Gymnodinium catenatum Paralytic Shellfish Toxin Production and Photobiological Responses under Marine Heat Waves
Abstract
1. Introduction
2. Results
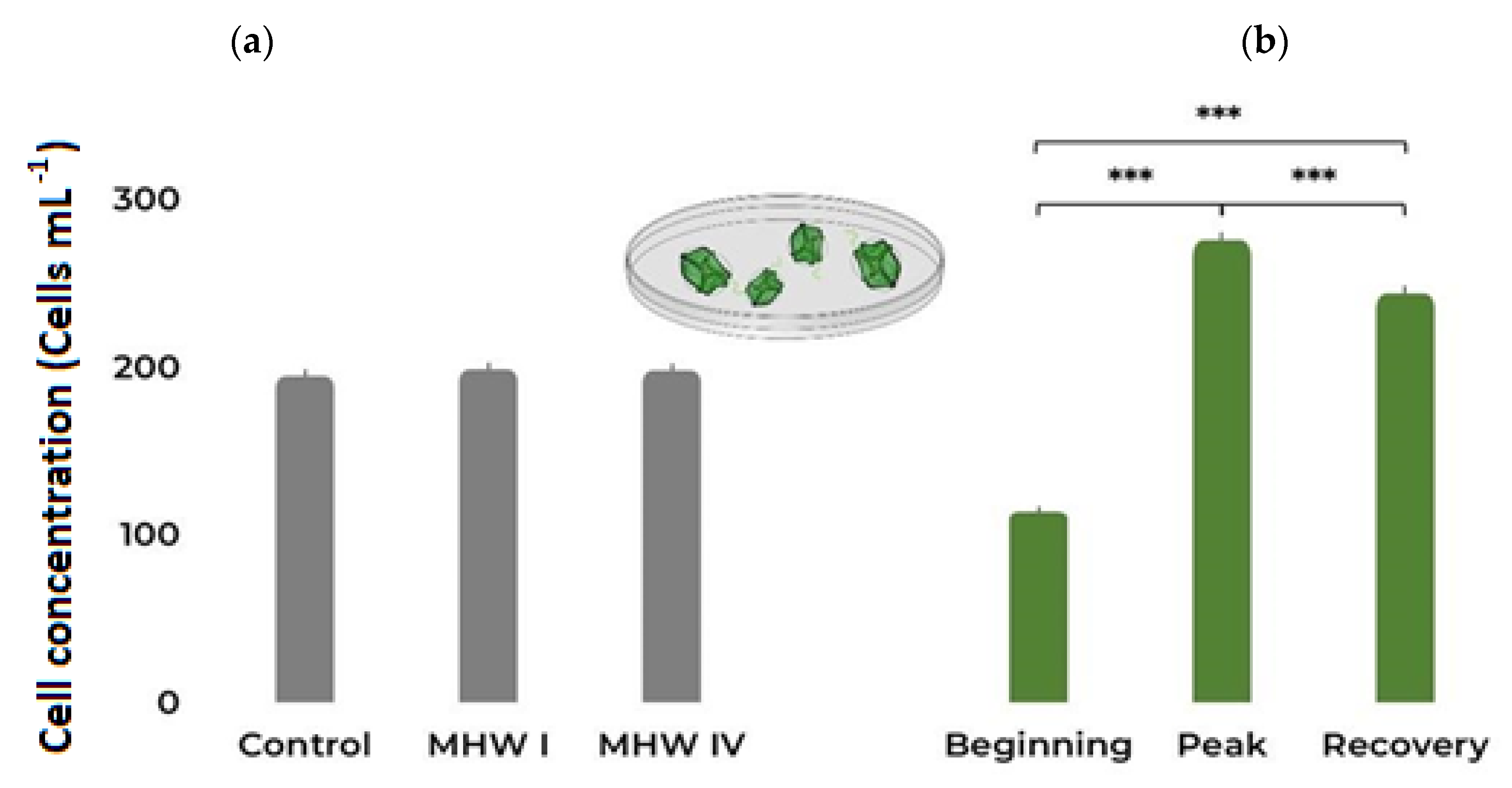
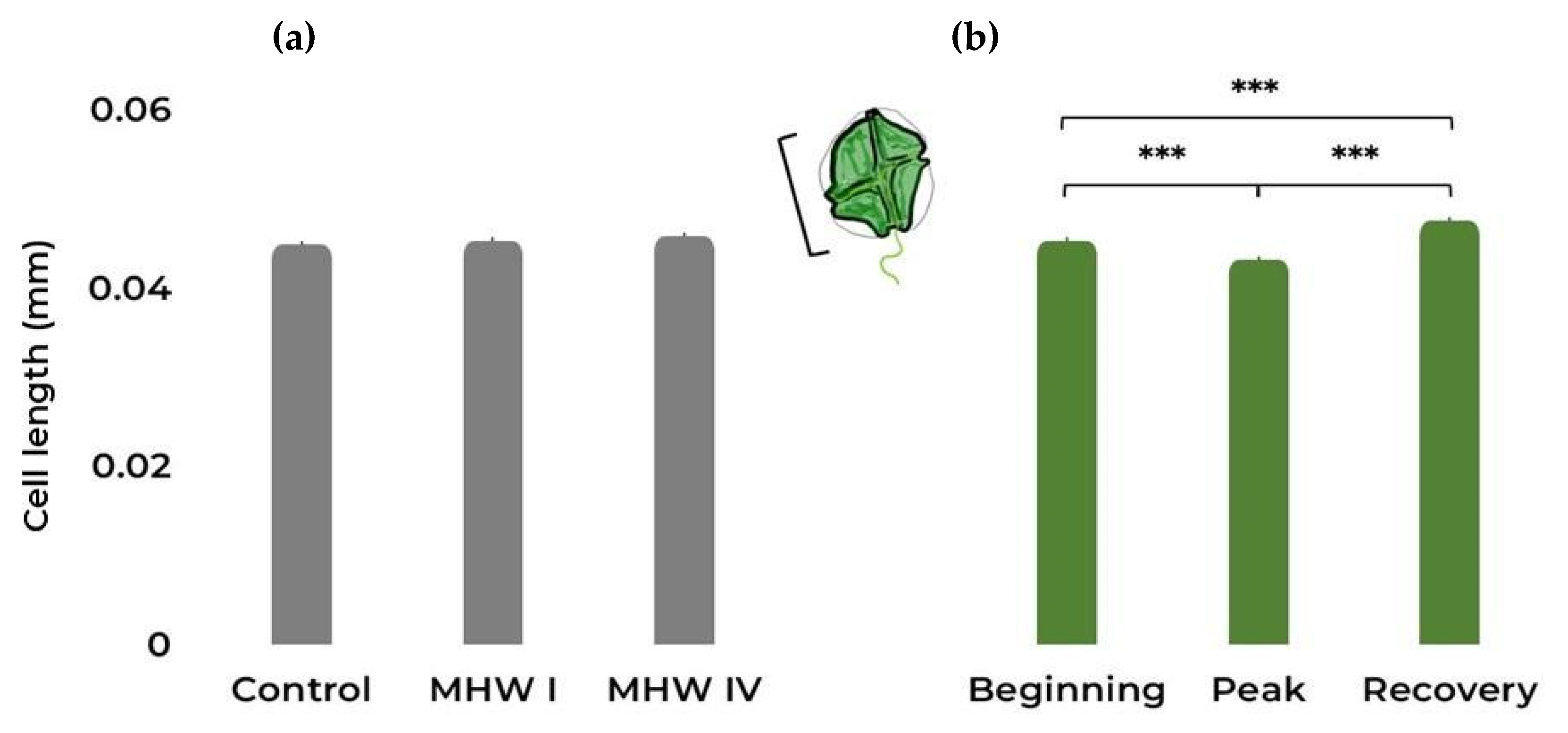
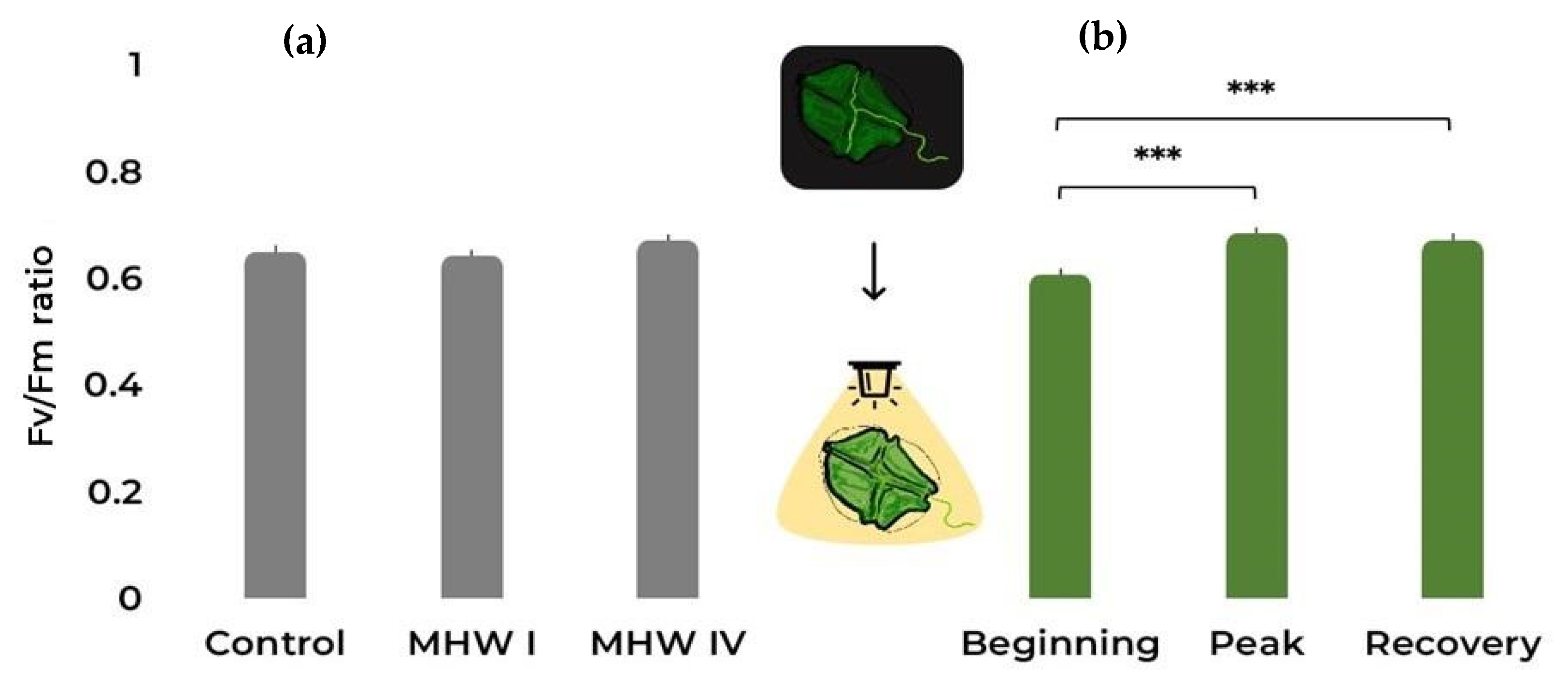
2.1. Cell Count, Size, and Photosynthetic Efficiency
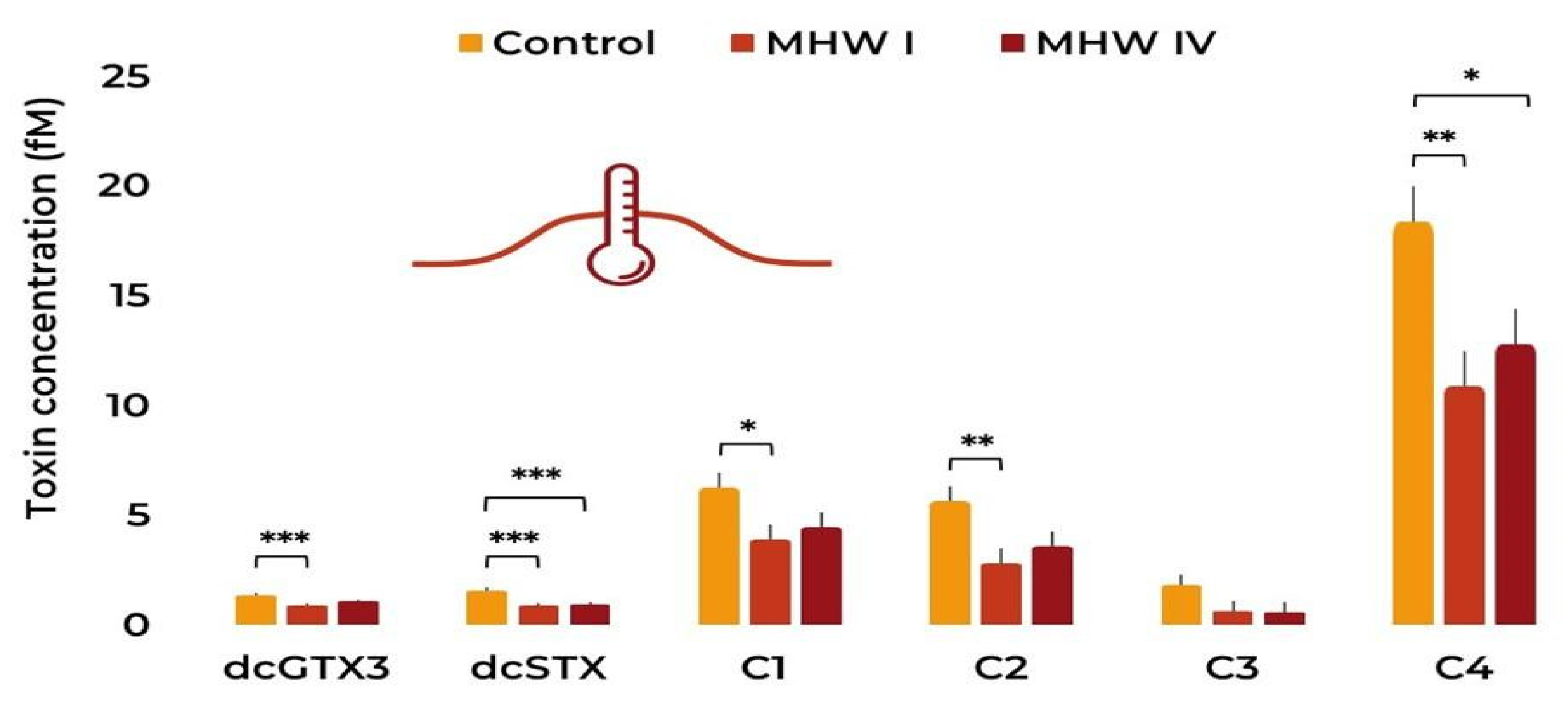
2.2. Toxin Concentration and Profile
3. Discussion
4. Materials and Methods
4.1. Strain Origin and Laboratory Acclimation
4.2. Experimental Design
4.3. Culture Maintenance and Sample Acquisition
4.4. Toxin Extraction and Quantification
4.4.1. Extraction
4.4.2. Solid-Phase Extraction (SPE) Clean-Up
4.4.3. LC-HRMS Analysis
4.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change (IPCC). Changing Ocean, Marine Ecosystems, and Dependent Communities. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2019; pp. 447–587. [Google Scholar] [CrossRef]
- Sampaio, E.; Santos, C.; Rosa, I.C.; Ferreira, V.; Pörtner, H.-O.; Duarte, C.M.; Levin, L.A.; Rosa, R. Impacts of hypoxic events surpass those of future ocean warming and acidification. Nat. Ecol. Evol. 2021, 5, 311–321. [Google Scholar] [CrossRef]
- Hobday, A.J.; Alexander, L.V.; Perkins, S.E.; Smale, D.A.; Straub, S.C.; Oliver, E.C.J.; Benthuysen, J.A.; Burrows, M.T.; Donat, M.G.; Feng, M.; et al. A hierarchical approach to defining marine heatwaves. Prog. Oceanogr. 2016, 141, 227–238. [Google Scholar] [CrossRef]
- Hobday, A.J.; Oliver, E.C.J.; Gupta, A.S.; Benthuysen, J.A.; Burrows, M.T.; Donat, M.G.; Holbrook, N.J.; Moore, P.J.; Thomsen, M.S.; Wernberg, T.; et al. Categorizing and Naming Marine Heatwaves. Oceanography 2018, 31, 162–173. [Google Scholar] [CrossRef]
- Collins, M.; Sutherland, M.; Bouwer, L.; Cheong, S.-M.; Frölicher, T.L.; Jacot Des Combes, H.; Roxy, M.K.; Losada, I.; McInnes, K.; Ratter, B.; et al. Extremes, Abrupt Changes and Managing Risks. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2019; pp. 589–655. [Google Scholar]
- Anderson, S.I.; Franzè, G.; Kling, J.D.; Wilburn, P.; Kremer, C.T.; Menden-Deuer, S.; Litchman, E.; Hutchins, D.A.; Rynearson, T.A. The interactive effects of temperature and nutrients on a spring phytoplankton community. Limnol. Oceanogr. 2022, 67, 634–645. [Google Scholar] [CrossRef]
- Fernández-González, C.; Tarran, G.A.; Schuback, N.; Woodward, E.M.S.; Arístegui, J.; Marañón, E. Phytoplankton responses to changing temperature and nutrient availability are consistent across the tropical and subtropical Atlantic. Commun. Biol. 2022, 5, 1035. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.L.; Trainer, V.L.; Smayda, T.J.; Karlson, B.S.; Trick, C.G.; Kudela, R.M.; Ishikawa, A.; Bernard, S.; Wulff, A.; Anderson, D.M.; et al. Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae 2015, 49, 68–93. [Google Scholar] [CrossRef] [PubMed]
- Woods Hole Oceanographic Institution. Harmful Algal Blooms Understanding the Threat and the Actions Being Taken to Address it. 2022, A Special Report from Woods Hole Oceanographic Institution. Available online: https://www.whoi.edu/wp-content/uploads/2022/01/2022WHOI-HarmfulAlgalBlooms-Report.pdf (accessed on 15 January 2023).
- Di Lorenzo, E.; Mantua, N. Multi-year persistence of the 2014/15 North Pacific marine heatwave. Nat. Clim. Chang. 2016, 6, 1042–1047. [Google Scholar] [CrossRef]
- McCabe, R.M.; Hickey, B.M.; Kudela, R.M.; Lefebvre, K.A.; Adams, N.G.; Bill, B.D.; Gulland, F.M.D.; Thomson, R.E.; Cochlan, W.P.; Trainer, V.L. An unprecedented coastwide toxic algal bloom linked to anomalous ocean conditions. Geophys. Res. Lett. 2016, 43, 10366–10376. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.P.; Kudela, R.M.; Birch, J.M.; Blum, M.; Bowers, H.A.; Chavez, F.P.; Doucette, G.J.; Hayashi, K.; Marin, R.; Mikulski, C.M.; et al. Causality of an extreme harmful algal bloom in Monterey Bay, California, during the 2014–2016 northeast Pacific warm anomaly. Geophys. Res. Lett. 2017, 44, 5571–5579. [Google Scholar] [CrossRef]
- Takagi, S.; Kuroda, H.; Hasegawa, N.; Watanabe, T.; Unuma, T.; Taniuchi, Y.; Yokota, T.; Izumida, D.; Nakagawa, T.; Kurokawa, T.; et al. Controlling factors of large-scale harmful algal blooms with Karenia selliformis after record-breaking marine heatwaves. Front. Mar. Sci. 2022, 9. [Google Scholar] [CrossRef]
- Kuroda, H.; Setou, T. Extensive Marine Heatwaves at the Sea Surface in the Northwestern Pacific Ocean in Summer 2021. Remote Sens. 2021, 13, 3989. [Google Scholar] [CrossRef]
- Hallegraeff, G.; Blackburn, S.; Doblin, M.; Bolch, C. Global toxicology, ecophysiology and population relationships of the chainforming PST dinoflagellate Gymnodinium catenatum. Harmful Algae 2012, 14, 130–143. [Google Scholar] [CrossRef]
- Band-Schmidt, C.J.; Bustillos-Guzmán, J.J.; Hernández-Sandoval, F.E.; Núñez-Vázquez, E.J.; López-Cortés, D.J. Effect of temperature on growth and paralytic toxin profiles in isolates of Gymnodinium catenatum (Dinophyceae) from the Pacific coast of Mexico. Toxicon 2014, 90, 199–212. [Google Scholar] [CrossRef]
- Lopes, V.; Costa, P.; Rosa, R. Effects of Harmful Algal Bloom Toxins on Marine Organisms. In Ecotoxicology of Marine Organisms; Duarte, B., Caçador, I., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 42–88. [Google Scholar] [CrossRef]
- Band-Schmidt, C.J.; Morquecho, L.; Lechuga-Devéze, C.H.; Anderson, D.M. Effects of growth medium, temperature, salinity and seawater source on the growth of Gymnodinium catenatum (Dinophyceae) from Bahia Concepcion, Gulf of California, Mexico. J. Plankton Res. 2004, 26, 1459–1470. [Google Scholar] [CrossRef]
- Oshima, Y.; Blackburn, S.I.; Hallegraeff, G.M. Comparative Study on Paralytic Shellfish Toxin of the Dinoflagellate Gymnodinium Catenatum from Three Different Countries Profiles. Mar. Biol. 1993, 116, 471–476. [Google Scholar] [CrossRef]
- Jentsch, A.; Kreyling, J.; Beierkuhnlein, C.A. New Generation of Climate-Change Experiments: Events, Not Trends. Front. Ecol. Environ. 2007, 5, 365–374. [Google Scholar] [CrossRef]
- Marañón, E.; Lorenzo, M.P.; Cermeño, P.; Mouriño-Carballido, B. Nutrient limitation suppresses the temperature dependence of phytoplankton metabolic rates. ISME J. 2018, 12, 1836–1845. [Google Scholar] [CrossRef] [PubMed]
- Lovecchio, S.; Climent, E.; Stocker, R.; Durham, W.M. Chain formation can enhance the vertical migration of phytoplankton through turbulence. Sci. Adv. 2019, 5, eaaw7879. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, D.; Ciotti, B.J.; Montagnes, D.J.S. Protists decrease in size linearly with temperature: Ca. 2.5% °C−1. Proc. R. Soc. B Boil. Sci. 2003, 270, 2605–2611. [Google Scholar] [CrossRef]
- Mai, G.; Liu, J.; Xia, X.; Pang, X.; Li, B.; Yu, L.; Tan, Y.; Song, X.; Li, G. Acutely Rising Temperature Reduces Photosynthetic Capacity of Phytoplankton Assemblages in Tropical Oceans: A Large-Scale Investigation. Front. Mar. Sci. 2021, 8, 902. [Google Scholar] [CrossRef]
- Milligan, A.J.; Halsey, K.H.; Behrenfeld, M.J. Advancing interpretations of 14C-uptake measurements in the context of phytoplankton physiology and ecology: Fig. 1. J. Plankton Res. 2015, 37, 692–698. [Google Scholar] [CrossRef]
- Brandenburg, K.M.; Velthuis, M.; Van de Waal, D.B. Meta-analysis reveals enhanced growth of marine harmful algae from temperate regions with warming and elevated CO2 levels. Glob. Chang. Biol. 2019, 25, 2607–2618. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, S.; Takatani, T.; Arakawa, O.; Noguchi, T. Effects of temperature on the PSP-excreting activity of toxic dinoflagellate Gymnodinium catenatum. Fish. Sci. 2002, 68, 625–626. [Google Scholar] [CrossRef]
- Navarro, J.; Muñoz, M.; Contreras, A. Temperature as a factor regulating growth and toxin content in the dinoflagellate Alexandrium catenella. Harmful Algae 2006, 5, 762–769. [Google Scholar] [CrossRef]
- Costa, P.R.; Robertson, A.; Quilliam, M.A. Toxin Profile of Gymnodinium catenatum (Dinophyceae) from the Portuguese Coast, as Determined by Liquid Chromatography Tandem Mass Spectrometry. Mar. Drugs 2015, 13, 2046–2062. [Google Scholar] [CrossRef]
- Silva, T.; Caeiro, M.F.; Costa, P.R.; Amorim, A. Gymnodinium catenatum Graham isolated from the Portuguese coast: Toxin content and genetic characterization. Harmful Algae 2015, 48, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Albinsson, M.E.; Negri, A.; Blackburn, S.I.; Bolch, C.J.S. Bacterial Community Affects Toxin Production by Gymnodinium catenatum. PLoS ONE 2014, 9, e104623. [Google Scholar] [CrossRef]
- Smith, E.; Mackintosh, F.; Grant, F.; Gallacher, S. Sodium channel blocking (SCB) activity and transformation of paralytic shellfish toxins (PST) by dinoflagellate-associated bacteria. Aquat. Microb. Ecol. 2002, 29, 1–9. [Google Scholar] [CrossRef]
- Flynn, K.J.; Fynn, K.; John, E.H.; Reguera, B.; Reyero, I.; Franco, J.M. Changes in toxins, intracellular and dissolved free amino acids of the toxic dinoflagellate Gymnodinium catenatum in response to changes in inorganic nutrients and salinity. J. Plankton Res. 1996, 18, 2093–2111. [Google Scholar] [CrossRef]
- Schlegel, R.W.; Smit, A.J.; Hobday, R.; Alexander, L.V.; Perkins, S.E.; Smale, D.A.; Straub, S.C.; Oliver, E.C.; Benthuysen, J.A.; sen Gupta, A.; et al. heatwaveR: A central algorithm for the detection of heatwaves and cold-spells. J. Open Source Softw. 2018, 3, 821. [Google Scholar] [CrossRef]
- Harrison, P.; Berges, J. Marine Culture Media. In Algal Culturing Techniques; Andersen, R., Ed.; Elsevier Academic Press: Cambridge, MA, USA, 2005; pp. 21–35. [Google Scholar]
- Costa, P.R.; Braga, A.C.; Turner, A.D. Accumulation and Elimination Dynamics of the Hydroxybenzoate Saxitoxin Analogues in Mussels Mytilus galloprovincialis Exposed to the Toxic Marine Dinoflagellate Gymnodinium catenatum. Toxins 2018, 10, 428. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, I.; Alfonso, A.; González-Jartín, J.M.; Vieytes, M.R.; Botana, L.M. A single run UPLC-MS/MS method for detection of all EU-regulated marine toxins. Talanta 2018, 189, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Benford, D.; Boobis, A.; Ceccatelli, S.; Cravedi, J.-P.; di Domenico, A.; Doerge, D.; Dogliotti, E.; Edler, L.; Farmer, P.; et al. Marine biotoxins in shellfish—Summary on regulated marine biotoxins. EFSA J. 2009, 7, 1306. [Google Scholar] [CrossRef]
- Madsen, H.; Thyregod, P. Introduction to General and Generalized Linear Models; CRC Press: Boca Raton, FL, USA, 2010; pp. 1–291. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; ISBN 3-900051-07-0. Available online: http://www.R-project.org/ (accessed on 10 August 2022).


| Response | Model Distribution | Predictor | χ2 | Degrees of Freedom | p-Value |
|---|---|---|---|---|---|
| Cell concentration | Poisson | Treatment | 6.08 | 2 | 0.048 |
| Stage | 917.9 | 2 | <0.001 | ||
| Treatment:Stage | 65.2 | 4 | <0.001 | ||
| Chain formation | Binomial | Treatment | 390.0 | 2 | <0.001 |
| Stage | 145.8 | 2 | <0.001 | ||
| Replicate | 70.1 | 3 | <0.001 | ||
| Treatment:Date | 56.2 | 4 | <0.001 | ||
| Cell length | Gaussian | Treatment | 2.75 | 2 | 0.253 |
| Stage | 65.16 | 2 | <0.001 | ||
| Treatment:Stage | 86.07 | 4 | <0.001 | ||
| Fv/Fm | Gaussian | Treatment | 3.949 | 2 | 0.139 |
| Stage | 29.754 | 2 | <0.001 | ||
| PSP toxicity | Gaussian | Treatment | 9.814 | 2 | 0.007 |
| Stage | 16.21 | 2 | <0.001 | ||
| dcGTX3 (fM) | Gaussian | Treatment | 13.33 | 2 | 0.001 |
| Stage | 29.44 | 2 | <0.001 | ||
| dcGTX3 (MF) | Gaussian | Treatment | 0.66 | 2 | 0.718 |
| Stage | 27.29 | 2 | <0.001 | ||
| Treatment:Stage | 9.18 | 4 | 0.057 | ||
| dcSTX (fM) | Gaussian | Treatment | 19.49 | 2 | <0.001 |
| Stage | 17.84 | 2 | <0.001 | ||
| dcSTX (MF) | Gaussian | Treatment | 6.55 | 2 | 0.038 |
| Stage | 9.51 | 2 | 0.009 | ||
| C1 (fM) | Gaussian | Treatment | 6.39 | 2 | 0.041 |
| Stage | 23.97 | 2 | <0.001 | ||
| C1 (MF) | Gaussian | Treatment | 2.36 | 2 | 0.308 |
| Stage | 12.99 | 2 | 0.002 | ||
| Treatment:Stage | 9.80 | 4 | 0.044 | ||
| C2 (fM) | Gaussian | Treatment | 9.44 | 2 | 0.008 |
| Stage | 30.37 | 2 | <0.001 | ||
| C2 (MF) | Gaussian | Treatment | 6.55 | 2 | 0.038 |
| Stage | 9.51 | 2 | 0.009 | ||
| C3 (fM) | Gaussian | Treatment | 4.38 | 2 | 0.112 |
| Stage | 7.99 | 2 | 0.018 | ||
| C3 (MF) | Gaussian | Treatment | 4.65 | 2 | 0.098 |
| Stage | 4.08 | 2 | 0.130 | ||
| C4 (fM) | Gaussian | Treatment | 12.16 | 2 | 0.002 |
| Stage | 40.50 | 2 | <0.001 | ||
| Treatment:Stage | 9.44 | 4 | 0.051 | ||
| C4 (MF) | Gaussian | Treatment | 1.02 | 2 | 0.602 |
| Stage | 18.06 | 2 | <0.001 |
| Category I | Category IV | |
|---|---|---|
| Peak intensity (°C) | 19.9 °C | 24.1 °C |
| Rate of onset (°C day−1) | 0.13 | 0.55 |
| Duration (days) | 10 | 10 |
| Rate of offset (°C day−1) | 0.14 | 0.56 |
| Total duration (days) | 30 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, V.M.; Court, M.; Seco, M.C.; Borges, F.O.; Vicente, B.; Lage, S.; Braga, A.C.; Duarte, B.; Santos, C.F.; Amorim, A.; et al. Gymnodinium catenatum Paralytic Shellfish Toxin Production and Photobiological Responses under Marine Heat Waves. Toxins 2023, 15, 157. https://doi.org/10.3390/toxins15020157
Lopes VM, Court M, Seco MC, Borges FO, Vicente B, Lage S, Braga AC, Duarte B, Santos CF, Amorim A, et al. Gymnodinium catenatum Paralytic Shellfish Toxin Production and Photobiological Responses under Marine Heat Waves. Toxins. 2023; 15(2):157. https://doi.org/10.3390/toxins15020157
Chicago/Turabian StyleLopes, Vanessa M., Mélanie Court, Martim Costa Seco, Francisco O. Borges, Bernardo Vicente, Sandra Lage, Ana Catarina Braga, Bernardo Duarte, Catarina Frazão Santos, Ana Amorim, and et al. 2023. "Gymnodinium catenatum Paralytic Shellfish Toxin Production and Photobiological Responses under Marine Heat Waves" Toxins 15, no. 2: 157. https://doi.org/10.3390/toxins15020157
APA StyleLopes, V. M., Court, M., Seco, M. C., Borges, F. O., Vicente, B., Lage, S., Braga, A. C., Duarte, B., Santos, C. F., Amorim, A., Costa, P. R., & Rosa, R. (2023). Gymnodinium catenatum Paralytic Shellfish Toxin Production and Photobiological Responses under Marine Heat Waves. Toxins, 15(2), 157. https://doi.org/10.3390/toxins15020157









