Abstract
End-stage renal disease (ESRD) patients are a population with high rates of COVID-19 and mortality. These patients present a low response to anti-SARS-CoV-2 immunization, which is associated with immune dysfunction. ESRD patients also present high plasma titers of Fibroblast Growth Factor 23 (FGF23), a protein hormone that reduces immune response in vivo and in vitro. Increased FGF23 levels associate with higher infection-related hospitalizations and adverse infectious outcomes. Thus, we evaluated whether ESRD patients with high FGF23 titers have an increased rate of SARS-CoV-2 infection. Methods: We performed a prospective cohort of ESRD patients in hemodialysis who had measurements of plasma intact FGF23 in 2019. We determined COVID-19 infections, hospitalizations, and mortality between January 2020 and December 2021. Results: We evaluated 243 patients. Age: 60.4 ± 10.8 years. Female: 120 (49.3%), diabetes: 110 (45.2%). During follow-up, 45 patients developed COVID-19 (18.5%), 35 patients were hospitalized, and 12 patients died (mortality rate: 26.6%). We found that patients with higher FGF23 levels (defined as equal or above median) had a higher rate of SARS-CoV-2 infection versus those with lower levels (18.8% versus 9.9%; Hazard ratio: 1.92 [1.03–3.56], p = 0.039). Multivariate analysis showed that increased plasma FGF23 was independently associated with SARS-CoV-2 infection and severe COVID-19. Discussion: Our results suggest that high plasma FGF23 levels are a risk factor for developing COVID-19 in ESRD patients. These data support the potential immunosuppressive effects of high circulating FGF23 as a factor implicated in the association with worse clinical outcomes. Further data are needed to confirm this hypothesis.
Keywords:
end-stage renal disease; hemodialysis; FGF23; COVID-19; SARS-CoV-2; immune response; mortality Key Contribution:
In end-stage renal disease patients on hemodialysis, high plasma levels of Fibroblast Growth Factor 23 (FGF23) is a risk factor for SARS-CoV-2 infection and severe COVID-19.
1. Introduction
The SARS-CoV-2 pandemic has had a massive impact worldwide over the past two years, with over 620,000,000 infections and over 6,500,000 deaths as of October 2022 [1]. Patients with end-stage renal disease (ESRD) in renal replacement therapy, such as renal transplantation, peritoneal dialysis, and hemodialysis, are a population with higher infection rates and adverse outcomes, including hospitalizations, requirements of mechanical ventilation and deaths, compared to the general population [2,3,4,5].
ESRD patients develop an immune dysfunction that includes impairment in both innate and adaptive immune response [6], including decreased neutrophil bacterial activity, monocyte hypoactivity, impaired activity of T lymphocytes, and a decreased number of B lymphocytes [6]. This immune impairment is associated with a higher rate of infections and up to 100-fold higher infection-related mortality than the general population [7]. ESRD patients also present a high failure rate of immunization against viruses such as influenza, despite appropriate vaccination [6], and immunization against SARS-CoV-2 produced a lower humoral response in ESRD patients versus control subjects [7]. Patients on hemodialysis who were vaccinated with mRNA vaccines induce anti-SARS-CoV-2 antibodies [8], although at lower concentrations than healthy volunteers [9]. In addition, they have an early decrease in antibody titers compared to the general population [10,11,12]. The causal mechanisms leading to the immune impairment of ESRD patients are not entirely understood.
ESRD patients present high plasma levels of Fibroblast Growth Factor 23 (FGF23), a protein hormone involved in the regulation of vitamin D and phosphate metabolism [13]. In addition to its role in mineral homeostasis, recent evidence shows that FGF23 acts as an immunomodulatory hormone, affecting the immune response due to indirect actions on non-immune tissues and direct actions on immune cells, including macrophages and polymorphonuclear neutrophils. Elevated titers of FGF23 are associated with increased inflammation in patients [14], and increased levels of FGF23 induce immune dysfunction in experimental murine models and human white blood cells in vitro [15]. In addition, clinical studies in ESRD patients [16] and non-ESRD chronic kidney disease patients [17] show that high plasma levels of FGF23 are associated with an increased infection rate compared to patients with lower titers.
We propose that high baseline plasma levels of FGF23 are associated with increased risk and severity of SARS-CoV-2 infection in the ESRD population. Therefore, we evaluated a multicenter prospective cohort of adult ESRD patients in chronic hemodialysis to determine the association between high FGF23 titers and the incidence of SARS-CoV-2 infection and COVID-related adverse clinical outcomes.
2. Results
2.1. Baseline Characteristics
We evaluated 243 end-stage renal disease patients on chronic hemodialysis for the study. Table 1 presents the baseline characteristics of these patients. Age: 60.4 ± 10.8 years. Patients over 60 years: 132 (54.3%). Female: 120 (49.3%). Diabetes: 110 (45.2%), hemodialysis vintage: 25 [15–40] months. Baseline intact FGF23 levels: 319 [204–600] pg/mL.

Table 1.
Baseline characteristics of patients. Information on the total cohort and stratification by the presence of SARS-CoV-2 infection are presented. Data are expressed as number (N) and percentage (%) for categorical variables and as mean ± standard deviation or median [p25–p75] for continuous variables. The p-values of patients who presented SARS-CoV-2 infection vs. those without SARS-CoV-2 infection are detailed.
2.2. COVID-19-Related Events and Predictors of SARS-CoV-2 Infection
During follow-up (January 2020–December 2021), 45 patients (18.5%) had SARS-CoV-2 infection, and 35 patients (14.4%) presented COVID-related hospitalizations. Regarding mortality, 32 patients (13.1%) died during follow-up: 12 patients (4.9%) had COVID-related deaths, and 20 (8.2%) died of other causes, where cardiovascular diseases were the most frequent causes. Regarding the period of COVID-19 events, 40/45 of total SARS-CoV-2 infections (88.9%) and 10/12 of total COVID-related deaths (83.3%) occurred before the initiation of the vaccination campaign in hemodialysis patients (initiated in February 2021 in Chile).
When comparing patients who developed SARS-CoV-2 infection versus those without infection (Table 1), infected patients were older (66.2 ± 9.3 versus 59.1 ± 10.7 years, p < 0.001) and had a higher rate of diabetes (60.0 vs. 41.9%, p = 0.028). Concerning additional baseline variables before the SARS-CoV-2 infection, the only parameter which differed between both groups was plasma FGF23 which was higher in those who developed COVID-19 (436 [269–669] vs. 288 [195–580] pg/mL, p = 0.026). This association was sustained after statistical adjustment for age and other comorbidities.
2.3. Relation between Plasma FGF23 Levels and COVID-19-Related Events
When comparing according to their baseline plasma FGF23 levels (Table 2), patients who had higher plasma FGF23 levels (equal to or above p50 of the total sample) had lower single-pool Kt/V (1.27 ± 0.20 versus 1.35 ± 0.21 years, p = 0.003), higher use of recombinant erythropoietin (88.5 vs. 78.5%, p = 0.035) and increased parathormone (PTH) levels (598 [465–703] vs. 204 [160–267] pg/mL, p < 0.001). In addition, people who had higher FGF23 titers had increased infection rates (23.7 vs. 13.2%, p = 0.034; Figure 1) and increased rates of severe COVID-19 (patients with COVID-related hospitalizations and deaths) (19.6 vs. 9.9%, p = 0.032; Figure 2).

Table 2.
Comparison of patients according to plasma FGF23 levels. Information on the total cohort and stratification by baseline plasma intact FGF23 levels are presented. Data are expressed as number (N) and percentage (%) for categorical variables and as mean ± standard deviation or median [p25–p75] for continuous variables. p-values of low FGF23 vs. high FGF23 are detailed.
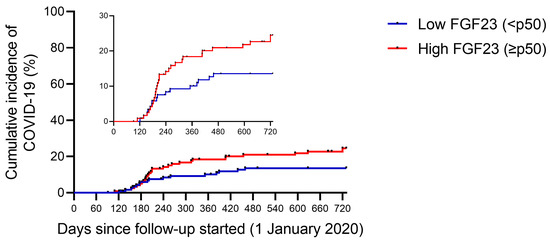
Figure 1.
Infection rate of SARS-CoV-2 during follow-up, stratified by baseline plasma FGF23 levels. Cumulative incidence rate of SARS-CoV-2 infection in end-stage renal disease patients in hemodialysis. Patients were classified according to baseline plasma intact FGF23 levels: low FGF23 (n = 121—blue line) and high FGF23 (n = 122—red line).
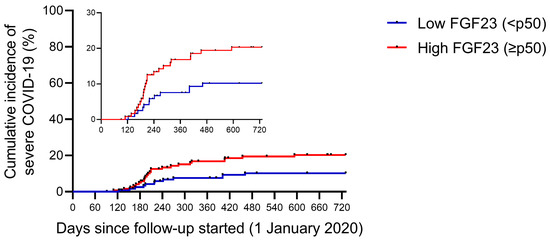
Figure 2.
Severe COVID-19 rate during follow-up, according to baseline plasma FGF23 levels. Cumulative incidence of severe COVID-19 in end-stage renal disease patients in hemodialysis. Severe COVID-19 is defined as the requirements for hospitalizations or COVID-related deaths. Patients were classified according to baseline plasma intact FGF23 levels: low FGF23 (n = 121—blue line) and high FGF23 (n = 122—red line).
Multivariate analysis indicates that SARS-CoV-2 infection predictors (Table 3) included age above 60 years (HR: 2.63 [1.35–5.11], p = 0.004), presence of diabetes (HR: 1.92 [1.05–3.48], p = 0.033) and high plasma FGF23 levels (HR: 1.92 [1.03–3.56], p = 0.039). No association was detected with another baseline clinical and laboratory variables. Finally, multivariate analysis indicates that the predictors of severe COVID-19 (Table 4) also included age above 60 years (HR: 2.65 [1.22–5.77], p = 0.014), presence of diabetes (HR: 2.52 [1.25–5.06], p = 0.009) and high plasma FGF23 levels (HR: 2.12 [1.04–4.28], p = 0.036).

Table 3.
Determination of predictors of SARS-CoV-2 infection during a 2-year follow-up. We present a univariate and multivariate logistic regression of several clinical, demographic, and laboratory parameters of patients. The variables which were included in the multivariate model were those which had a p-value below 0.2 in the univariate analysis. Variables that were considered predictors of SARS-CoV-2 infection were those with a p-value below 0.05 in the final model. Hazard ratios, the 95% confidence interval (95%CI), and the p-value are detailed.

Table 4.
Determination of predictors of severe COVID-19 during a 2-year follow-up. We present a univariate and multivariate logistic regression of several clinical, demographic, and laboratory variables of patients. Severe COVID-19 is defined as the requirements for hospitalizations or COVID-related deaths. The variables which were included in the multivariate model were those which had a p-value below 0.2 in the univariate analysis. Variables that were considered predictors of severe COVID-19 were those which had a p-value below 0.05 in the final model. Hazard ratios, the 95% confidence interval (95%CI), and the p-value are detailed.
3. Discussion
3.1. Association between Plasma Levels of FGF23 and COVID-19-Related Outcomes
This multicenter observational study showed that patients with end-stage renal disease in chronic hemodialysis who presented high baseline plasma levels of FGF23 had a higher rate of SARS-CoV-2 infection and severe COVID-19, including hospitalizations and deaths. In addition, this risk factor was independent of other variables associated with increased infections, such as age and diabetes. This study is one of the first clinical studies that evaluated the association between plasma FGF23 levels and COVID-19 in patients on renal replacement therapy. Currently, most studies evaluating the association between plasma FGF23 levels and infectious morbidity were performed before the SARS-CoV-2 pandemic and evaluated other infection-related hospitalizations caused by severe bacterial and viral infections [16,17]. A recent study determined that patients with asymptomatic SARS-CoV-2 infection had higher values of fibroblast growth factors (including FGF19, FGF21, and FGF23) compared to those with mild symptomatic COVID-19 [18].
3.2. Association between High Baseline FGF23 Levels and Development of Severe Infections in Humans
A posthoc analysis of the Hemodialysis Study (HEMO study) in ESRD patients on hemodialysis found that patients with high baseline FGF23 levels had an increased rate of a combined outcome of first infectious hospitalization or infectious death in a median follow-up of three years [16]. This association was independent of other associated variables. In addition, a posthoc analysis of the Chronic Kidney Insufficiency Cohort Study (CRIC study) in non-terminal chronic kidney disease (CKD) patients found that patients who had high baseline plasma titers of C-terminal FGF23 had an increased risk of first-time hospitalization with a severe infection, including pneumonia, urinary tract infection, and septicemia [17]. This association was independent of other inflammatory and bone mineral metabolism parameters. In addition, a post hoc analysis of the Cardiovascular Health Study (CHS study) in community-dwelling adults over 65 years (with or without CKD) found that those with high FGF23 levels had a higher rate of first infection-related hospitalization [19]. This association was observed in both patients with CKD and without CKD. These results, including our data, support the potential role of increased baseline FGF23 levels as a risk factor for severe infections.
3.3. High FGF23 Levels and Severe Infections: Correlation or Causality?
Previous data have shown that high FGF23 titers are associated with increased rates of cardiovascular events in CKD patients [13,20,21] and the general population [22]. FGF23 has deleterious cardiovascular effects, including cardiac hypertrophy [23,24,25], arterial wall calcification, and alteration of intracellular calcium and smooth muscle cell contractility [26]. There is currently limited evidence concerning the potential mechanisms related to the association of high baseline FGF23 levels and increased risk of severe infections. FGF23 is a negative modulator of the 1-alpha hydroxylase, the enzyme responsible for active vitamin D synthesis (1,25 dihydroxy vitamin D), both in renal [27,28] and extrarenal tissue, including monocytes [29]. Vitamin D is a hormone that modules immune response [30], and low vitamin D levels plus high FGF23 levels (a common condition in ESRD patients) are associated with adverse infectious outcomes [16]. A translational study demonstrated that FGF23 per se causes an immune dysfunction in experimental murine models and white blood cells obtained from healthy volunteers [15,31]. These effects include decreased neutrophil selectin-mediated rolling and chemokine-induced recruitment in vitro. In addition, in experimental models of bacterial pneumonia in CKD mice, the administration of FGF23 exacerbated disease activity and decreased murine survival [15]. Moreover, pharmacological blockage of FGF23 using specific anti-FGF23 antibodies [15] and short-acting small molecules reversibly inhibiting FGF23 [30,32] prevent cardiac and immune effects in vitro and in CKD mice. The mechanisms have not been clarified yet. It has been proposed that FGF23 may activate the FGFR2 receptor in neutrophil cells and cause indirect effects mediated by the FGFR receptors in other cells of the inflammatory milieu [30].
3.4. Limitations and Strengths of the Study
Concerning the strengths of this work is the prospective design, which evaluated this group of patients before and during the first two years of the SARS-CoV-2 pandemic, including relevant demographic, clinical, and laboratory baseline data, that was associated with clinical outcomes in ESRD patients. In addition, we had detailed data on COVID-19-related events obtained by direct information from hemodialysis centers and the national COVID-19 database, with follow-up data of all patients during the study period.
In relation to the limitations of this study, its (non-interventional) observational design has biases, as some potential influencing variables are difficult to isolate. For example, people who had a higher incidence of COVID-19, besides higher levels of plasma FGF23, also were older and had increased rates of diabetes, known risk factors for COVID-19 in the general population and ESRD patients [5,33], which may have influenced the differences in clinical outcomes. Additionally, patients with high FGF23 levels also had high PTH levels. PTH is modulated by FGF23 [13], and low PTH levels have been associated with increased infection rates in ESRD patients [34,35]. To decrease this bias, we used statistical adjustments, including potential confounding variables, such as multivariate regressions, to evaluate the association of FGF23 levels and adverse clinical outcomes regardless of other demographical and clinical variables. Another limitation is the lack of data on C-terminal FGF23 levels in these patients. Similar to intact FGF23, high C-terminal FGF23 is associated with worse clinical outcomes and increased mortality [13,14] and has been associated with increased rates of severe infections [17]. Currently, no published data compares intact versus C-terminal FGF23 and its association with severe infections, including COVID-19.
4. Conclusions
Our results suggest that high baseline plasma FGF23 levels in end-stage renal disease patients on chronic hemodialysis are associated with an increased risk for SARS-CoV-2 infection and severe COVID-19, including hospitalization and death. This is one of the first reports that evaluate the relationship between FGF23 and COVID-19 in this population. These data support the potential immunosuppressive effects of high circulating FGF23 as a factor associated with worse clinical outcomes in ESRD and non-ESRD patients. Further data from translational research and clinical studies with larger populations are needed to confirm this hypothesis.
5. Materials and Methods
5.1. Study Design
The CATALINA-HD study is a multicenter observational cohort of end-stage renal disease patients on chronic hemodialysis performed in Chile. The study was initiated in 2018 to evaluate the association of FGF23 with clinical outcomes, including hospitalizations, major cardiovascular events, infectious events, and death. The information included demographic, laboratory, and clinical data, which were collected from patients. Measurements of plasma FGF23 were performed in 2019. We evaluated the association of plasma FGF23 levels before the SARS-CoV-2 pandemic with clinical outcomes, including SARS-CoV-2 infection and severe COVID-19, including COVID-related hospitalizations and deaths. The study was approved by the local Institutional Ethics Committee.
5.2. Inclusion and Exclusion Criteria
We included people older than 18 years with a previous diagnosis of end-stage renal disease who were on chronic hemodialysis before 1 January 2020 and had measurements of plasma FGF23 levels during 2019. The analysis included the final measurement if a patient had more than one measurement. We excluded: terminal patients with palliative management, pregnancy or breastfeeding women, and patients with incomplete data.
5.3. Evaluation of Patients
Determination of plasma levels of intact FGF23 was performed using an enzyme-linked immunosorbent assay (ELISA) technique (human iFGF23: cat#60–6600, Quidel, San Diego, CA, USA). Blood samples were collected during the hemodialysis session, before the initiation of hemodialysis, by the puncture of the vascular access (arteriovenous fistula or central venous catheter) using a 4-mL EDTA tube. Samples were centrifugated, and plasma was extracted and then used for measurements. Patients were classified according to their plasma FGF23 levels, as low level (FGF23 below p50 of the whole sample) and high level (FGF23 greater than or equal to p50 of the whole sample).
Patient follow-up was carried out between 1 January 2020 and 31 December 2021. SARS-CoV-2 infection was confirmed by a polymerase chain reaction (PCR) test. This information was reported on the Epivigila platform (the method used by the Chilean Ministry of Health to perform patient follow-ups) [36]. We evaluated patients who had COVID-related hospitalization or COVID-related death with PCR confirmation, corresponding to the U07.1 code in the International Classification of Diseases, 10th Revision [37].
5.4. Statistical Analysis
Continuous variables are expressed as arithmetic mean ± standard deviation or median [percentile 25—percentile 75], and discrete variables are expressed as absolute values (percentages). To compare baseline data between groups, we used the chi-square test for discrete variables or the Student t-test for paired or unpaired groups. In addition, calculations of Hazard Ratios and their 95% confidence intervals (95% CI), Kaplan-Meier analysis, and Cox proportional tests were performed for survival analysis. Finally, we performed a multivariate analysis using logistic regression to evaluate predictors of SARS-CoV-2 infection or severe COVID-19, evaluating several clinical, demographical, and laboratory variables. In those models, we included variables that had a p-value below 0.20 in univariate analysis. Data analysis was executed using GraphPad Prism v.6.0 (GraphPad Software, La Jolla, CA, USA) and Stata/SE v.17.0 (Stata Software, College Station, TX, USA). All the analyses were two-tailed, and we considered a statistically significant difference with a p-value below 5% (p < 0.05).
Author Contributions
L.T. performed patient recruitment, clinical follow-up, and data analysis. L.M., M.A., M.E.S., R.T., L.E., S.C., V.R. and R.T. recruited patients and collected clinical data. P.A. performed ELISA measurements. A.P.-L., G.M.-V., E.V., C.P. and I.B. collected clinical data related to COVID-19 infection and mortality. L.T. and A.P.-L. created the figures. L.T. and L.M. designed the clinical study and drafted the manuscript, which was revised by all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FONDECYT de Iniciacion 11171141 (L.T.) and FONDECYT Regular 1221571 (L.T.) of the Agencia Nacional de Investigacion y Desarrollo (ANID). This institution did not participate in the study design, data analysis, or writing of the manuscript.
Institutional Review Board Statement
The study was performed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Universidad de Chile (protocol code N°033/16 approved in August 2016).
Informed Consent Statement
We obtained informed consent from all the patients who participated in the study.
Data Availability Statement
The information that supports the findings of our study is available upon reasonable request to the corresponding author, L.T. This information is not publicly available because it contains data that may compromise the privacy of the research participants and third-party restrictions.
Acknowledgments
We thank all the members of the FUTAC-RENAL group Team and the Chilean Society of Nephrology.
Conflicts of Interest
The authors declare no conflict of interest.
References
- John Hopkins University & Medicine Coronavirus Resource Center. COVID-19 Map. 2022. Available online: https://coronavirus.jhu.edu/map.html (accessed on 21 October 2022).
- Yi, Y.; Lagniton, P.N.P.; Ye, S.; Li, E.; Xu, R.H. COVID-19: What has been learned and to be learned about the novel coronavirus disease. Int. J. Biol. Sci. 2020, 16, 1753–1766. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Diao, B.; Lv, X.; Zhu, J.; Chen, C.; Liu, L.; Zhang, S.; Shen, B.; Wang, H. Epidemiological, Clinical, and Immunological Features of a Cluster of COVID-19-Contracted Hemodialysis Patients. Kidney Int. Rep. 2020, 5, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.H.; Hirsch, J.S.; Wanchoo, R.; Sachdeva, M.; Sakhiya, V.; Hong, S.; Jhaveri, K.D.; Fishbane, S.; Abate, M.; Andrade, H.P.; et al. Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int. 2020, 98, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.M.; Weiner, D.E.; Aweh, G.; Miskulin, D.C.; Manley, H.J.; Stewart, C.; Ladik, V.; Hosford, J.; Lacson, E.C.; Johnson, D.S.; et al. COVID-19 Among US Dialysis Patients: Risk Factors and Outcomes From a National Dialysis Provider. Am. J. Kidney Dis. 2021, 77, 748–756. [Google Scholar] [CrossRef]
- Kato, S.; Chmielewski, M.; Honda, H.; Pecoits-Filho, R.; Matsuo, S.; Yuzawa, Y.; Tranaeus, A.; Stenvinkel, P.; Lindholm, B. Aspects of immune dysfunction in end-stage renal disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Sarnak, M.J.; Jaber, B.L. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int. 2000, 58, 1758–1764. [Google Scholar] [CrossRef]
- Clavero, R.; Parra-Lucares, A.; Méndez-Valdés, G.; Villa, E.; Bravo, K.; Mondaca, E.; Aranda, J.; Brignardello, R.; Gajardo, C.; Ordenes, A.; et al. Humoral Immune Response of BNT162b2 and CoronaVac Vaccinations in Hemodialysis Patients: A Multicenter Prospective Cohort. Vaccines 2022, 10, 1542. [Google Scholar] [CrossRef]
- Simon, B.; Rubey, H.; Treipl, A.; Gromann, M.; Hemedi, B.; Zehetmayer, S.; Kirsch, B. Haemodialysis patients show a highly diminished antibody response after COVID-19 mRNA vaccination compared with healthy controls. Nephrol. Dial. Transplant. 2021, 36, 1709–1716. [Google Scholar] [CrossRef]
- Alcázar-Arroyo, R.; Portolés, J.; López-Sánchez, P.; Zalamea, F.; Furaz, K.; Méndez, Á.; Nieto, L.; Sánchez-Hernández, R.; Pizarro, S.; García, A.; et al. Rapid decline of anti-SARS-CoV-2 antibodies in patients on haemodialysis: The COVID-FRIAT study. Clin. Kidney J. 2021, 14, 1835–1844. [Google Scholar] [CrossRef]
- Yen, C.-C.; Lin, S.-Y.; Chen, S.-C.; Chiu, Y.-W.; Chang, J.-M.; Hwang, S.-J. COVID-19 Vaccines in Patients with Maintenance Hemodialysis. J. Pers. Med. 2021, 11, 789. [Google Scholar] [CrossRef]
- Jahn, M.; Korth, J.; Dorsch, O.; Anastasiou, O.E.; Krawczyk, A.; Brochhagen, L.; van de Sand, L.; Sorge-Hädicke, B.; Tyczynski, B.; Witzke, O.; et al. Decline of Humoral Responses 6 Months after Vaccination with BNT162b2 (Pfizer-BioNTech) in Patients on Hemodialysis. Vaccines 2022, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Kurpas, A.; Supeł, K.; Idzikowska, K.; Zielińska, M. FGF23: A Review of Its Role in Mineral Metabolism and Renal and Cardiovascular Disease. Dis. Markers 2021, 2021, 8821292. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.M.; Isakova, T.; Cai, X.; Bayes, L.Y.; Faul, C.; Scialla, J.J.; Lash, J.P.; Chen, J.; He, J.; Navaneethan, S.; et al. Inflammation and elevated levels of fibroblast growth factor 23 are independent risk factors for death in chronic kidney disease. Kidney Int. 2017, 91, 711–719. [Google Scholar] [CrossRef]
- Rossaint, J.; Oehmichen, J.; Van Aken, H.; Reuter, S.; Pavenstädt, H.J.; Meersch, M.; Unruh, M.; Zarbock, A. FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J. Clin. Investig. 2016, 126, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Chonchol, M.; Greene, T.; Zhang, Y.; Hoofnagle, A.N.; Cheung, A.K. Low Vitamin D and High Fibroblast Growth Factor 23 Serum Levels Associate with Infectious and Cardiac Deaths in the HEMO Study. J. Am. Soc. Nephrol. 2016, 27, 227–237. [Google Scholar] [CrossRef]
- Ishigami, J.; Taliercio, J.T.; Feldman, H.I.; Srivastava, A.; Townsend, R.R.; Cohen, D.L.; Horwitz, E.J.; Rao, P.; Charleston, J.; Fink, J.C.; et al. Fibroblast Growth Factor 23 and Risk of Hospitalization with Infection in Chronic Kidney Disease: The Chronic Renal Insufficiency Cohort (CRIC) Study. J. Am. Soc. Nephrol. 2020, 31, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Soares-Schanoski, A.; Sauerwald, N.; Goforth, C.W.; Periasamy, S.; Weir, D.L.; Lizewski, S.; Lizewski, R.; Ge, Y.; Kuzmina, N.A.; Nair, V.D.; et al. Asymptomatic SARS-CoV-2 Infection Is Associated with Higher Levels of Serum IL-17C, Matrix Metalloproteinase 10 and Fibroblast Growth Factors than Mild Symptomatic COVID-19. Front. Immunol. 2022, 13, 821730. [Google Scholar] [CrossRef]
- Nowak, K.L.; Bartz, T.M.; Dalrymple, L.; de Boer, I.H.; Kestenbaum, B.; Shlipak, M.G.; Garimella, P.S.; Ix, J.H.; Chonchol, M. Fibroblast Growth Factor 23 and the Risk of Infection-Related Hospitalization in Older Adults. J. Am. Soc. Nephrol. 2017, 28, 1239–1246. [Google Scholar] [CrossRef]
- Scialla, J.J. Epidemiologic insights on the role of fibroblast growth factor 23 in cardiovascular disease. Curr. Opin. Nephrol. Hypertens. 2015, 24, 260–267. [Google Scholar] [CrossRef]
- Nakano, C.; Hamano, T.; Fujii, N.; Obi, Y.; Matsui, I.; Tomida, K.; Mikami, S.; Inoue, K.; Shimomura, A.; Nagasawa, Y.; et al. Intact fibroblast growth factor 23 levels predict incident cardiovascular event before but not after the start of dialysis. Bone 2012, 50, 1266–1274. [Google Scholar] [CrossRef]
- Lutsey, P.L.; Alonso, A.; Selvin, E.; Pankow, J.S.; Michos, E.D.; Agarwal, S.K.; Loehr, L.R.; Eckfeldt, J.H.; Coresh, J. Fibroblast growth factor-23 and incident coronary heart disease, heart failure, and cardiovascular mortality: The Atherosclerosis Risk in Communities study. J. Am. Heart Assoc. 2014, 3, e000936. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, G.S.; Reuter, S.; Kentrup, D.; Grabner, A.; Amaral, A.P.; Fobker, M.; Stypmann, J.; Pavenstädt, H.; Wolf, M.; Faul, C.; et al. Treatment of established left ventricular hypertrophy with fibroblast growth factor receptor blockade in an animal model of CKD. Nephrol. Dial. Transplant. 2014, 29, 2028–2035. [Google Scholar] [CrossRef] [PubMed]
- Figurek, A.; Rroji, M.; Spasovski, G. The Complexity of FGF23 Effects on Cardiomyocytes in Normal and Uremic Milieu. Cells 2021, 10, 1266. [Google Scholar] [CrossRef] [PubMed]
- Kuczera, P.; Adamczak, M.; Wiecek, A. Fibroblast Growth Factor-23—A Potential Uremic Toxin. Toxins 2016, 8, 369. [Google Scholar] [CrossRef]
- Six, I.; Okazaki, H.; Gross, P.; Cagnard, J.; Boudot, C.; Maizel, J.; Drueke, T.B.; Massy, Z.A. Direct, acute effects of Klotho and FGF23 on vascular smooth muscle and endothelium. PLoS ONE 2014, 9, e93423. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Shiizaki, K.; Kuro-o, M.; Moe, O.W. Fibroblast growth factor 23 and Klotho: Physiology and pathophysiology of an endocrine network of mineral metabolism. Annu. Rev. Physiol. 2013, 75, 503–533. [Google Scholar] [CrossRef] [PubMed]
- Gattineni, J.; Twombley, K.; Goetz, R.; Mohammadi, M.; Baum, M. Regulation of serum 1,25(OH)2 vitamin D3 levels by fibroblast growth factor 23 is mediated by FGF receptors 3 and 4. Am. J. Physiol. Ren. Physiol. 2011, 301, F371–F377. [Google Scholar] [CrossRef]
- Bacchetta, J.; Sea, J.L.; Chun, R.F.; Lisse, T.S.; Wesseling-Perry, K.; Gales, B.; Adams, J.S.; Salusky, I.B.; Hewison, M. Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25-dihydroxyvitamin D in human monocytes. J. Bone Miner. Res. 2013, 28, 46–55. [Google Scholar] [CrossRef]
- Fitzpatrick, E.A.; Han, X.; Xiao, Z.; Quarles, L.D. Role of Fibroblast Growth Factor-23 in Innate Immune Responses. Front. Endocrinol. 2018, 9, 320. [Google Scholar] [CrossRef]
- Mace, M.L.; Olgaard, K.; Lewin, E. New Aspects of the Kidney in the Regulation of Fibroblast Growth Factor 23 (FGF23) and Mineral Homeostasis. Int. J. Mol. Sci. 2020, 21, 8810. [Google Scholar] [CrossRef]
- Xiao, Z.; Riccardi, D.; Velazquez, H.A.; Chin, A.L.; Yates, C.R.; Carrick, J.D.; Smith, J.C.; Baudry, J.; Quarles, L.D. A computationally identified compound antagonizes excess FGF-23 signaling in renal tubules and a mouse model of hypophosphatemia. Sci. Signal. 2016, 9, ra113. [Google Scholar] [CrossRef] [PubMed]
- Kakkanattu, T.J.; Sankarasubbaiyan, S.; Yadav, A.K.; Kundu, M.; Bg, M.G.; Kumar, V.; Shah, K.; Jha, V. Outcome and Determinants of Outcome of COVID-19 Infection among Hemodialysis Patients: Findings from a National Dialysis Network Program in India. Kidney Int. Rep. 2021, 6, 1429–1432. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-T.; Zheng, C.-M.; Lin, Y.-C.; Wu, M.-Y.; Lin, Y.-F.; Hsu, Y.-H.; Hsu, C.-C.; Wu, M.-S. Aberrant Serum Parathyroid Hormone, Calcium, and Phosphorus as Risk Factors for Peritonitis in Peritoneal Dialysis Patients. Sci. Rep. 2021, 11, 1171. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.A.; Kim, J.H.; Kim, Y.K.; Chang, Y.K.; Park, C.W.; Kim, S.Y.; Kim, Y.S.; Kang, S.-W.; Kim, N.-H.; Kim, Y.-L.; et al. Low Parathyroid Hormone Level Predicts Infection-Related Mortality in Incident Dialysis Patients: A Prospective Cohort Study. Korean J. Intern. Med. 2020, 35, 160–170. [Google Scholar] [CrossRef]
- Chilean Ministry of Health. Epidemiological Surveillance System EPIVIGILA. 2021. Available online: http://epi.minsal.cl/sistema-de-vigilancia-epidemiologica-epivigila-antecedentes/ (accessed on 21 October 2022).
- World Health Organization. COVID-19 Coding in ICD-10. Available online: https://www.who.int/classifications/icd/COVID-19-coding-icd10.pdf (accessed on 31 October 2021).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).