebite Patients Demonstrated Distinct Immunological Profiles at T2
2.5. Snakebite Patients Exhibit Complex Integrative Networks and Interactions between the Elements of Peripheral Blood and Blister Exudate
2.6. Evaluation of Blister Exudate in Severe Bothrops atrox Snakebite Patients Revealed Cellular Infiltration and Inflammatory Status
2.7. Snakebite Patients Exhibit Complex Integrative Networks and Interactions among the Elements of Peripheral Blood and Blister Exudate
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Population and Design
5.2. Ethical Issues
5.3. Collection of Biological Samples and Acquisition of Clinical and Laboratory Data
5.4. Immunophenotypic Characterization and Analysis
5.5. Quantification of the Soluble Immunological Mediators
5.6. Conventional Statistical Analysis
5.7. Correlation Network Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Ethical Approvals and Consent to Participate
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Snakebite Envenoming; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Williams, D.J.; Faiz, M.A.; Abela-Ridder, B.; Ainsworth, S.; Bulfone, T.C.; Nickerson, A.D.; Habib, A.G.; Junghanss, T.; Fan, H.W.; Turner, M.; et al. Strategy for a globally coordinated response to a priority neglected tropical disease: Snakebite envenoming. PLoS Negl. Trop. Dis. 2019, 13, 12–14. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, S.S.; de Sampaio, V.; de Sachett, J.; Alves, E.C.; da Silva, V.C.; de Lima, J.A.A.; da Silva, I.M.; de Ferreira, L.C.; Fan, H.W.; Lacerda, M.V.G.; et al. Snakebites in the Brazilian Amazon: Current Knowledge and Perspectives. In Clinical Toxinology; Academia: San Francisco, CA, USA, 2017; p. 22. ISBN 9789400762886. [Google Scholar]
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, E.S.; Sampaio, V.; Sachett, J.; Castro, D.B.; Noronha, M.D.; Lozano, J.L.; Muniz, E.; Ferreira, L.C.; Lacerda, M.V.; Monteiro, W.M. Snakebites as a largely neglected problem in the brazilian amazon: Highlights of the epidemiological trends in the state of amazonas. Rev. Soc. Bras. Med. Trop. 2015, 48, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Ministério da Saúde Acidentes de trabalho por animais peçonhentos entre trabalhadores do campo, floresta e águas, Brasil 2007 a 2017. Bol. Epidemiol. 2019, 50, 1–50. Available online: http://portalarquivos2.saude.gov.br/images/pdf/2019/marco/29/2018-059.pdf (accessed on 10 January 2023).
- Roriz, K.R.; Zaqueo, K.D.; Setubal, S.S.; Katsuragawa, T.H.; Silva, R.R.; Fernandes, C.F.; Cardoso, L.A.; Rodrigues, M.M.; Soares, A.M.; Stábeli, R.G.; et al. Epidemiological study of snakebite cases in Brazilian western Amazonia. Rev. Soc. Bras. Med. Trop. 2018, 51, 338–346. [Google Scholar] [CrossRef] [PubMed]
- SINAN. Sistema de Informação de Agravos de Notificação—Notificação de Acidentes Botrópicos no Brasil Segundo Região. Available online: https://portalsinan.saude.gov.br/ (accessed on 17 February 2023).
- Neiva, M.; Arraes, F.B.; de Souza, J.V.; Radis-Baptista, G.; da Silva, A.R.; Walter, M.E.; de Macedo Brigido, M.; Yamane, T.; Lopez-Lozano, J.L.; Astolfi-Filho, S. Transcriptome analysis of the Amazonian viper Bothrops atrox venom gland using expressed sequence tags (ESTs). Toxicon 2009, 53, 427–436. [Google Scholar] [CrossRef]
- Sousa, L.F.; Portes-Junior, J.A.; Nicolau, C.A.; Bernardoni, J.L.; Nishiyama-Jr, M.Y.; Amazonas, D.R.; Freitas-de-Sousa, L.A.; Mourão, R.H.; Chalkidis, H.M.; Valente, R.H.; et al. Functional proteomic analyses of Bothrops atrox venom reveals phenotypes associated with habitat variation in the Amazon. J. Proteomics 2017, 159, 32–46. [Google Scholar] [CrossRef]
- Rucavado, A.; Nicolau, C.A.; Escalante, T.; Kim, J.; Herrera, C.; Gutiérrez, J.M.; Fox, J.W. Viperid envenomationwound exudate contributes to increased vascular permeability via a DAMPs/TLR-4 mediated pathway. Toxins 2016, 8, 349. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Escalante, T.; Rucavado, A.; Herrera, C.; Fox, J.W. A comprehensive view of the structural and functional alterations of extracellular matrix by snake venom metalloproteinases (SVMPs): Novel perspectives on the pathophysiology of envenoming. Toxins 2016, 8, 304. [Google Scholar] [CrossRef]
- Jiménez, N.; Escalante, T.; Gutiérrez, J.M.; Rucavado, A. Skin pathology induced by snake venom metalloproteinase: Acute damage, revascularization, and re-epithelization in a mouse ear model. J. Invest. Dermatol. 2008, 128, 2421–2428. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Escalante, T.; Rucavado, A.; Herrera, C. Hemorrhage Caused by Snake Venom Metalloproteinases: A Journey of Discovery and Understanding. Toxins 2016, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Menaldo, D.L.; Bernardes, C.P.; Jacob-ferreira, A.L.; Nogueira-santos, C.G.; Casare-ogasawara, T.M.; Pereira-crott, L.S.; Sampaio, S.V. Effects of Bothrops atrox venom and two isolated toxins on the human complement system: Modulation of pathways and generation of anaphylatoxins. Mol. Immunol. 2016, 80, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Rucavado, A.; Núñez, J.; Gutiérrez, J.M. Blister formation and skin damage induced by BaP1, a haemorrhagic metalloproteinase from the venom of the snake Bothrops asper. Int. J. Exp. Pathol. 1998, 79, 245–254. [Google Scholar]
- Herrera, C.; Escalante, T.; Voisin, M.B.; Rucavado, A.; Morazán, D.; Macêdo, J.K.A.; Calvete, J.J.; Sanz, L.; Nourshargh, S.; Gutiérrez, J.M.; et al. Tissue Localization and Extracellular Matrix Degradation by PI, PII and PIII Snake Venom Metalloproteinases: Clues on the Mechanisms of Venom-Induced Hemorrhage. PLoS Negl. Trop. Dis. 2015, 9, e0003731. [Google Scholar] [CrossRef]
- De Brito Sousa, J.D.; de Oliveira, S.S.; Sachett, J.; Fan, H.W.; Monteiro, W.M. Low accuracy of microscopic hematuria in detecting coagulopathy from Bothrops pit viper bites, Brazilian Amazon. Clin. Toxicol. 2019, 57, 816–818. [Google Scholar] [CrossRef]
- De Cássia Mothé Escocard, R.; Kanashiro, M.M.; Petretski, J.H.; Azevedo-Silva, J.; Queiroz de Carvalho, E.C.; Dias da Silva, W.; Kipnis, T.L. Neutrophils regulate the expression of cytokines, chemokines and nitric oxide synthase/nitric oxide in mice injected with Bothrops atrox venom. Immunobiology 2006, 211, 37–46. [Google Scholar] [CrossRef]
- Alves, E.C.; Sachett, J.D.; Sampaio, V.S.; Sousa, J.D.; Oliveira, S.S.; Nascimento, E.F.; Santos, A.D.; da Silva, I.M.; da Silva, A.M.; Wen, F.H.; et al. Predicting acute renal failure in Bothrops snakebite patients in a tertiary reference center, Western Brazilian Amazon. PLoS ONE 2018, 13, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Baramova, E.N.; Shannon, J.D.; Bjarnason, J.B.; Fox, J.W. Degradation of extracellular matrix proteins by hemorrhagic metalloproteinases. Arch. Biochem. Biophys. 1989, 275, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Ownby, C.L. Skeletal muscle degeneration induced by venom phospholipases A 2: Insights into the mechanisms of local and systemic myotoxicity. Toxicon 2003, 42, 915–931. [Google Scholar] [CrossRef]
- Montecucco, C.; Gutiérrez, J.M.; Lomonte, B. Cellular pathology induced by snake venom phospholipase A2 myotoxins and neurotoxins: Common aspects of their mechanisms of action. Cell. Mol. Life Sci. 2008, 65, 2897–2912. [Google Scholar] [CrossRef]
- Wellmann, I.A.M.; Ibiapina, H.N.S.; Sachett, J.A.G.; Sartim, M.A.; Silva, I.M.; Oliveira, S.S.; Tarragô, A.M.; Moura-da-Silva, A.M.; Lacerda, M.V.G.; Ferreira, L.C.d.L.; et al. Correlating Fibrinogen Consumption and Profiles of Inflammatory Molecules in Human Envenomation’s by Bothrops atrox in the Brazilian Amazon. Front. Immunol. 2020, 11, 1874. [Google Scholar] [CrossRef] [PubMed]
- Moreira, V.; Dos-Santos, M.C.; Nascimento, N.G.; da Silva, H.B.; Fernandes, C.M.; D’Império Lima, M.R.; Teixeira, C. Local inflammatory events induced by Bothrops atrox snake venom and the release of distinct classes of inflammatory mediators. Toxicon 2012, 60, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Leon, G.; Sanchez, L.; Hernandez, A.; Villalta, M.; Herrera, M.; Segura, A.; Estrada, R.; Maria Gutierrez, J. Immune Response Towards Snake Venoms. Inflamm. Allergy Drug Targets 2012, 10, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Menaldo, D.L.; Bernardes, C.P.; Zoccal, K.F.; Jacob-ferreira, A.L.; Costa, T.R.; Del, M.P.F.M.; Naal, R.M.Z.G.; Frantz, F.G.; Faccioli, L.H.; Sampaio, S.V. Immune cells and mediators involved in the inflammatory responses induced by a P-I metalloprotease and a phospholipase A 2 from Bothrops atrox venom. Mol. Immunol. 2017, 85, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.; Cury, Y.; Moreira, V.; Picolo, G.; Chaves, F.; Picado, I.C.; Rica, U.D.C.; Jose, S.; Rica, C. Inflammation induced by Bothrops asper venom. Toxicon 2009, 54, 67–76. [Google Scholar] [CrossRef]
- Ibiapina, H.N.; Costa, A.G.; Sachett, J.A.; Silva, I.M.; Tarragô, A.M.; Neves, J.C.; Kerr, M.W.; Santana, M.F.; Martins-Filho, O.A.; Lacerda, M.V.; et al. An Immunological Stairway to Severe Tissue Complication Assembly in Bothrops atrox Snakebites. Front. Immunol. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Ministério da Saúde do Brasil. Guia de Vigilância em Saúde 3a Edição; Ministério da Saúde, Secretaria de Vigilância em Saúde, Coordenação-Geral de Desenvolvimento da Epidemiologia em Serviços: Brasilia, Brasil, 2019; p. 741.
- Da Silva, I.M.; Tavares, A.M. Comparative evaluation of adverse effects in the use of powder trivalent antivenom and liquid antivenoms in Bothrops snake bites. Rev. Soc. Bras. Med. Trop. 2012, 45, 523–525. [Google Scholar] [CrossRef]
- Zychar, B.; Dale, C.; Dermarchi, D.; Gonçalves, L. Contribution of metalloproteases, serine proteases and phospholipases A 2 to the inflammatory reaction induced by Bothrops jararaca crude venom in mice. Toxicon 2010, 55, 227–234. [Google Scholar] [CrossRef]
- Hamilton, K.K.; Hattori, R.; Esmon, C.T.; Sims, P.J. Complement proteins C5b-9 induce vesiculation of the endothelial plasma membrane and expose catalytic surface for assembly of the prothrombinase enzyme complex. J. Biol. Chem. 1990, 265, 3809–3814. [Google Scholar] [CrossRef]
- Bhaumik, S.; Beri, D.; Tyagi, J.; Clarke, M.; Sharma, S.K.; Williamson, P.R.; Jagnoor, J. Outcomes in intervention research on snakebite envenomation: A systematic review. F1000Research 2022, 11, 628. [Google Scholar] [CrossRef]
- Nicoleti, A.F.; de Medeiros, C.R.; Duarte, M.R.; França, F.O.d.S. Comparison of Bothropoides jararaca bites with and without envenoming treated at the vital brazil hospital of the Butantan institute, state of São Paulo, Brazil. Rev. Soc. Bras. Med. Trop. 2010, 43, 657–661. [Google Scholar] [CrossRef]
- Santoro, M.L.; Sano-Martins, I.S.; Fan, H.W.; Cardoso, J.L.C.; Theakston, R.D.G.; Warrell, D.A. Haematological evaluation of patients bitten by the jararaca, Bothrops jararaca, in Brazil. Toxicon 2008, 51, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.L.C.; Fan, H.W.; Franca, F.O.S.; Jorge, M.T.; Leite, R.P.; Nishioka, S.A.; Avila, A.; Tomy, S.C.; Santoro, M.L. Randomized comparative trial of three antivenoms in the treatment of envenoming by lance-headed vipers (Bothrops jararaca) in Sao Paulo, Brazil. Quartely J. Med. 1993, 86, 315–325. [Google Scholar]
- Cardoso, J.L.; Haddad, V., Jr.; França, F.S.; Wen, F.H. Animais Peçonhentos no Brasil—Biologia, Clínica e Terapêutica dos Acidentes, 2nd ed.; Sarvier: São Paulo, Brasil, 2009. [Google Scholar]
- Maria Fernandes, C.; Regina Zamuner, S.; Pavan Zuliani, J.; Rucavado, A.; Maria Gutiérrez, J.; de Fátima Pereira Teixeira, C. Inflammatory effects of BaP1 a metalloproteinase isolated from Bothrops asper snake venom: Leukocyte recruitment and release of cytokines. Toxicon 2006, 47, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Suwansrinon, K.; Khow, O.; Mitmoonpitak, C.; Daviratanasilpa, S.; Chaiyabutr, N.; Sitprija, V. Effects of Russell’s viper venom fractions on systemic and renal hemodynamics. Toxicon 2007, 49, 82–88. [Google Scholar] [CrossRef]
- Ravindran, M.; Khan, M.A.; Palaniyar, N. Neutrophil extracellular trap formation: Physiology, pathology, and pharmacology. Biomolecules 2019, 9, 365. [Google Scholar] [CrossRef]
- Schofield, L.; Grau, G.E. Immunological processes in malaria pathogenesis. Nat. Rev. Immunol. 2005, 5, 722–735. [Google Scholar] [CrossRef]
- Qiu, Q.; Xiong, W.; Yang, C.; Dai, X.; Dan, X.; Yang, Z.; Jiao, Y.; Xiang, Y.; Liu, G.; Hardy, P. Lymphocyte-derived microparticles induce apoptosis of airway epithelial cells through activation of p38 MAPK and production of arachidonic acid. Apoptosis 2014, 19, 1113–1127. [Google Scholar] [CrossRef]
- Liew, P.X.; Kubes, P. The Neutrophil’s role during health and disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef]
- Köse, A.; Akdeniz, A.; Babus, S.B.; Göçmen, M.; Temel, G.O. The Usefulness of Platelet Distribution Width and Platelet Distribution Width to Lymphocyte Ratio in Predicting Severity and Outcomes in Patients with Snakebite. Wilderness Environ. Med. 2021, 32, 284–292. [Google Scholar] [CrossRef]
- Lomonte, B. Tissue Damage and Inflamation Induced by Snake Venons; Tryckt & Bunden: Goteborg, Sweden, 1994. [Google Scholar]
- Carneiro, A.S.; Ribeiro, O.G.; Cabrera, W.H.K.; Vorraro, F.; De Franco, M.; Ibañez, O.M.; Starobinas, N. Bothrops jararaca venom (BjV) induces differential leukocyte accumulation in mice genetically selected for acute inflammatory reaction: The role of host genetic background on expression of adhesion molecules and release of endogenous mediators. Toxicon 2008, 52, 619–627. [Google Scholar] [CrossRef]
- D’Amélio, F.; Vigerelli, H.; Kerkis, I. Bothrops moojeni venom and its components–An overview. J. Venom Res. 2021, 11, 26–33. [Google Scholar]
- Soehnlein, O.; Zernecke, A.; Eriksson, E.E.; Rothfuchs, A.G.; Pham, C.T.; Herwald, H.; Bidzhekov, K.; Rottenberg, M.E.; Weber, C.; Lindbom, L. Neutrophil secretion products pave the way for inflammatory monocytes. Blood 2008, 112, 1461–1471. [Google Scholar] [CrossRef]
- Pires, W.L.; Kayano, A.M.; de Castro, O.B.; Paloschi, M.V.; Lopes, J.A.; Boeno, C.N.; Pereira, S.D.; Antunes, M.M.; Rodrigues, M.M.; Stábeli, R.G.; et al. Lectin isolated from Bothrops jararacussu venom induces IL-10 release by TCD4+ cells and TNF-α release by monocytes and natural killer cells. J. Leukoc. Biol. 2019, 106, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Barraviera, B.; Lomonte, B.; Tarkowski, A.; Hanson, L.Å.; Meira, D.A. Acute-Phase Reactions, Including Cytokines, in Patients Bitten by Bothrops and Crotalus Snakes in Brazil. J. Venom. Anim. Toxins 1995, 1, 11–22. [Google Scholar] [CrossRef]
- De Vries, J.E. Immunosuppressive and anti-inflammatory properties of interleukin 10. Ann. Med. 1995, 27, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Farsky, S.H.P.; Walber, J.; Costa-Cruz, M.; Curry, Y.; Teixeira, C.F.P. Leukocyte response induced by Bothrops jararaca crude venom: In vivo and in vitro studies. Toxicon 1997, 35, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.C.F.; Najibe, H.I.S.; Magalhães-gama, F.; Almeida, J.; Sachett, G.; Coelho, K.F.; Alves, E.C.; Tarragô, A.M.; Carlos, L.; Ferreira, D.L.; et al. CCL-2 and CXCL-8: Potential Prognostic Biomarkers of Acute Kidney Injury after a Bothrops atrox Snakebite. Hindawi Mediat. Inflamm. 2022, 2022, 14. [Google Scholar] [CrossRef]
- Ministerio da Saúde. Manual de Diagnóstico e Tratamento de Acidentes por Animais Peçonhentos, 2nd ed.; Funasa, Ed.; Ministerio da Saúde: Brasília, Brasil, 2001. [Google Scholar]
 ) and severe (
) and severe ( ) groups, and in healthy donors (
) groups, and in healthy donors ( ). The frequency of the cell populations and the levels of the soluble immunological mediators were measured using flow cytometry and CBA, respectively. The results are presented using bar plots, with a linear scale. Statistical analyses were performed using the Mann–Whitney test. Significant differences are indicated by connecting lines and asterisks for p < 0.001 (***), p < 0.01 (**) or p < 0.05 (*).
). The frequency of the cell populations and the levels of the soluble immunological mediators were measured using flow cytometry and CBA, respectively. The results are presented using bar plots, with a linear scale. Statistical analyses were performed using the Mann–Whitney test. Significant differences are indicated by connecting lines and asterisks for p < 0.001 (***), p < 0.01 (**) or p < 0.05 (*).
 ) and severe (
) and severe ( ) groups, and in healthy donors (
) groups, and in healthy donors ( ). The frequency of the cell populations and the levels of the soluble immunological mediators were measured using flow cytometry and CBA, respectively. The results are presented using bar plots, with a linear scale. Statistical analyses were performed using the Mann–Whitney test. Significant differences are indicated by connecting lines and asterisks for p < 0.001 (***), p < 0.01 (**) or p < 0.05 (*).
). The frequency of the cell populations and the levels of the soluble immunological mediators were measured using flow cytometry and CBA, respectively. The results are presented using bar plots, with a linear scale. Statistical analyses were performed using the Mann–Whitney test. Significant differences are indicated by connecting lines and asterisks for p < 0.001 (***), p < 0.01 (**) or p < 0.05 (*).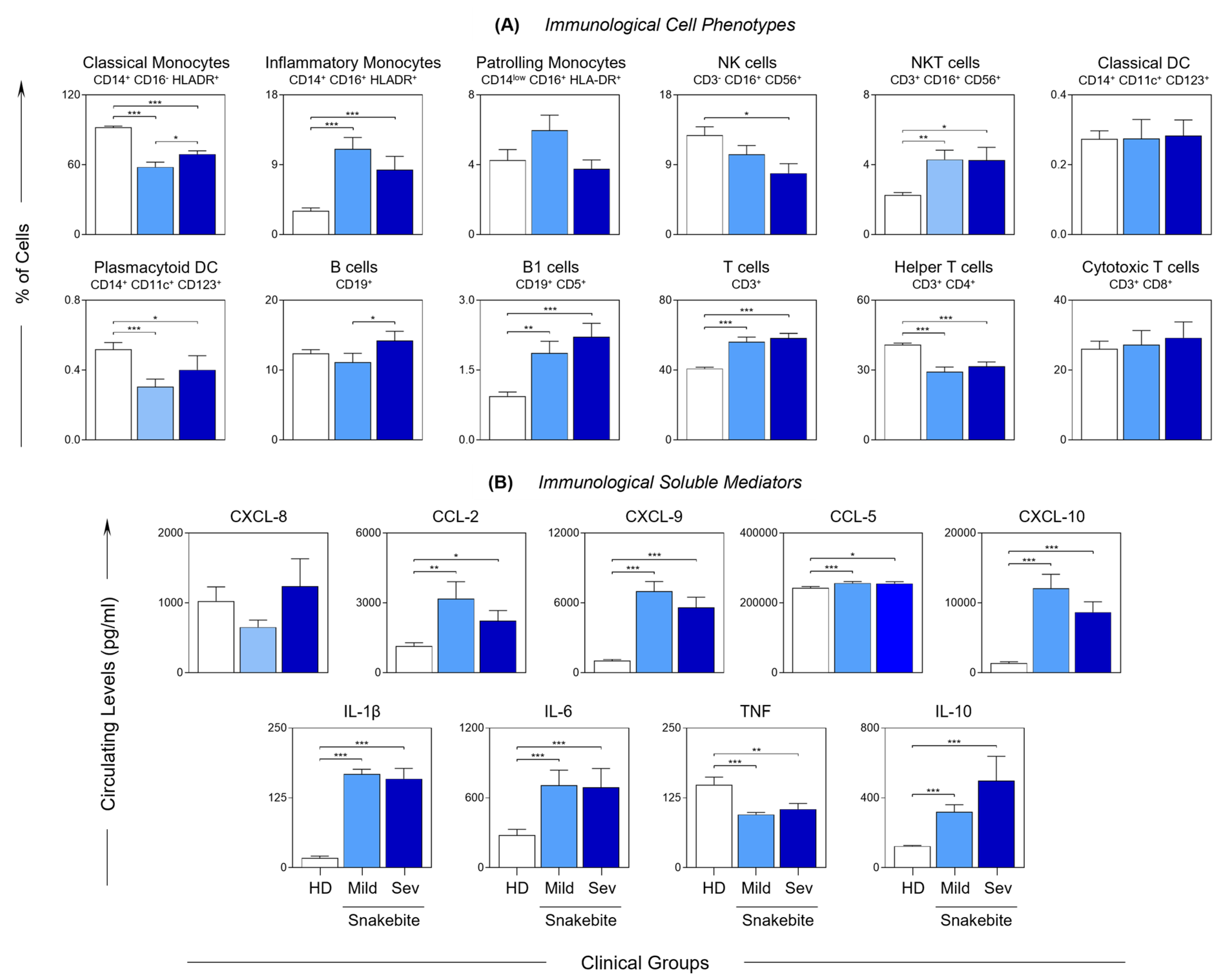
 ) and severe (
) and severe ( ) groups. The frequency of cell populations and the levels of soluble immunological mediators were measured using flow cytometry and CBA, respectively. The results are presented using bar plots, with a linear scale. The interquartile ranges (25–75°) for the results observed in the control group were used as a reference interval (gray background—
) groups. The frequency of cell populations and the levels of soluble immunological mediators were measured using flow cytometry and CBA, respectively. The results are presented using bar plots, with a linear scale. The interquartile ranges (25–75°) for the results observed in the control group were used as a reference interval (gray background— ). Statistical analyses were performed using the Mann–Whitney test. Significant differences are indicated by asterisks for p < 0.001 (***), p < 0.01 (**) and p < 0.05 (*).
). Statistical analyses were performed using the Mann–Whitney test. Significant differences are indicated by asterisks for p < 0.001 (***), p < 0.01 (**) and p < 0.05 (*).
 ) and severe (
) and severe ( ) groups. The frequency of cell populations and the levels of soluble immunological mediators were measured using flow cytometry and CBA, respectively. The results are presented using bar plots, with a linear scale. The interquartile ranges (25–75°) for the results observed in the control group were used as a reference interval (gray background—
) groups. The frequency of cell populations and the levels of soluble immunological mediators were measured using flow cytometry and CBA, respectively. The results are presented using bar plots, with a linear scale. The interquartile ranges (25–75°) for the results observed in the control group were used as a reference interval (gray background— ). Statistical analyses were performed using the Mann–Whitney test. Significant differences are indicated by asterisks for p < 0.001 (***), p < 0.01 (**) and p < 0.05 (*).
). Statistical analyses were performed using the Mann–Whitney test. Significant differences are indicated by asterisks for p < 0.001 (***), p < 0.01 (**) and p < 0.05 (*).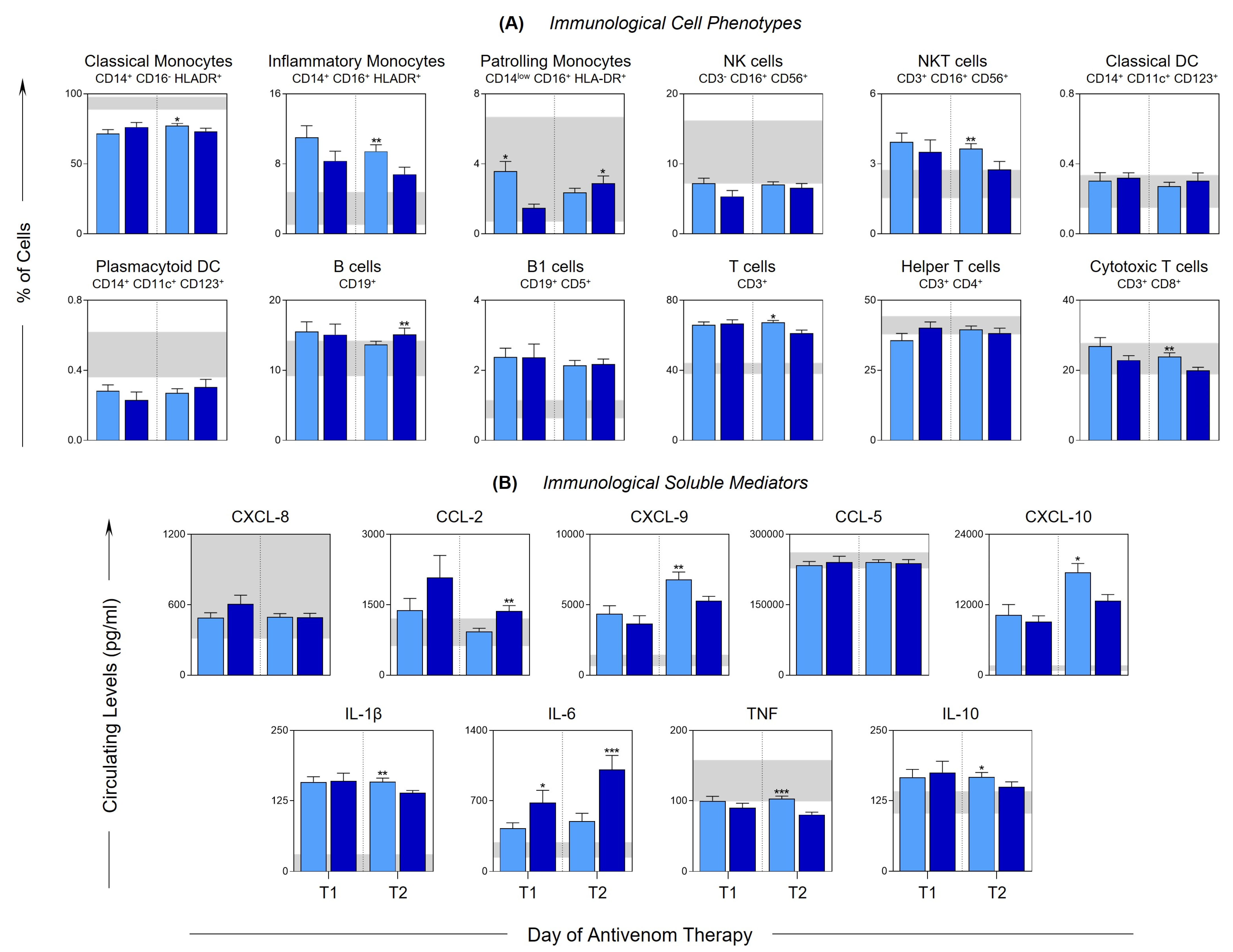
 ) are used to identify healthy donors, light blue nodes (
) are used to identify healthy donors, light blue nodes ( ) are used to identify the MILD group, while dark blue (
) are used to identify the MILD group, while dark blue ( ) is used to identify the SEVERE group. Solid lines between the elements indicate a positive correlation, while dashed lines indicate a negative correlation. The thickness of the lines indicates the strength of the correlation. The correlation index (r) was used to categorize the strength of the correlation as weak (r ≤ 0.35), moderate (r ≥ 0.36 to r ≤ 0.67) or strong (r ≥ 0.68).
) is used to identify the SEVERE group. Solid lines between the elements indicate a positive correlation, while dashed lines indicate a negative correlation. The thickness of the lines indicates the strength of the correlation. The correlation index (r) was used to categorize the strength of the correlation as weak (r ≤ 0.35), moderate (r ≥ 0.36 to r ≤ 0.67) or strong (r ≥ 0.68).
 ) are used to identify healthy donors, light blue nodes (
) are used to identify healthy donors, light blue nodes ( ) are used to identify the MILD group, while dark blue (
) are used to identify the MILD group, while dark blue ( ) is used to identify the SEVERE group. Solid lines between the elements indicate a positive correlation, while dashed lines indicate a negative correlation. The thickness of the lines indicates the strength of the correlation. The correlation index (r) was used to categorize the strength of the correlation as weak (r ≤ 0.35), moderate (r ≥ 0.36 to r ≤ 0.67) or strong (r ≥ 0.68).
) is used to identify the SEVERE group. Solid lines between the elements indicate a positive correlation, while dashed lines indicate a negative correlation. The thickness of the lines indicates the strength of the correlation. The correlation index (r) was used to categorize the strength of the correlation as weak (r ≤ 0.35), moderate (r ≥ 0.36 to r ≤ 0.67) or strong (r ≥ 0.68).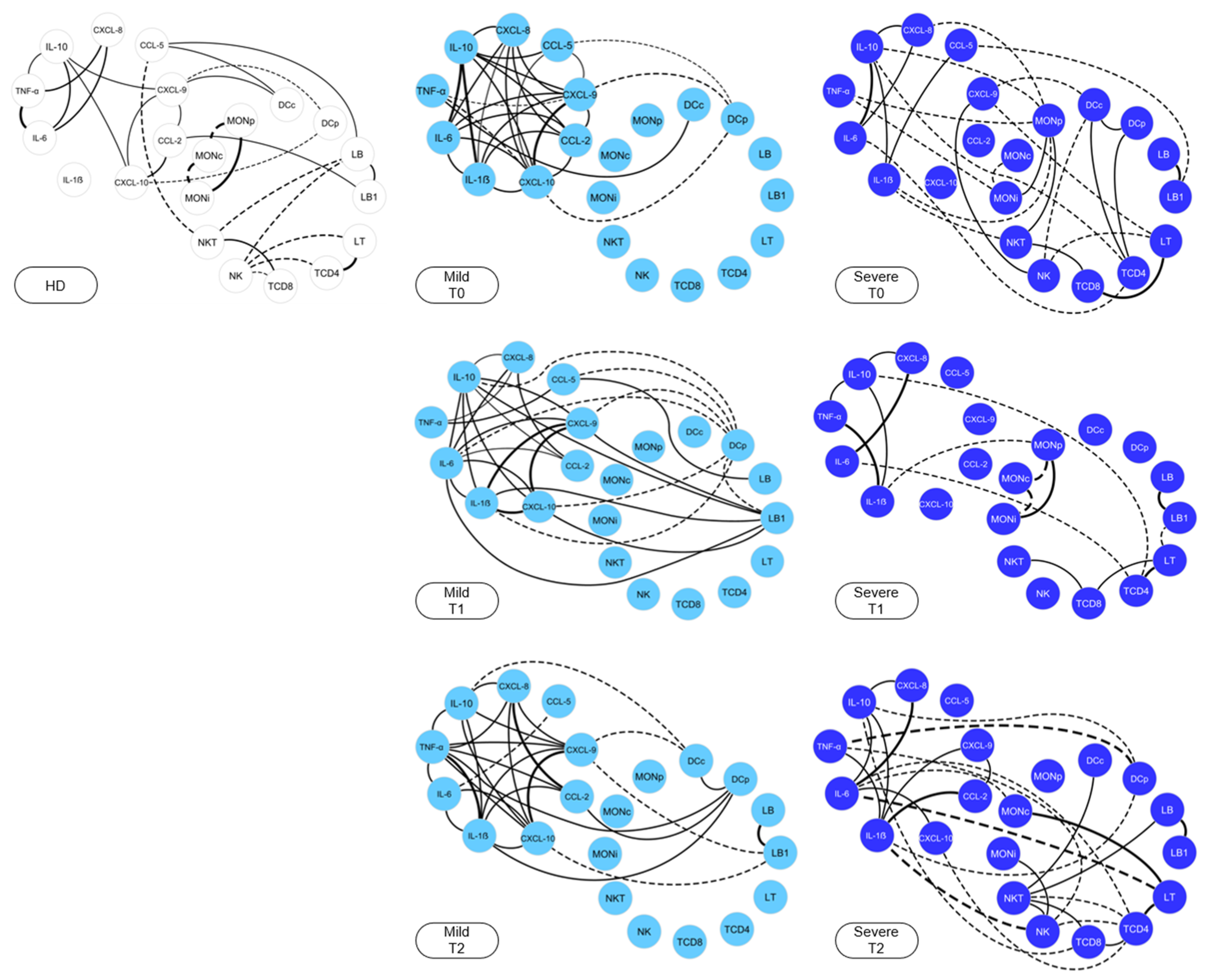
 ) and blister (
) and blister ( ) compartment of severe Bothrops snakebite patients. The frequency of cell populations and the levels of soluble immunological mediators were measured using flow cytometry and CBA, respectively. The results are presented using bar plots, with a linear scale. Statistical analyses were performed using Wilcoxon’s matched pairs signed rank test. Significant differences are indicated by asterisks for p < 0.01 (**) and p < 0.05 (*).
) compartment of severe Bothrops snakebite patients. The frequency of cell populations and the levels of soluble immunological mediators were measured using flow cytometry and CBA, respectively. The results are presented using bar plots, with a linear scale. Statistical analyses were performed using Wilcoxon’s matched pairs signed rank test. Significant differences are indicated by asterisks for p < 0.01 (**) and p < 0.05 (*).
 ) and blister (
) and blister ( ) compartment of severe Bothrops snakebite patients. The frequency of cell populations and the levels of soluble immunological mediators were measured using flow cytometry and CBA, respectively. The results are presented using bar plots, with a linear scale. Statistical analyses were performed using Wilcoxon’s matched pairs signed rank test. Significant differences are indicated by asterisks for p < 0.01 (**) and p < 0.05 (*).
) compartment of severe Bothrops snakebite patients. The frequency of cell populations and the levels of soluble immunological mediators were measured using flow cytometry and CBA, respectively. The results are presented using bar plots, with a linear scale. Statistical analyses were performed using Wilcoxon’s matched pairs signed rank test. Significant differences are indicated by asterisks for p < 0.01 (**) and p < 0.05 (*).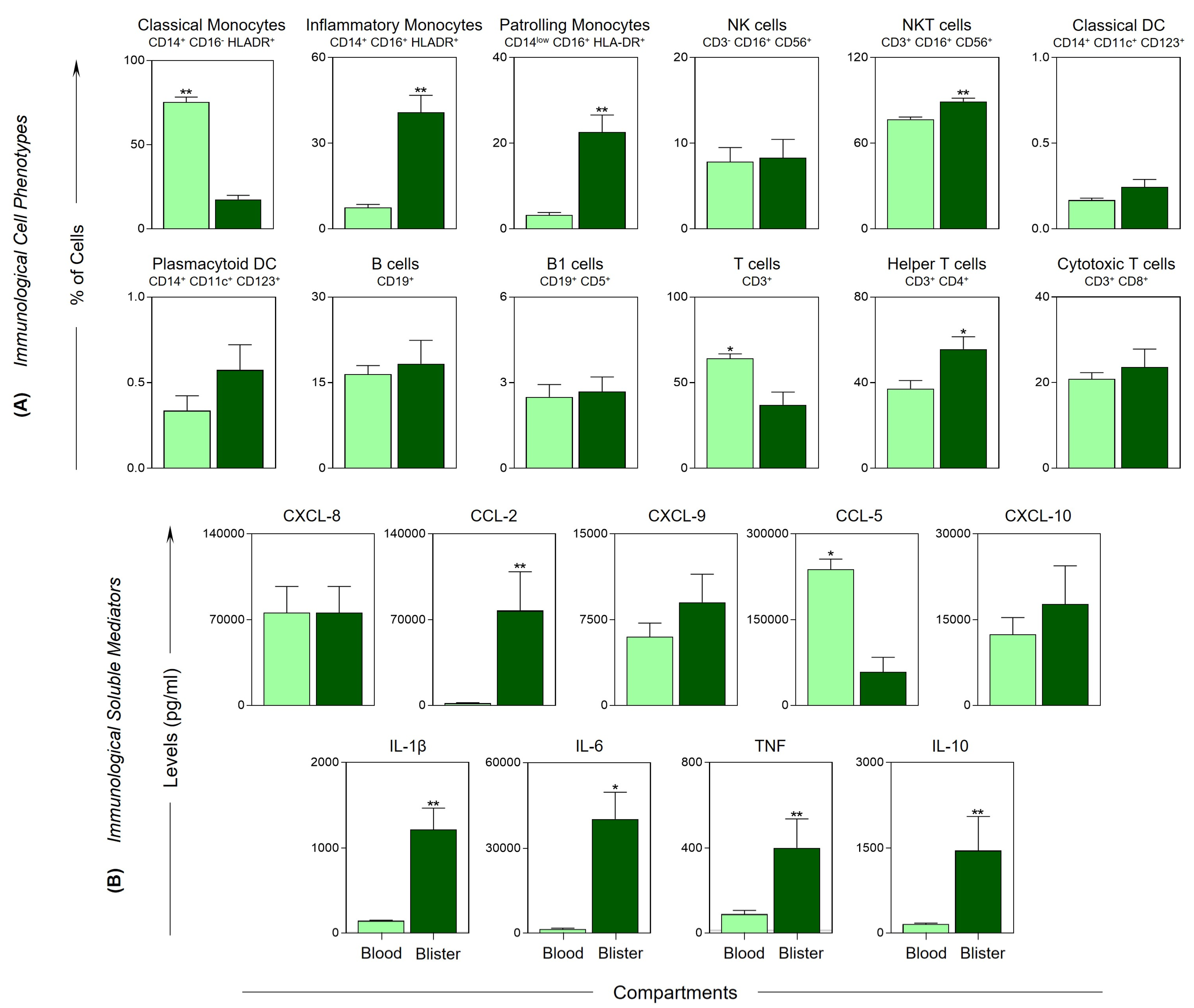
 ) represent the elements of blister exudate, and blue nodes (
) represent the elements of blister exudate, and blue nodes ( ) are used to identify the elements of peripheral blood. Solid lines between the elements indicate a positive correlation, while dashed lines indicate a negative correlation. The red lines indicate the correlations between the blister exudate and peripheral blood. The thickness of lines indicates the strength of the correlation. The correlation index (r) was used to categorize the strength of the correlation as weak (r ≤ 0.35), moderate (r ≥ 0.36 to r ≤ 0.67) or strong (r ≥ 0.68).
) are used to identify the elements of peripheral blood. Solid lines between the elements indicate a positive correlation, while dashed lines indicate a negative correlation. The red lines indicate the correlations between the blister exudate and peripheral blood. The thickness of lines indicates the strength of the correlation. The correlation index (r) was used to categorize the strength of the correlation as weak (r ≤ 0.35), moderate (r ≥ 0.36 to r ≤ 0.67) or strong (r ≥ 0.68).
 ) represent the elements of blister exudate, and blue nodes (
) represent the elements of blister exudate, and blue nodes ( ) are used to identify the elements of peripheral blood. Solid lines between the elements indicate a positive correlation, while dashed lines indicate a negative correlation. The red lines indicate the correlations between the blister exudate and peripheral blood. The thickness of lines indicates the strength of the correlation. The correlation index (r) was used to categorize the strength of the correlation as weak (r ≤ 0.35), moderate (r ≥ 0.36 to r ≤ 0.67) or strong (r ≥ 0.68).
) are used to identify the elements of peripheral blood. Solid lines between the elements indicate a positive correlation, while dashed lines indicate a negative correlation. The red lines indicate the correlations between the blister exudate and peripheral blood. The thickness of lines indicates the strength of the correlation. The correlation index (r) was used to categorize the strength of the correlation as weak (r ≤ 0.35), moderate (r ≥ 0.36 to r ≤ 0.67) or strong (r ≥ 0.68).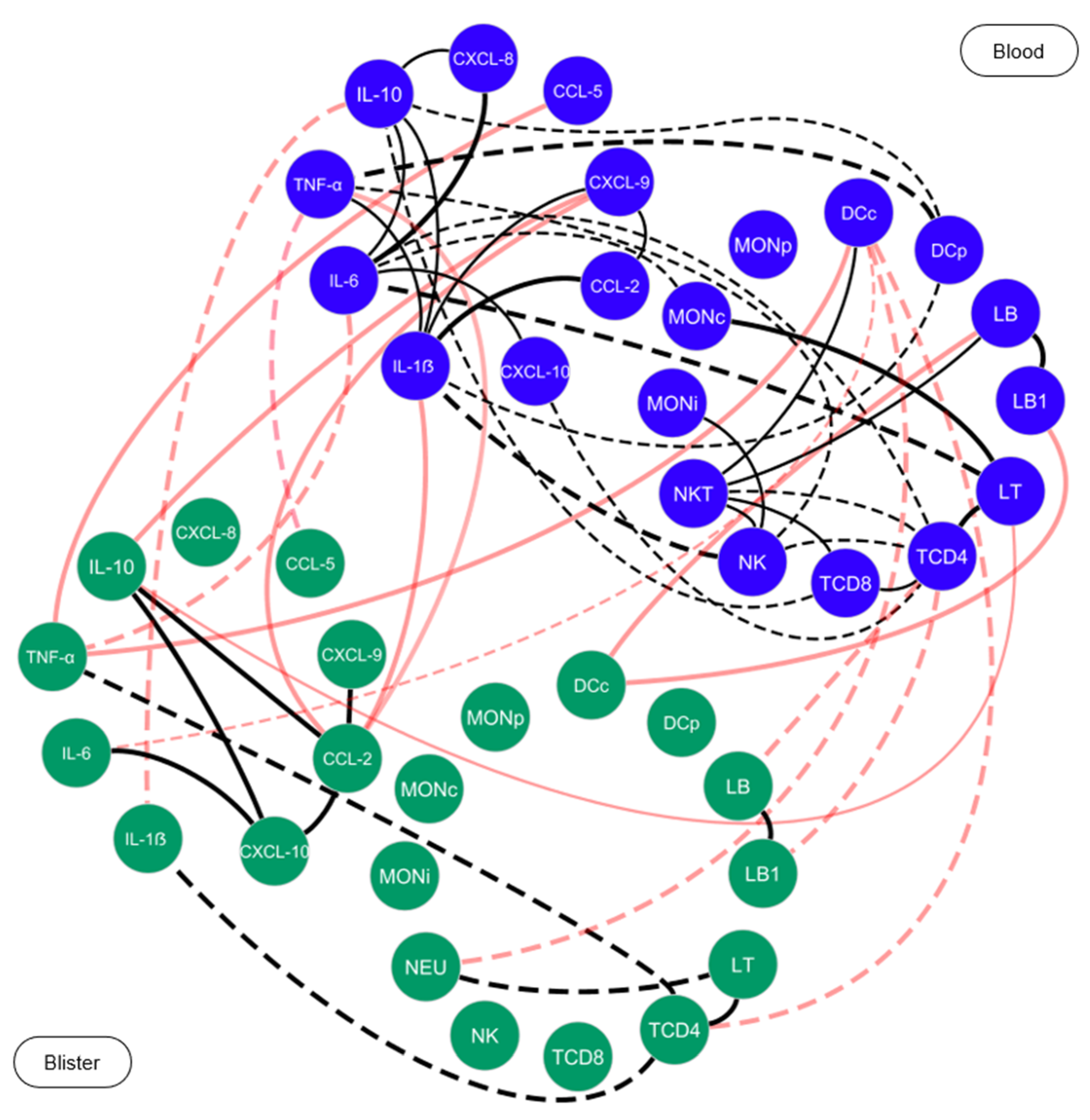
| Demographic and Clinical Characteristics | HD (n = 48) | B. atrox Patients | p-Value | |
|---|---|---|---|---|
| Mild (n = 29) | Severe (n = 19) | |||
| Gender (n, male/female) | 34/14 | 26/3 | 15/4 | 0.248 |
| Age (median and (IQR)) | 33(23–44) | 46 (28–60) | 53 (30–63) | 0.0067 # |
| Previous snakebite (n, yes/no) | - | 3/26 | 5/14 | 0.146 |
| Zone of occurrence (n, rural/urban) | - | 26/3 | 15/4 | 0.304 |
| Anatomical site of the snakebite (n, upper/lower) limb) | - | 4/25 | 9/10 | 0.010 # |
| Anatomical site of the snakebite (n, right/left) | - | 11/18 | 12/7 | 0.087 |
| Work-related accident (n, yes/no) | - | 10/19 | 7/12 | 0.867 |
| Adverse reaction to the antivenom (n, yes/no) * | - | 8/21 | 11/8 | 0.035 # |
| Time between snakebite and administration of the antivenom (hours, median (IQR)) | - | 3 (2–6) | 6 (3–9) | 0.129 |
| Parameters | Mild (n = 29) | Severe (n = 19) | HD (n = 48) | ||||
|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T0 | T1 | T2 | T0 | |
| Red blood cells | 5.2 (4.8–5.3) | 4.6 (4.4–4.9) a | 3.2 (2.5–4.5) a.b | 5.2 (4.9–5.4) | 4.8 (4.6–5.1) a | 3.7 (0.7–4.7) a.b | 5.0 (4.6–5.4) |
| Hemoglobin | 15.0 (14.3–15.4) | 13.6 (12.1–14.2) a | 8.8 (6.8–13.0) a.b | 15.2 (14.5–15.6) | 13.9 (13.2–15.4) a | 11.4 (1.9–13.5) a.b | 14.9 (13.6–16.0) |
| Platelets | 221 (167–259) § | 191 (182–232) | 117 (97–171) a.b.# | 185 (149–231) § | 202 (151–243) | 61 (31–193) a.b | 243 (211–282) |
| Total leukocytes | 10.9 (8.4–14.6) § | 11.6 (8.7–15.0) | 4.8 (3.8–7.6) a.b | 13.3 (9.7–15.6) § | 12.3 (9.1–15.0) | 8.3 (1.3–11.5) a.b | 14.9 (13.6–16.0) |
| Neutrophils | 77.0 (67.7–88.9) § | 67.8 (62.9–77.8) a | 47.4 (36.8–62.3) a.b | 80.3 (69.0–89.0) § | 71.0 (64.4–82.9) | 60.2 (10.0–77.7) a.b | 56.1 (50.0–61.5) |
| Lymphocytes | 13.0 (5.0–21.3) § | 16.9 (13.3–25.5) a | 13.6 (11.3–20.5) # | 12.1 (5.3–20.3) § | 15.5 (10.0–22.0) a | 8.5 (1.3–10.7) b | 29.7 (26.4–34.6) |
| Monocytes | 4.0 (3.5–5.7) § | 5.6 (4.5–6.7) a | 4.1 (3.3–5.7) b | 4.1 (3.1–5.1) § | 5.4 (4.0–6.6) | 4.4 (0.7–5.3) b | 6.0 (5.1–6.6) |
| Eosinophils | 2.0 (0.9–4.7) § | 1.8 (1.0–2.6) | 2.8 (2.2–3.2) b | 1.8 (0.4–5.8) § | 2.3 (1.2–3.5) | 1.8 (1.0–4.9) | 3.3 (2.3–6.1) |
| Basophils | 0.4 (0.2–0.8) | 0.3 (0.2–0.4) a | 0.3 (0.2–0.3) # | 0.3 (0.2–0.4) § | 0.3 (0.2–0.4) | 0.1 (0.0–0.3) a.b | 0.5 (0.3–0.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, K.F.; Neves, J.C.F.; Ibiapina, H.N.S.; Magalhães-Gama, F.; Barbosa, F.B.A.; Silva, F.S.; Wellmann, I.A.M.; Sachett, J.A.G.; Tarragô, A.M.; Ferreira, L.C.L.; et al. Exploring the Profile of Cell Populations and Soluble Immunological Mediators in Bothrops atrox Envenomations. Toxins 2023, 15, 196. https://doi.org/10.3390/toxins15030196
Coelho KF, Neves JCF, Ibiapina HNS, Magalhães-Gama F, Barbosa FBA, Silva FS, Wellmann IAM, Sachett JAG, Tarragô AM, Ferreira LCL, et al. Exploring the Profile of Cell Populations and Soluble Immunological Mediators in Bothrops atrox Envenomations. Toxins. 2023; 15(3):196. https://doi.org/10.3390/toxins15030196
Chicago/Turabian StyleCoelho, Kerolaine Fonseca, Juliana Costa Ferreira Neves, Hiochelson Najibe Santos Ibiapina, Fábio Magalhães-Gama, Fabiane Bianca Albuquerque Barbosa, Flavio Souza Silva, Irmgardt Alicia María Wellmann, Jacqueline Almeida Gonçalves Sachett, Andréa Monteiro Tarragô, Luiz Carlos Lima Ferreira, and et al. 2023. "Exploring the Profile of Cell Populations and Soluble Immunological Mediators in Bothrops atrox Envenomations" Toxins 15, no. 3: 196. https://doi.org/10.3390/toxins15030196
APA StyleCoelho, K. F., Neves, J. C. F., Ibiapina, H. N. S., Magalhães-Gama, F., Barbosa, F. B. A., Silva, F. S., Wellmann, I. A. M., Sachett, J. A. G., Tarragô, A. M., Ferreira, L. C. L., Malheiro, A., Monteiro, W. M., & Costa, A. G. (2023). Exploring the Profile of Cell Populations and Soluble Immunological Mediators in Bothrops atrox Envenomations. Toxins, 15(3), 196. https://doi.org/10.3390/toxins15030196






