Absorption and Transport Mechanism of Red Meat-Derived N-glycolylneuraminic Acid and Its Damage to Intestinal Barrier Function through the NF-κB Signaling Pathway
Abstract
1. Introduction
2. Results
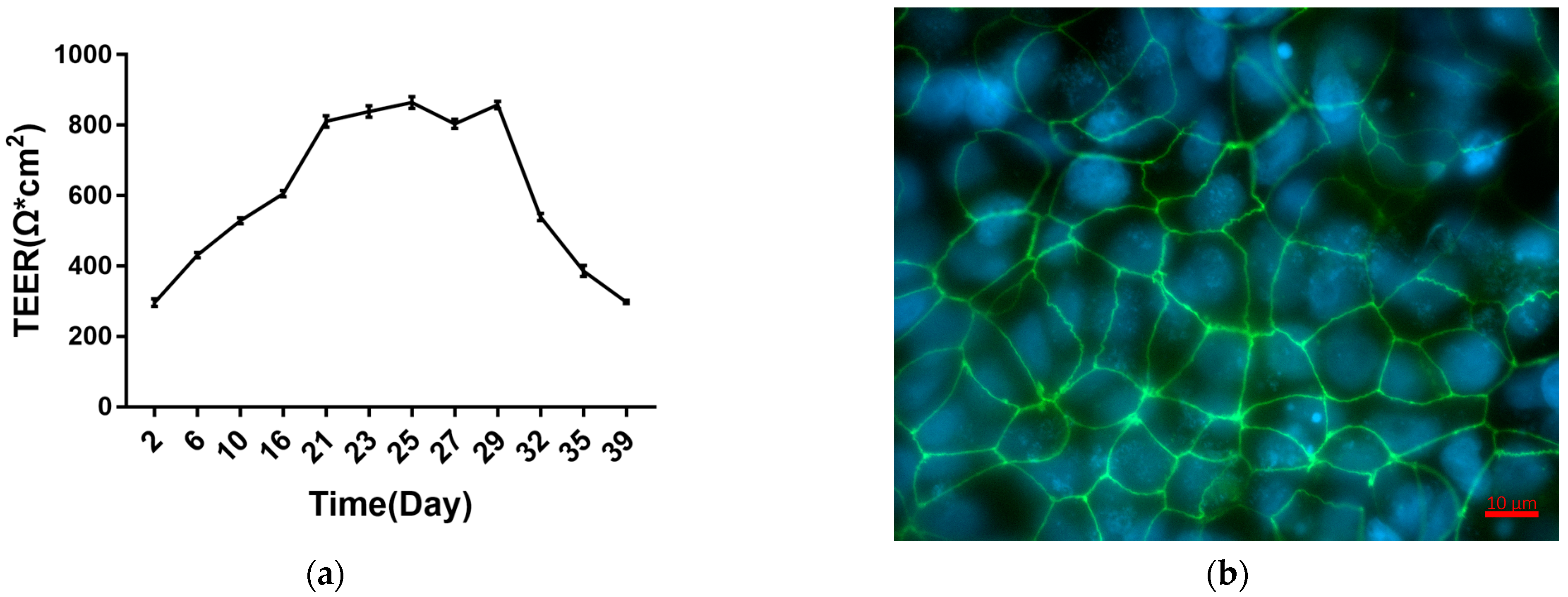
2.1. Establishment and Characterization of Caco-2 Cell Monolayers
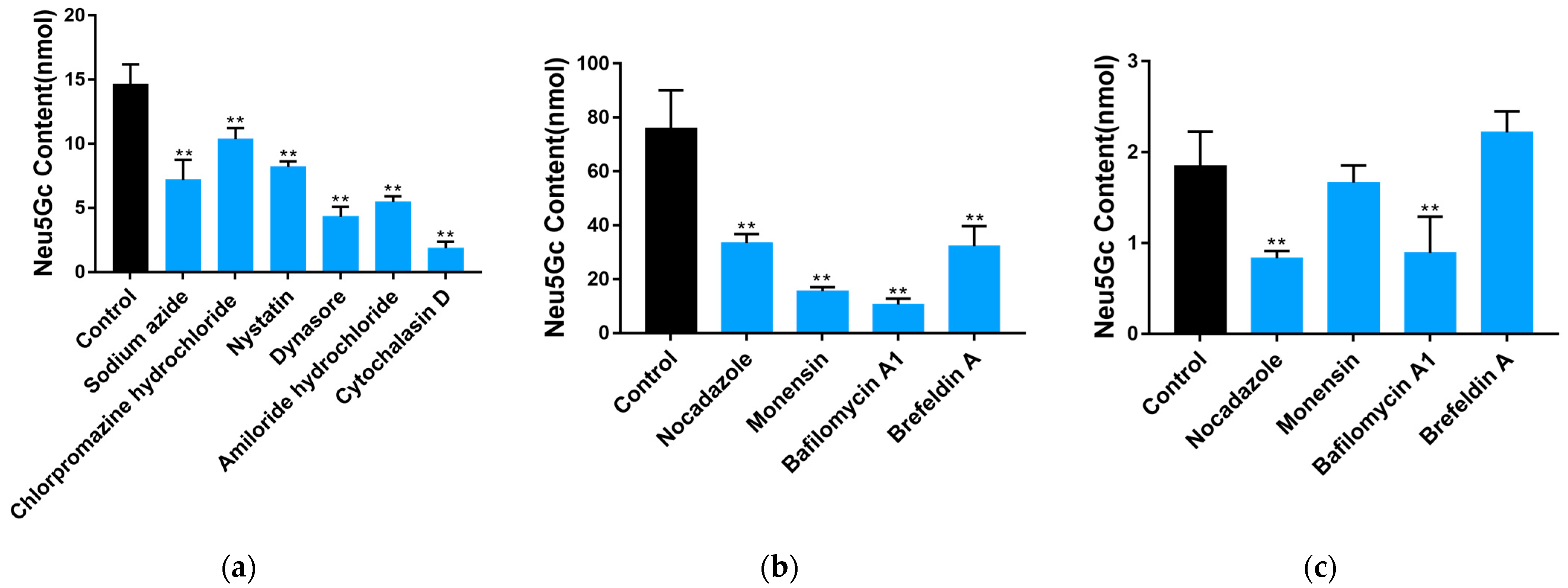
2.2. Uptake and Transport of Neu5Gc by Caco-2 Cells
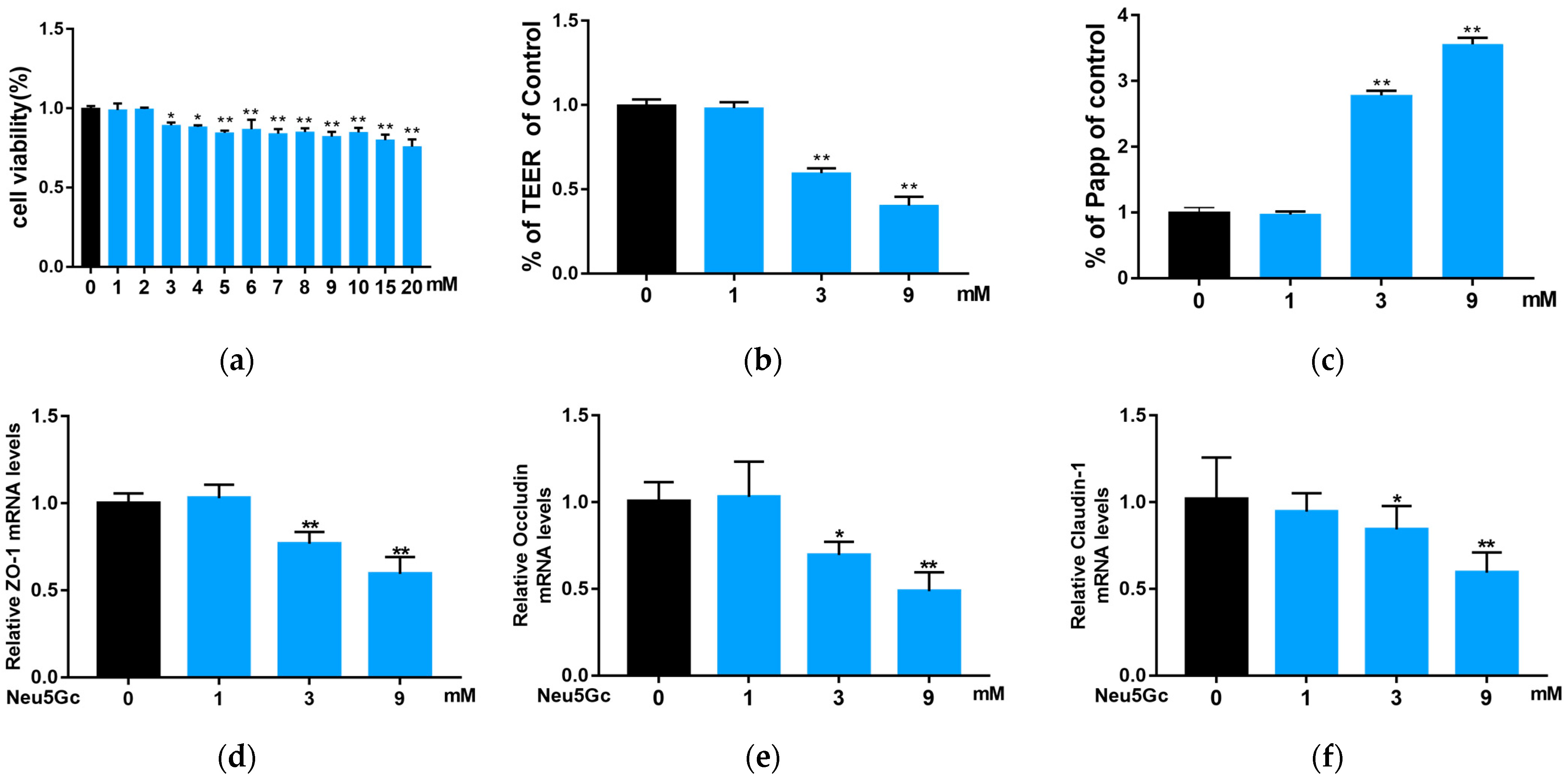
2.3. Effect of Neu5Gc on Intestinal Barrier Function
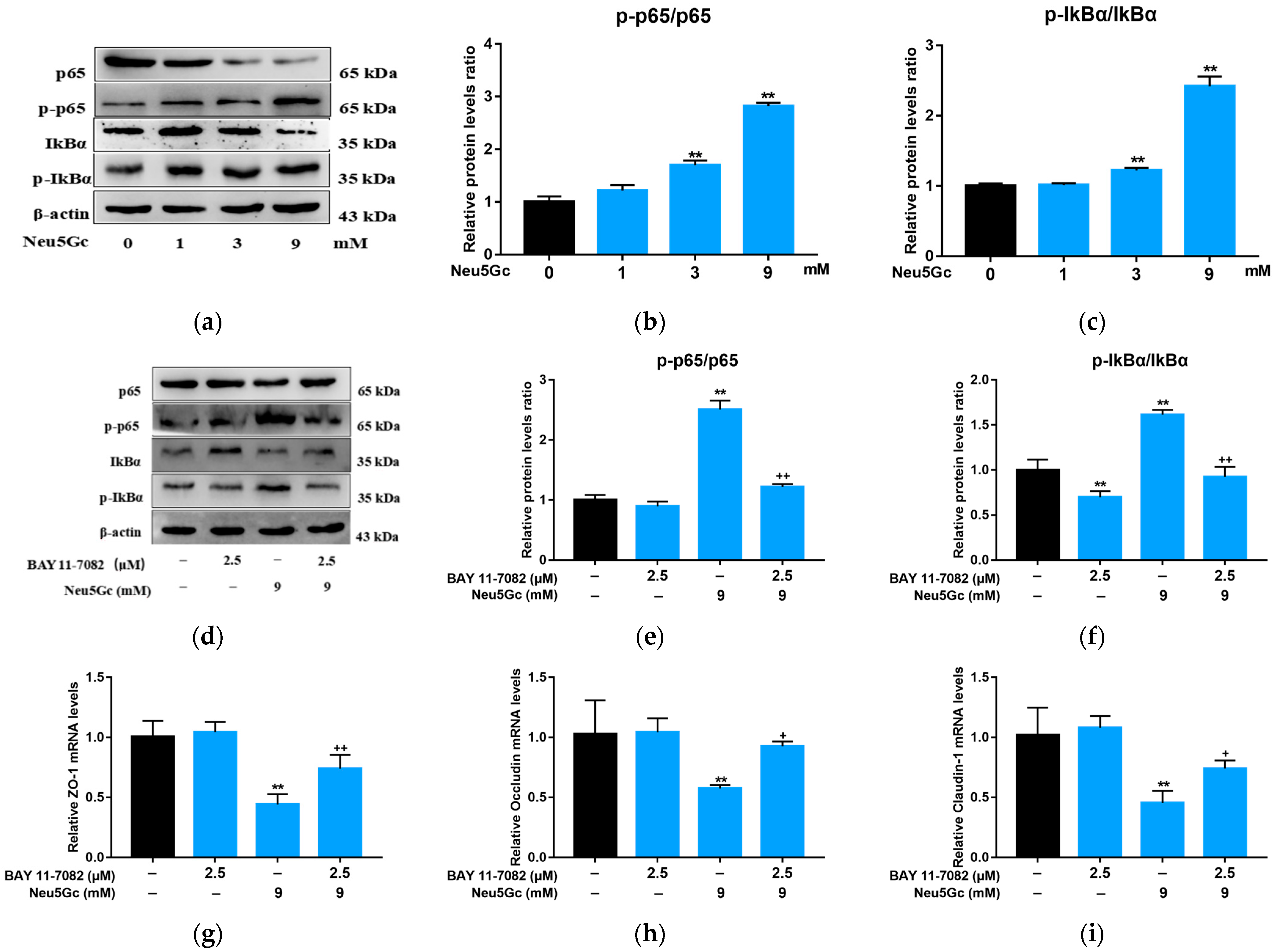
2.4. Neu5Gc Regulates the mRNA Expression of Inflammatory Factors in Intestinal Epithelial Cells through the Nuclear Factor kappa-B (NF-κB) Signaling Pathway
2.5. Activation of NF-κB Signaling Is Required by Neu5Gc-Induced Intestinal Epithelial Cell Barrier Dysfunction
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Reagents
5.2. Cell Culture and Neu5Gc Treatment
5.3. TEER Analysis
5.4. Measurement of Papp
5.5. Immunofluorescence
5.6. Endocytosis and Exocytosis Pathways of Neu5Gc in Caco-2 Cells
5.7. Quantification of Neu5Gc by DMB-HPLC
5.8. Endocytosis and Exocytosis Pathways of Neu5Gc in Caco-2 Cell Monolayers
5.9. Cell Viability Analysis
5.10. Quantitative Real-Time PCR
5.11. Western Blot Analysis
5.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, A.; Sun, Q.; Bernstein, A.M.; Schulze, M.B.; Manson, J.E.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Red Meat Consumption and Mortality: Results from 2 Prospective Cohort Studies. Arch. Intern. Med. 2012, 172, 555–563. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wolk, A.; Larsson, S.C.; Wolk, A. Meat Consumption and Risk of Colorectal Cancer: A Meta-Analysis of Prospective Studies. Int. J. Cancer 2006, 119, 2657–2664. [Google Scholar] [CrossRef]
- Norat, T.; Lukanova, A.; Ferrari, P.; Riboli, E. Meat Consumption and Colorectal Cancer Risk: Dose-Response Meta-Analysis of Epidemiological Studies. Int. J. Cancer 2002, 98, 241–256. [Google Scholar] [CrossRef]
- Alisson-Silva, F.; Kawanishi, K.; Varki, A. Human Risk of Diseases Associated with Red Meat Intake: Analysis of Current Theories and Proposed Role for Metabolic Incorporation of a Non-Human Sialic Acid. Mol. Asp. Med. 2016, 51, 16–30. [Google Scholar] [CrossRef]
- Turesky, R.J. Mechanistic Evidence for Red Meat and Processed Meat Intake and Cancer Risk: A Follow-up on the International Agency for Research on Cancer Evaluation of 2015. Chimia 2018, 72, 78. [Google Scholar] [CrossRef]
- Dhar, C.; Sasmal, A.; Varki, A. From “Serum Sickness” to “Xenosialitis”: Past, Present, and Future Significance of the Non-Human Sialic Acid Neu5Gc. Front. Immunol. 2019, 10, 807. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.H.; Hayakawa, T.; Diaz, S.; Krings, M.; Indriati, E.; Leakey, M.; Paabo, S.; Satta, Y.; Takahata, N.; Varki, A. Inactivation of CMP-N-Acetylneuraminic Acid Hydroxylase Occurred Prior to Brain Expansion during Human Evolution. Proc. Natl. Acad. Sci. USA 2002, 99, 11736–11741. [Google Scholar] [CrossRef]
- Hayakawa, T.; Aki, I.; Varki, A.; Satta, Y.; Takahata, N. Fixation of the Human-Specific CMP-N-Acetylneuraminic Acid Hydroxylase Pseudogene and Implications of Haplotype Diversity for Human Evolution. Genetics 2006, 172, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Tangvoranuntakul, P.; Gagneux, P.; Diaz, S.; Bardor, M.; Varki, N.; Varki, A.; Muchmore, E. Human Uptake and Incorporation of an Immunogenic Nonhuman Dietary Sialic Acid. Proc. Natl. Acad. Sci. USA 2003, 100, 12045–12050. [Google Scholar] [CrossRef] [PubMed]
- Banda, K.; Gregg, C.J.; Chow, R.; Varki, N.M.; Varki, A. Metabolism of Vertebrate Amino Sugars with N-Glycolyl Groups: Mechanisms Underlying Gastrointestinal Incorporation of the Non-Human Sialic Acid Xeno-Autoantigen N-Glycolylneuraminic Acid. J. Biol. Chem. 2012, 287, 28852–28864. [Google Scholar] [CrossRef]
- Rombouts, C.; Meulebroek, L.; De Spiegeleer, M.; Goethals, S.; Van Hecke, T.; De Smet, S.; De Vos, W.; Vanhaecke, L. Untargeted Metabolomics Reveals Elevated L-Carnitine Metabolism in Pig and Rat Colon Tissue Following Red Versus White Meat Intake. Mol. Nutr. Food Res. 2021, 65, 2000463. [Google Scholar] [CrossRef] [PubMed]
- Samraj, A.N.; Pearce, O.M.T.; Läubli, H.; Crittenden, A.N.; Bergfeld, A.K.; Banda, K.; Gregg, C.J.; Bingman, A.E.; Secrest, P.; Diaz, S.L.; et al. A Red Meat-Derived Glycan Promotes Inflammation and Cancer Progression. Proc. Natl. Acad. Sci. USA 2014, 112, 542–547. [Google Scholar] [CrossRef]
- Hedlund, M.; Padler-Karavani, V.; Varki, N.M.; Varki, A. Evidence for a Human-Specific Mechanism for Diet and Antibody-Mediated Inflammation in Carcinoma Progression. Proc. Natl. Acad. Sci. USA 2008, 105, 18936–18941. [Google Scholar] [CrossRef] [PubMed]
- Scobie, L.; Padler-Karavani, V.; Le Bas-Bernardet, S.; Crossan, C.; Blaha, J.; Matouskova, M.; Hector, R.D.; Cozzi, E.; Vanhove, B.; Charreau, B.; et al. Long-Term IgG Response to Porcine Neu5Gc Antigens without Transmission of PERV in Burn Patients Treated with Porcine Skin Xenografts. J. Immunol. 2013, 191, 2907–2915. [Google Scholar] [CrossRef]
- Pearce, O.; Laubli, H.; Verhagen, A.; Secrest, P.; Zhang, J.; Varki, N.M.; Crocker, P.R.; Bui, J.D.; Varki, A. Inverse Hormesis of Cancer Growth Mediated by Narrow Ranges of Tumor-Directed Antibodies. Proc. Natl. Acad. Sci. USA 2014, 111, 5998. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Fezeu, L.K.; Ben-Arye, S.L.; Yehuda, S.; Padler-Karavani, V. Association between Neu5Gc Carbohydrate and Serum Antibodies against It Provides the Molecular Link to Cancer: French NutriNet-Santé Study. BMC Med. 2020, 18, 262. [Google Scholar] [CrossRef]
- Camilleri, M.; Madsen, K.; Spiller, R.; Meerveld, B.G.V.; Verne, G.N. Intestinal Barrier Function in Health and Gastrointestinal Disease. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2012, 24, 503–512. [Google Scholar] [CrossRef]
- Bardor, M.; Nguyen, D.H.; Diaz, S.; Varki, A. Mechanism of Uptake and Incorporation of the Non-Human Sialic Acid N-Glycolylneuraminic Acid into Human Cells. J. Biol. Chem. 2005, 280, 4228–4237. [Google Scholar] [CrossRef]
- Chai, G.-H.; Xu, Y.; Chen, S.-Q.; Cheng, B.; Hu, F.-Q.; You, J.; Du, Y.-Z.; Yuan, H. Transport Mechanisms of Solid Lipid Nanoparticles across Caco-2 Cell Monolayers and Their Related Cytotoxicology. ACS Appl. Mater. Interfaces 2016, 8, 5929–5940. [Google Scholar] [CrossRef]
- Chen, Y. Nystain Regulates the Endocytosis and Uptake of Endostatin and EGFR Antibody. Ph.D. Thesis, Tsinghua University, Beijing, China, 2014. [Google Scholar]
- Wang, S. Elucidating the Cellular Uptake Mechanism of Graphene-Isolated-Au-Nano-Crystal. Master’s Thesis, Hunan University, Changsha, China, 2016. [Google Scholar]
- Duerr, C.U.; Hornef, M.W. The Mammalian Intestinal Epithelium as Integral Player in the Establishment and Maintenance of Host-Microbial Homeostasis. Semin. Immunol. 2012, 24, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Everard, A.; Duparc, T. Gut Microbiota, Enteroendocrine Functions and Metabolism. Curr. Opin. Pharmacol. 2013, 13, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Schoultz, I.; Keita, Å. Cellular and Molecular Therapeutic Targets in Inflammatory Bowel Disease—Focusing on Intestinal Barrier Function. Cells 2019, 8, 193. [Google Scholar] [CrossRef] [PubMed]
- Hurh, S.; Kang, B.; Choi, I.; Cho, B.; Lee, E.M.; Kim, H.; Kim, Y.J.; Chung, Y.S.; Jeong, J.C.; Hwang, J.-I.; et al. Human Antibody Reactivity against Xenogeneic N-Glycolylneuraminic Acid and Galactose-α-1,3-Galactose Antigen. Xenotransplantation 2016, 23, 279–292. [Google Scholar] [CrossRef]
- Reuven, E.M.; Leviatan Ben-Arye, S.; Marshanski, T.; Breimer, M.E.; Yu, H.; Fellah-Hebia, I.; Roussel, J.-C.; Costa, C.; Galiñanes, M.; Mañez, R.; et al. Characterization of Immunogenic Neu5Gc in Bioprosthetic Heart Valves. Xenotransplantation 2016, 23, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Mirakhur, B.; Chan, E.; Le, Q.-T.; Berlin, J.; Morse, M.; Murphy, B.A.; Satinover, S.M.; Hosen, J.; Mauro, D.; et al. Cetuximab-Induced Anaphylaxis and IgE Specific for Galactose-α-1,3-Galactose. N. Engl. J. Med. 2008, 358, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Naito-Matsui, Y.; Davies, L.R.L.; Takematsu, H.; Chou, H.-H.; Tangvoranuntakul, P.; Carlin, A.F.; Verhagen, A.; Heyser, C.J.; Yoo, S.-W.; Choudhury, B.; et al. Physiological Exploration of the Long Term Evolutionary Selection against Expression of N-Glycolylneuraminic Acid in the Brain. J. Biol. Chem. 2017, 292, 2557–2570. [Google Scholar] [CrossRef]
- Padlerkaravani, V.; Hedlund, M.; Hai, Y.; Cao, H.; Chokhawala, H.; Karp, F.; Data, A.; Xi, C.; Varki, N.; Varki, A. Neu5Gc and Human Anti-Neu5Gc Antibodies in Inflammation-Induced Carcinoma Progression. Cancer Res. 2008, 68, 5397. [Google Scholar]
- Tahara, H.; Ide, K.; Basnet, N.B.; Tanaka, Y.; Matsuda, H.; Takematsu, H.; Kozutsumi, Y.; Ohdan, H. Immunological Property of Antibodies against N-Glycolylneuraminic Acid Epitopes in Cytidine Monophospho-N-Acetylneuraminic Acid Hydroxylase-Deficient Mice. J. Immunol. 2010, 184, 3269–3275. [Google Scholar] [CrossRef]
- Padler-Karavani, V.; Yu, H.; Cao, H.; Chokhawala, H.; Karp, F. Diversity in Specificity, Abundance, and Composition of Anti-Neu5Gc Antibodies in Normal Humans: Potential Implications for Disease. Glycobiology 2008, 18, 818–830. [Google Scholar] [CrossRef]
- Coussens, L.M.; Zitvogel, L.; Palucka, A.K. Neutralizing Tumor-Promoting Chronic Inflammation: A Magic Bullet? Science 2013, 339, 286–291. [Google Scholar] [CrossRef]
- de Visser, K.E.; Eichten, A.; Coussens, L.M. Paradoxical Roles of the Immune System during Cancer Development. Nat. Rev. Cancer 2006, 6, 24–37. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-Related Inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, M.; Ng, E.; Varki, A.; Varki, N.M. α 2-6 Linked Sialic Acids on N-Glycans Modulate Carcinoma Differentiation In Vivo. Cancer Res. 2008, 68, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Padler-Karavani, V.; Varki, A. Potential Impact of the Non-Human Sialic Acid N-Glycolylneuraminic Acid on Transplant Rejection Risk. Xenotransplantation 2011, 18, 1–5. [Google Scholar] [CrossRef]
- Hopkins, A.P.; Hawkhead, J.A.; Thomas, G.H. Transport and Catabolism of the Sialic Acids N-Glycolylneuraminic Acid and 3-Keto-3-Deoxy-d-Glycero-d-Galactonononic Acid by Escherichia coli K-12. FEMS Microbiol. Lett. 2013, 347, 14–22. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Guo, S.; Ye, D.; Rawat, M.; Ma, T.Y. TNF-α Modulation of Intestinal Tight Junction Permeability Is Mediated by NIK/IKK-α Axis Activation of the Canonical NF-ΚB Pathway. Am. J. Pathol. 2016, 186, 1151–1165. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Ye, D.; Said, H.M.; Ma, T.Y. IL-1β-Induced Increase in Intestinal Epithelial Tight Junction Permeability Is Mediated by MEKK-1 Activation of Canonical NF-ΚB Pathway. Am. J. Pathol. 2010, 177, 2310–2322. [Google Scholar] [CrossRef]
- Ying, C.; Hong, W.; Nianhui, Z.; Chunlei, W.; Kehe, H.; Cuiling, P. Nontoxic Concentrations of OTA Aggravate DON-Induced Intestinal Barrier Dysfunction in IPEC-J2 Cells via Activation of NF-ΚB Signaling Pathway. Toxicol. Lett. 2019, 311, 114–124. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, W.; Wang, H.; Cao, M.; Li, M.; Zhao, J.; Hu, Y.; Wang, Y.; Li, S.; Xie, Y.; et al. Inhibition of CREB-Mediated ZO-1 and Activation of NF-ΚB-Induced IL-6 by Colonic Epithelial MCT4 Destroys Intestinal Barrier Function. Cell Prolif. 2019, 52, e12673. [Google Scholar] [CrossRef]
- Nenci, A.; Becker, C.; Wullaert, A.; Gareus, R.; van Loo, G.; Danese, S.; Huth, M.; Nikolaev, A.; Neufert, C.; Madison, B.; et al. Epithelial NEMO Links Innate Immunity to Chronic Intestinal Inflammation. Nature 2007, 446, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Ye, D.; Dokladny, K.; Ma, T.Y. Mechanism of IL-1β-Induced Increase in Intestinal Epithelial Tight Junction Permeability. J. Immunol. 2008, 180, 5653–5661. [Google Scholar] [CrossRef] [PubMed]
- Verhoeckx, K.; Cotter, P.; Lopez-Exposito, I.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H.J. The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer International Publishing AG: Cham, Switzerland, 2015. [Google Scholar]
- Ivanov, A.I. (Ed.) Exocytosis and Endocytosis; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2008; ISBN 978-1-58829-865-2. [Google Scholar]
- Hara, S.; Yamaguchi, M.; Takemori, Y.; Furuhata, K.; Ogura, H.; Nakamura, M. Determination of Mono-O-Acetylated N-Acetylneuraminic Acids in Human and Rat Sera by Fluorometric High-Performance Liquid Chromatography. Anal. Biochem. 1989, 179, 162–166. [Google Scholar] [CrossRef] [PubMed]





| Indicators | Monolayer 1 | Monolayer 2 | Monolayer 3 | Monolayer 4 | Monolayer 5 | Monolayer 6 | Monolayer 7 |
|---|---|---|---|---|---|---|---|
| Papp (×10−6 cm/s) | 0.0210 ± 0.0014 | 0.0322 ± 0.0039 | 0.0305 ± 0.0029 | 0.0283 ± 0.0025 | 0.0268 ± 0.0025 | 0.0286 ± 0.0028 | 0.0257 ± 0.0027 |
| TEER (Ω*cm2) | 974.40 ± 20.08 | 859.79 ± 44.82 | 808.27 ± 39.46 | 949.01 ± 30.79 | 936.69 ± 40.51 | 961.71 ± 29.04 | 1022.56 ± 17.51 |
| Inhibitor | Classification | Concentration | Function |
|---|---|---|---|
| Sodium azide | Endocytosis | 1 mg/mL | Inhibit energy-dependent active transportation |
| Chlorpromazine | Endocytosis | 30 μM | Inhibit clathrin-dependent endocytosis |
| Nystatin | Endocytosis | 25 μg/mL | Inhibit caveolin-dependent endocytosis |
| Amiloride hydrochloride | Endocytosis | 3 mM | Inhibit macropinocytosis and phagocytosis |
| Dynasore | Endocytosis | 80 μM | Inhibit both clathrin- and caveolin-dependent endocytosis |
| Cytochalasin D | Endocytosis | 10 μM | Inhibit macropinocytosis and phagocytosis |
| Nocodazole | Exocytosis | 6 μg/mL | Inhibit microtubule-vesicle mediated protein transport to membranes |
| Monensin | Exocytosis | Monensin | Inhibit the transportation between Golgi apparatus and cell membranes |
| Bafilomycin A1 | Exocytosis | 100 nM | Inhibit the maturation process of early endosomes to lysosomes |
| Brefeldin A | Exocytosis | 25 μg/mL | Inhibit the transportation between endoplasmic reticulum (ER) and Golgi apparatus |
| Genes | Accession Number | Primers | Sequences | Product Length (bp) |
|---|---|---|---|---|
| ZO-1 | NM_001301025.3 | Forward Reverse | CAGAAATACCTGACGGTGCT TCCATTGCTGTGCTAGTGAG | 233 |
| Occludin | NM_021101.4 | Forward Reverse | CCGGCGACAACATCGTGAC CGGGTTGCTTGCAATGTGC | 136 |
| Claudin-1 | NM_002538.2 | Forward Reverse | AAGAGTTGACAGTCCCATGGCATAC ATCCACAGGCGAAGTTAATGGAAG | 133 |
| Claudin-4 | NM_001305.5 | Forward Reverse | AGACTTCTACAATCCGCTGG ACCTTACACGTAGTTGCTGG | 199 |
| IL-Iβ | NM_000576.3 | Forward Reverse | AGGCACAAGGCACAACAGGCT GTCCTGGAAGGAGCACTTCATCTGT | 187 |
| IL-6 | NM_001371096.1 | Forward Reverse | AGACAGCCACTCACCTCTTCAG TTCTGCCAGTGCCTCTTTGCTG | 132 |
| TNF-α | NM_000594.4 | Forward Reverse | CTCTTCTGCCTGCTGCACTTTG ATGGGCTACAGGCTTGTCACTC | 135 |
| TGF-β | NM_000660.7 | Forward Reverse | AACCCACAACGAAATCTATGAC GCTGAGGTATCGCCAGGAAT | 205 |
| IL-10 | NM_000572.3 | Forward Reverse | GCTCTTGCAAAACCAAACCAC TGGCAACCCAGGTAACCCTTA | 286 |
| GAPDH | NM_001357943.2 | Forward Reverse | GTCTCCTCTGACTTCAACAGCG ACCACCCTGTTGCTGTAGCCAA | 131 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, E.; Quan, W.; Luo, J.; Liu, C.; Zheng, W.; Shen, Q. Absorption and Transport Mechanism of Red Meat-Derived N-glycolylneuraminic Acid and Its Damage to Intestinal Barrier Function through the NF-κB Signaling Pathway. Toxins 2023, 15, 132. https://doi.org/10.3390/toxins15020132
He E, Quan W, Luo J, Liu C, Zheng W, Shen Q. Absorption and Transport Mechanism of Red Meat-Derived N-glycolylneuraminic Acid and Its Damage to Intestinal Barrier Function through the NF-κB Signaling Pathway. Toxins. 2023; 15(2):132. https://doi.org/10.3390/toxins15020132
Chicago/Turabian StyleHe, Enqi, Wei Quan, Jie Luo, Chuxin Liu, Wanting Zheng, and Qingwu Shen. 2023. "Absorption and Transport Mechanism of Red Meat-Derived N-glycolylneuraminic Acid and Its Damage to Intestinal Barrier Function through the NF-κB Signaling Pathway" Toxins 15, no. 2: 132. https://doi.org/10.3390/toxins15020132
APA StyleHe, E., Quan, W., Luo, J., Liu, C., Zheng, W., & Shen, Q. (2023). Absorption and Transport Mechanism of Red Meat-Derived N-glycolylneuraminic Acid and Its Damage to Intestinal Barrier Function through the NF-κB Signaling Pathway. Toxins, 15(2), 132. https://doi.org/10.3390/toxins15020132





